Qualitative and Quantitative Sex-Related Differences in the Perception of Single Molecules from Coffee Headspace
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Olfactory Sensitivity Screening
2.3. Dynamic Headspace Sampling
2.4. Mass Spectrometry/Gas Chromatography–Olfactometry (MS/GC-O) Analysis
2.5. Statistical Analysis
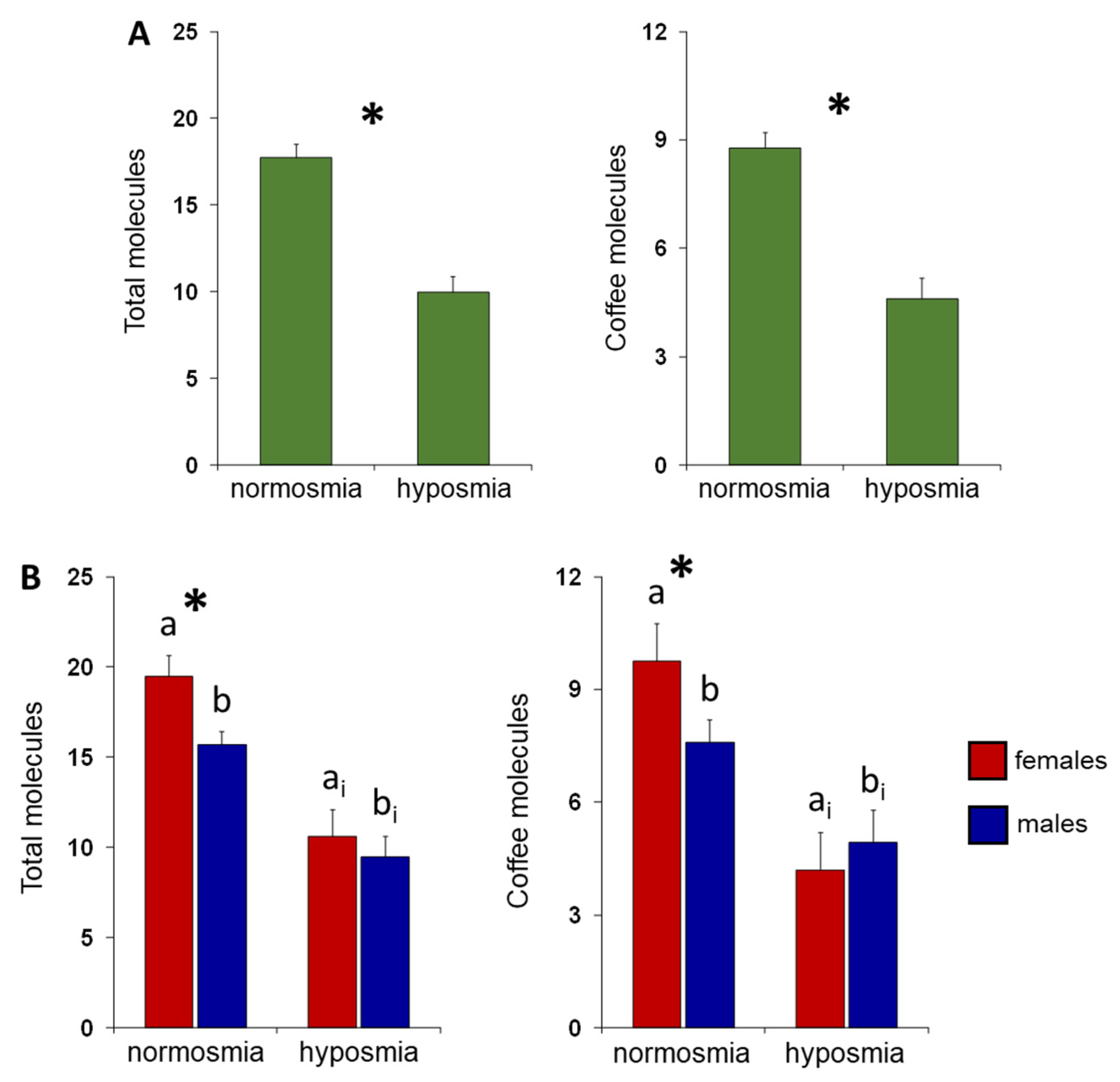
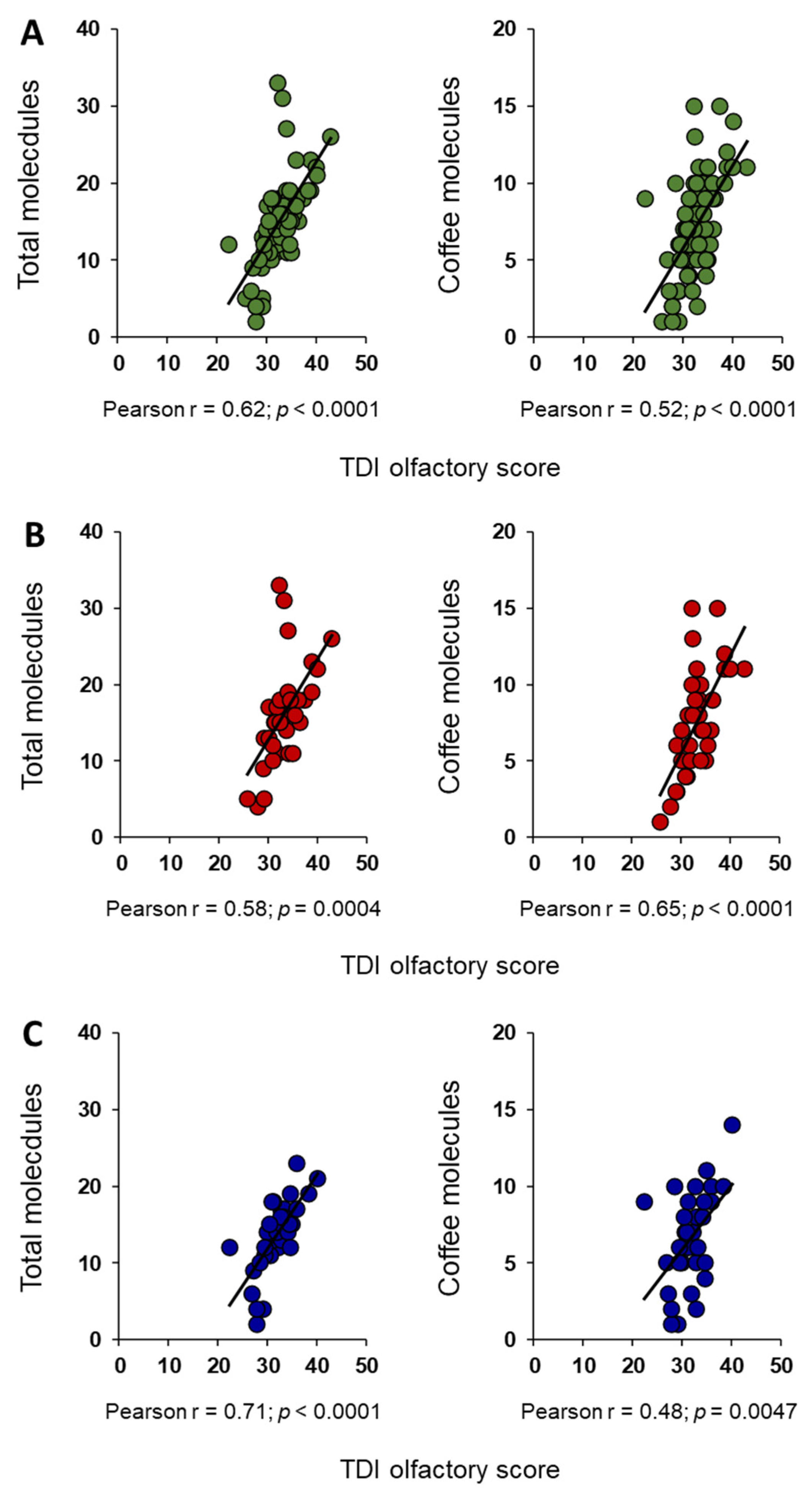
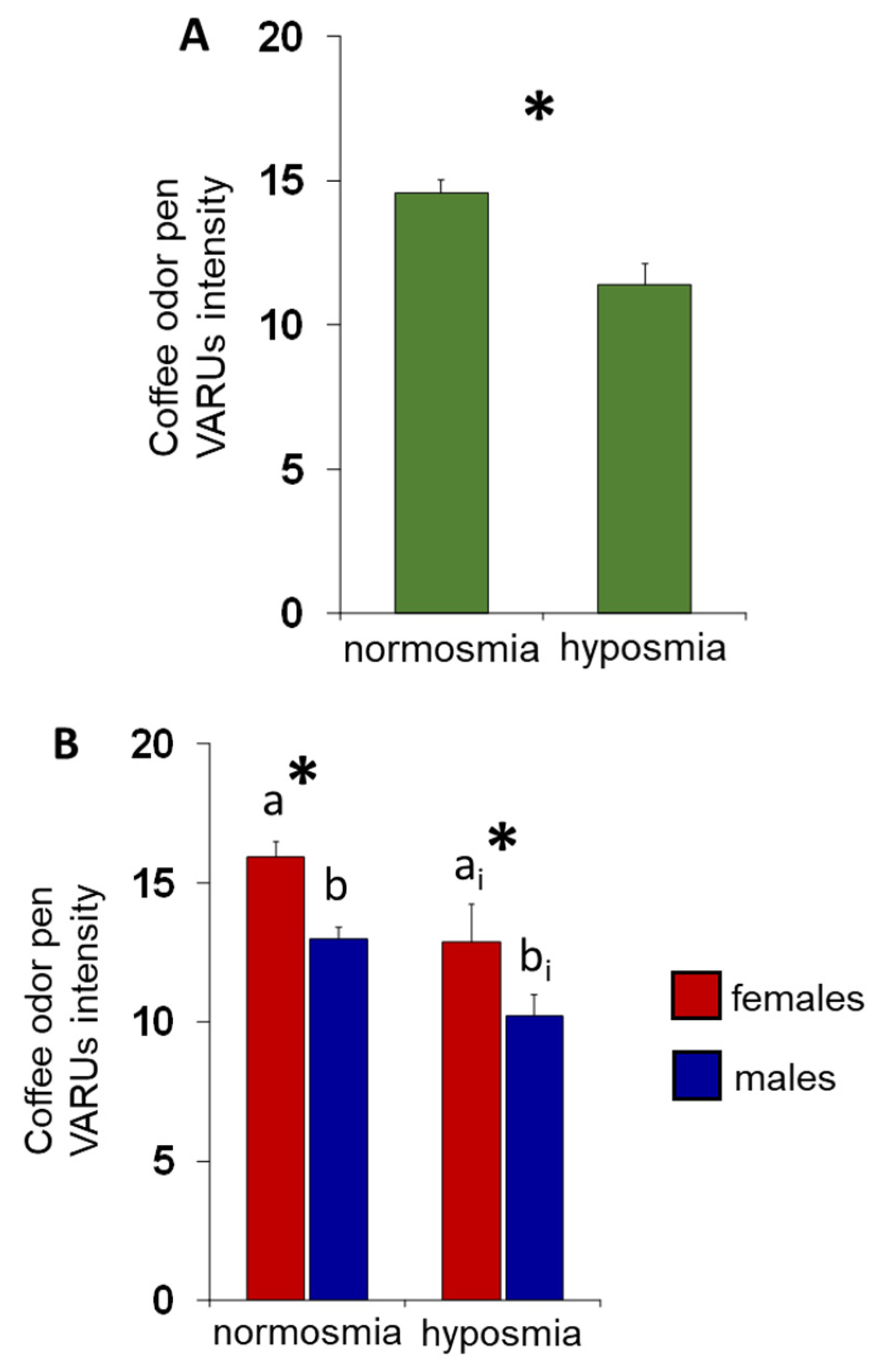
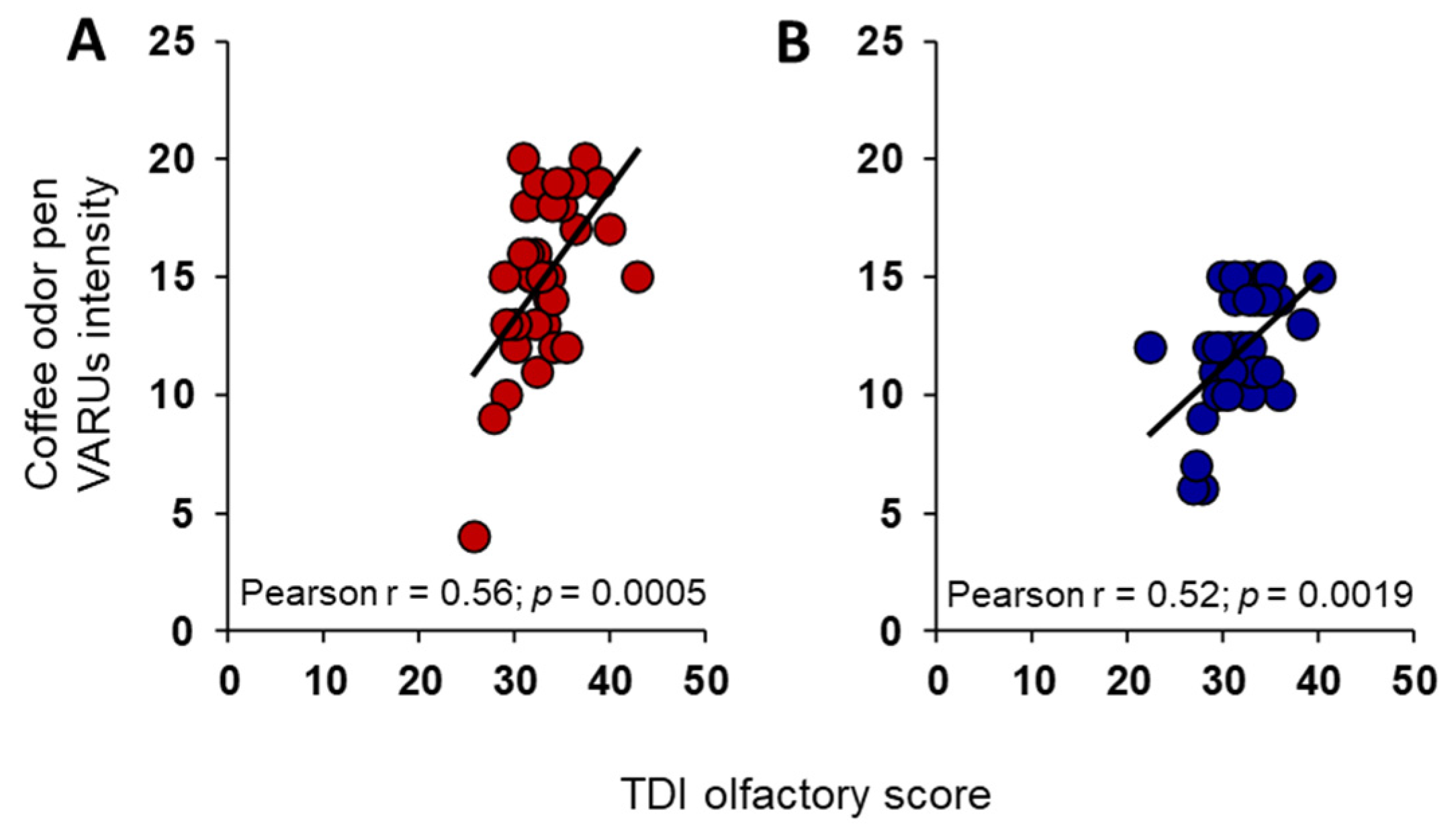
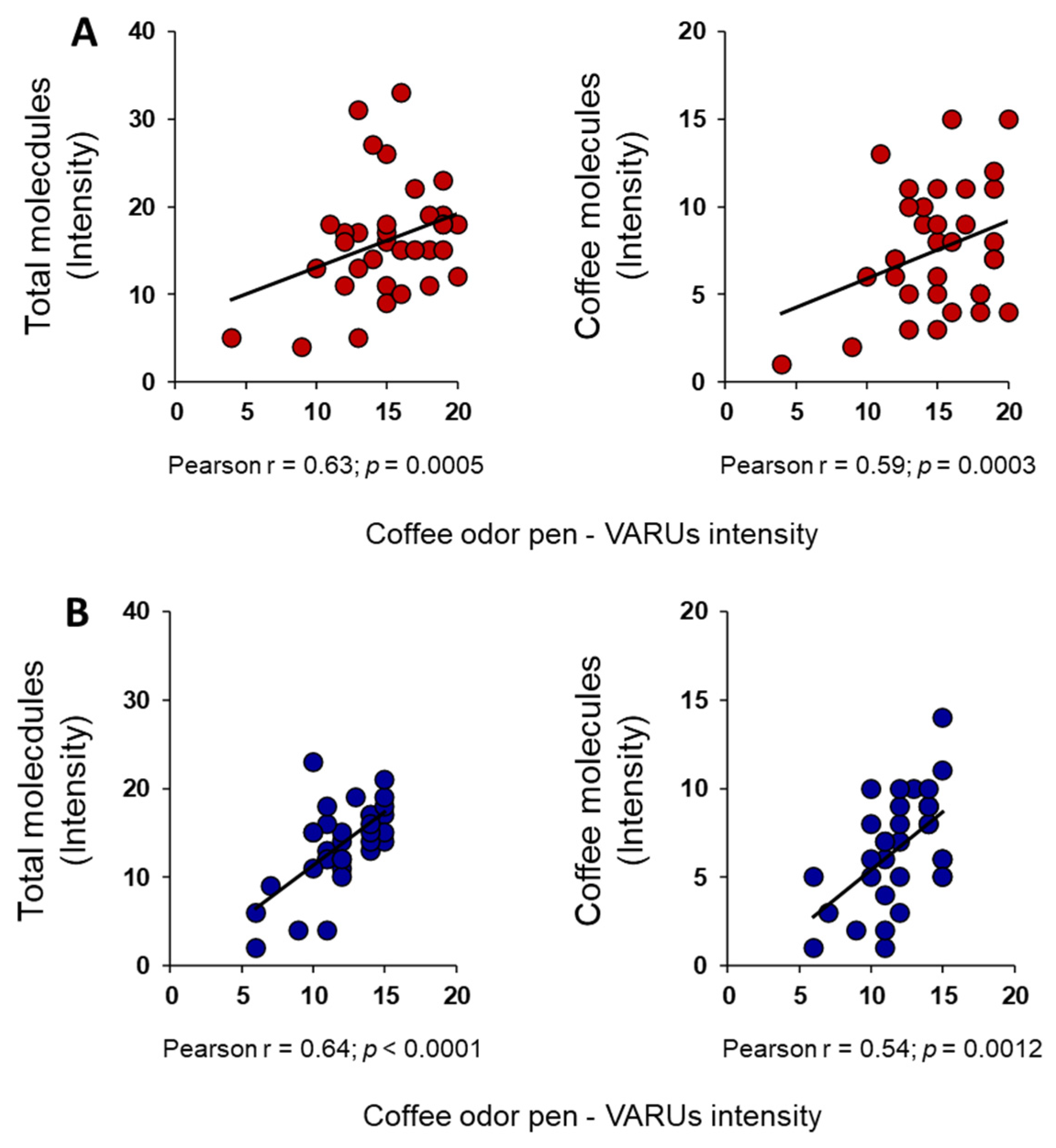
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.S.; Hummel, T. The influence of olfactory loss on dietary behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef]
- Connor, E.E.; Zhou, Y.; Liu, G.E. The essence of appetite: Does olfactory receptor variation play a role? J. Anim. Sci. 2018, 96, 1551–1558. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory Disorders and Quality of Life—An Updated Review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef]
- Hummel, T.; Nordin, S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005, 125, 116–121. [Google Scholar] [CrossRef]
- Landolt, P.J.; Heath, R.R.; Chambers, D.L. Oriented flight responses of female Mediterranean fruit flies to calling males, odor of calling males, and a synthetic pheromone blend. Entomol. Exp. Appl. 1992, 65, 259–266. [Google Scholar] [CrossRef]
- Lebreton, S.; Borrero-Echeverry, F.; Gonzalez, F.; Solum, M.; Wallin, E.A.; Hedenström, E.; Hansson, B.S.; Gustavsson, A.-L.; Bengtsson, M.; Birgersson, G.; et al. A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 2017, 15, 88. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Zhang, L.; Xu, X.; Cao, Z.; Zhang, L. Expressions of Olfactory Proteins in Locust Olfactory Organs and a Palp Odorant Receptor Involved in Plant Aldehydes Detection. Front. Physiol. 2018, 9, 663. [Google Scholar] [CrossRef]
- Solari, P.; Corda, V.; Sollai, G.; Kreissl, S.; Galizia, C.G.; Crnjar, R. Morphological characterization of the antennal lobes in the Mediterranean fruit fly Ceratitis capitata. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2016, 202, 131–146. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Crnjar, R. Olfactory sensitivity to major, intermediate and trace components of sex pheromone in Ceratitis capitata is related to mating and circadian rhythm. J. Insect Physiol. 2018, 110, 23–33. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Crnjar, R. Differences in the Olfactory Sensitivity of Ceratitis capitata to Headspace of Some Host Plants in Relation to Sex, Mating Condition and Population. Diversity 2020, 12, 207. [Google Scholar] [CrossRef]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory perception: Receptors, cells, and circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J. Am. Diet. Assoc. 1995, 95, 879–884, quiz 885–876. [Google Scholar] [CrossRef] [PubMed]
- Erskine, S.E.; Philpott, C.M. An unmet need: Patients with smell and taste disorders. Clin. Otolaryngol. 2020, 45, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ferris, A.M.; Duffy, V.B. Effect of olfactory deficits on nutritional status. Does age predict persons at risk? Ann. N. Y. Acad. Sci. 1989, 561, 113–123. [Google Scholar] [CrossRef]
- Gaillet-Torrent, M.; Sulmont-Rossé, C.; Issanchou, S.; Chabanet, C.; Chambaron, S. Impact of a non-attentively perceived odour on subsequent food choices. Appetite 2014, 76, 17–22. [Google Scholar] [CrossRef]
- Manesse, C.; Ferdenzi, C.; Sabri, M.; Bessy, M.; Rouby, C.; Faure, F.; Bellil, D.; Jomain, S.; Landis, B.N.; Hugentobler, M.; et al. Dysosmia-Associated Changes in Eating Behavior. Chemosens. Percept. 2017, 10, 104–113. [Google Scholar] [CrossRef]
- Postma, E.; Graaf, C.; Boesveldt, S. Food preferences and intake in a population of Dutch individuals with self-reported smell loss: An online survey. Food Qual. Prefer. 2019, 79, 103771. [Google Scholar] [CrossRef]
- Seo, H.S.; Guarneros, M.; Hudson, R.; Distel, H.; Min, B.C.; Kang, J.K.; Croy, I.; Vodicka, J.; Hummel, T. Attitudes toward Olfaction: A Cross-regional Study. Chem. Senses 2011, 36, 177–187. [Google Scholar] [CrossRef]
- Stroebele, N.; De Castro, J.M. Effect of ambience on food intake and food choice. Nutrition 2004, 20, 821–838. [Google Scholar] [CrossRef]
- Albrecht, J.; Schreder, T.; Kleemann, A.M.; Schöpf, V.; Kopietz, R.; Anzinger, A.; Demmel, M.; Linn, J.; Kettenmann, B.; Wiesmann, M. Olfactory detection thresholds and pleasantness of a food-related and a non-food odour in hunger and satiety. Rhinology 2009, 47, 160–165. [Google Scholar] [PubMed]
- Bolhuis, D.P.; Lakemond, C.M.; de Wijk, R.A.; Luning, P.A.; de Graaf, C. Effect of salt intensity in soup on ad libitum intake and on subsequent food choice. Appetite 2012, 58, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Egecioglu, E.; Skibicka, K.P.; Hansson, C.; Alvarez-Crespo, M.; Friberg, P.A.; Jerlhag, E.; Engel, J.A.; Dickson, S.L. Hedonic and incentive signals for body weight control. Rev. Endocr. Metab. Disord. 2011, 12, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Schulkin, J. Anticipatory physiological regulation in feeding biology: Cephalic phase responses. Appetite 2008, 50, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, M.G.; Boesveldt, S.; Lakemond, C.M.; van Boekel, M.A.; Luning, P.A. Odors: Appetizing or satiating? Development of appetite during odor exposure over time. Int. J. Obes. 2014, 38, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.D. Olfactory Specific Satiety depends on degree of association between odour and food. Appetite 2016, 98, 63–66. [Google Scholar] [CrossRef][Green Version]
- Yin, W.; Hewson, L.; Linforth, R.; Taylor, M.; Fisk, I.D. Effects of aroma and taste, independently or in combination, on appetite sensation and subsequent food intake. Appetite 2017, 114, 265–274. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: A flavour chemistry view. Part 2: Qualitative aspects. A review. Flavour Fragr. J. 2012, 27, 201–215. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: A flavour chemistry view. Part 1: Intensity and detectability. A review. Flavour Fragr. J. 2012, 27, 124–140. [Google Scholar] [CrossRef]
- Frank, M.E.; Fletcher, D.B.; Hettinger, T.P. Recognition of the Component Odors in Mixtures. Chem. Senses 2017, 42, 537–546. [Google Scholar] [CrossRef]
- Iannario, M.; Manisera, M.; Piccolo, D.; Zuccolotto, P. Sensory analysis in the food industry as a tool for marketing decisions. Adv. Data Anal. Classif. 2012, 6, 303–321. [Google Scholar] [CrossRef]
- Ruijschop, R.M.; Boelrijk, A.E.; de Ru, J.A.; de Graaf, C.; Westerterp-Plantenga, M.S. Effects of retro-nasal aroma release on satiation. Br. J. Nutr. 2008, 99, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.; Kaiser, R.; Natsch, A.; Gautschi, M. Investigation of odors in the fragrance industry. Chemoecology 2010, 20, 135–147. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J.P. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef]
- Jordán, M.J.; Tandon, K.; Shaw, P.E.; Goodner, K.L. Aromatic profile of aqueous banana essence and banana fruit by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). J. Agric. Food Chem. 2001, 49, 4813–4817. [Google Scholar] [CrossRef]
- Mayol, A.R.; Acree, T.E. Advances in Gas Chromatography-Olfactometry. In Gas Chromatography-Olfactometry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 782, pp. 1–10. [Google Scholar]
- Nuzzi, M.; Lo Scalzo, R.; Testoni, A.; Rizzolo, A. Evaluation of Fruit Aroma Quality: Comparison Between Gas Chromatography–Olfactometry (GC–O) and Odour Activity Value (OAV) Aroma Patterns of Strawberries. Food Anal. Methods 2008, 1, 270–282. [Google Scholar] [CrossRef]
- van Ruth, S.M. Methods for gas chromatography-olfactometry: A review. Biomol. Eng. 2001, 17, 121–128. [Google Scholar] [CrossRef]
- Crnjar, R.; Solari, P.; Sollai, G. The Human Nose as a Chemical Sensor in the Perception of Coffee Aroma: Individual Variability. Chemosensors 2023, 11, 248. [Google Scholar] [CrossRef]
- Melis, M.; Tomassini Barbarossa, I.; Hummel, T.; Crnjar, R.; Sollai, G. Effect of the rs2890498 polymorphism of the OBPIIa gene on the human ability to smell single molecules. Behav. Brain Res. 2021, 402, 113127. [Google Scholar] [CrossRef]
- Sollai, G.; Tomassini Barbarossa, I.; Usai, P.; Hummel, T.; Crnjar, R. Association between human olfactory performance and ability to detect single compounds in complex chemical mixtures. Physiol. Behav. 2020, 217, 112820. [Google Scholar] [CrossRef]
- Cain, W.S.; Gent, J.F. Olfactory sensitivity: Reliability, generality, and association with aging. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, E.; Bercovich, D.; Avidan, N.; Halbertal, S.; Haim, L.; Gross-Isseroff, R.; Goshen, S.; Lancet, D. Mutations in olfactory signal transduction genes are not a major cause of human congenital general anosmia. Chem. Senses 2007, 32, 21–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hasin-Brumshtein, Y.; Lancet, D.; Olender, T. Human olfaction: From genomic variation to phenotypic diversity. Trends Genet. TIG 2009, 25, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jafek, B.W.; Gordon, A.S.; Moran, D.T.; Eller, P.M. Congenital anosmia. Ear Nose Throat J. 1990, 69, 331–337. [Google Scholar] [PubMed]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and Aging: A Mini-Review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef]
- Cain, W.S.; Stevens, J.C. Uniformity of olfactory loss in aging. Ann. N. Y. Acad. Sci. 1989, 561, 29–38. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Henríquez-Roldán, C.; Osnaya, N.; González-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderon, R.; Herritt, L.; Brooks, D.; Keefe, S.; et al. Urban air pollution: Influences on olfactory function and pathology in exposed children and young adults. Exp. Toxicol. Pathol. 2010, 62, 91–102. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Applebaum, S.L.; Giberson, R.; Siksorski, L.; Rosenberg, L. Smell identification ability: Changes with age. Science 1984, 226, 1441–1443. [Google Scholar] [CrossRef]
- Keller, A.; Zhuang, H.; Chi, Q.; Vosshall, L.B.; Matsunami, H. Genetic variation in a human odorant receptor alters odour perception. Nature 2007, 449, 468–472. [Google Scholar] [CrossRef]
- Melis, M.; Mastinu, M.; Sollai, G. Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups. Nutrients 2024, 16, 821. [Google Scholar] [CrossRef]
- Melis, M.; Tomassini Barbarossa, I.; Crnjar, R.; Sollai, G. Olfactory Sensitivity Is Associated with Body Mass Index and Polymorphism in the Voltage-Gated Potassium Channels Kv1.3. Nutrients 2022, 14, 4986. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Kim, S.M.; Han, D.H.; Kim, K.S. The sniffing bead system, an olfactory dysfunction screening tool for geriatric subjects: A cross-sectional study. BMC Geriatr. 2021, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.; Larsson, M.; Bäckman, L. Differential sex effects in olfactory functioning: The role of verbal processing. J. Int. Neuropsychol. Soc. 2002, 8, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.R.; Fischer, M.E.; Pinto, A.A.; Klein, B.E.K.; Klein, R.; Tweed, T.S.; Cruickshanks, K.J. Sensory Impairments and Risk of Mortality in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Silva Teixeira, C.S.; Cerqueira, N.M.; Silva Ferreira, A.C. Unravelling the Olfactory Sense: From the Gene to Odor Perception. Chem. Senses 2016, 41, 105–121. [Google Scholar] [CrossRef]
- Sollai, G.; Crnjar, R. Age-Related Olfactory Decline Is Associated With Levels of Exercise and Non-exercise Physical Activities. Front. Aging Neurosci. 2021, 13, 695115. [Google Scholar] [CrossRef]
- Sollai, G.; Crnjar, R. Association among Olfactory Function, Lifestyle and BMI in Female and Male Elderly Subjects: A Cross-Sectional Study. Nutrients 2023, 15, 2492. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Magri, S.; Usai, P.; Hummel, T.; Tomassini Barbarossa, I.; Crnjar, R. Association between the rs2590498 polymorphism of Odorant Binding Protein (OBPIIa) gene and olfactory performance in healthy subjects. Behav. Brain Res. 2019, 372, 112030. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Tomassini Barbarossa, I.; Crnjar, R. A polymorphism in the human gene encoding OBPIIa affects the perceived intensity of smelled odors. Behav. Brain Res. 2022, 427, 113860. [Google Scholar] [CrossRef]
- Sorokowska, A.; Sorokowski, P.; Frackowiak, T. Determinants of human olfactory performance: A cross-cultural study. Sci. Total Environ. 2015, 506–507, 196–200. [Google Scholar] [CrossRef]
- Sorokowska, A.; Sorokowski, P.; Hummel, T. Cross-Cultural Administration of an Odor Discrimination Test. Chemosens. Percept. 2014, 7, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sorokowski, P.; Karwowski, M.; Misiak, M.; Marczak, M.K.; Dziekan, M.; Hummel, T.; Sorokowska, A. Sex Differences in Human Olfaction: A Meta-Analysis. Front. Psychol. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Cameron, E.L. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 2009, 97, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, J.K.; Nordin, S. Gender Differences in Chemosensory Perception and Event-related Potentials. Chem. Senses 2004, 29, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Bugaud, C.; Alter, P. Volatile and non-volatile compounds as odour and aroma predictors in dessert banana (Musa spp.). Postharvest Biol. Technol. 2016, 112, 14–23. [Google Scholar] [CrossRef]
- Aydın, E.; Tekeli, H.; Karabacak, E.; Altunay, İ.K.; Aydın, Ç.; Çerman, A.A.; Altundağ, A.; Salihoğlu, M.; Çayönü, M. Olfactory functions in patients with psoriasis vulgaris: Correlations with the severity of the disease. Arch. Dermatol. Res. 2016, 308, 409–414. [Google Scholar] [CrossRef]
- Besser, G.; Erlacher, B.; Aydinkoc-Tuzcu, K.; Liu, D.T.; Pablik, E.; Niebauer, V.; Koenighofer, M.; Renner, B.; Mueller, C.A. Body-Mass-Index Associated Differences in Ortho- and Retronasal Olfactory Function and the Individual Significance of Olfaction in Health and Disease. J. Clin. Med. 2020, 9, 366. [Google Scholar] [CrossRef]
- Croy, I.; Symmank, A.; Schellong, J.; Hummel, C.; Gerber, J.; Joraschky, P.; Hummel, T. Olfaction as a marker for depression in humans. J. Affect. Disord. 2014, 160, 80–86. [Google Scholar] [CrossRef]
- Graves, A.B.; Bowen, J.D.; Rajaram, L.; McCormick, W.C.; McCurry, S.M.; Schellenberg, G.D.; Larson, E.B. Impaired olfaction as a marker for cognitive decline: Interaction with apolipoprotein E epsilon4 status. Neurology 1999, 53, 1480–1487. [Google Scholar] [CrossRef]
- Palouzier-Paulignan, B.; Lacroix, M.C.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem. Senses 2012, 37, 769–797. [Google Scholar] [CrossRef]
- Pastor, A.; Fernández-Aranda, F.; Fitó, M.; Jiménez-Murcia, S.; Botella, C.; Fernández-Real, J.M.; Frühbeck, G.; Tinahones, F.J.; Fagundo, A.B.; Rodriguez, J.; et al. A Lower Olfactory Capacity Is Related to Higher Circulating Concentrations of Endocannabinoid 2-Arachidonoylglycerol and Higher Body Mass Index in Women. PLoS ONE 2016, 11, e0148734. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.M.; DelGaudio, J.M.; Wise, S.K. Higher Body Mass Index Is Associated with Subjective Olfactory Dysfunction. Behav. Neurol. 2015, 2015, 675635. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Shoenfeld, N.; Agmon-Levin, N.; de Carolis, C.; Perricone, R.; Shoenfeld, Y. Smell and autoimmunity: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.M.; Wroblewski, K.E.; Kern, D.W.; Schumm, L.P.; McClintock, M.K. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE 2014, 9, e107541. [Google Scholar] [CrossRef]
- Poessel, M.; Freiherr, J.; Wiencke, K.; Villringer, A.; Horstmann, A. Insulin Resistance Is Associated with Reduced Food Odor Sensitivity across a Wide Range of Body Weights. Nutrients 2020, 12, 2201. [Google Scholar] [CrossRef]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Mastinu, M.; Paduano, D.; Chicco, F.; Magri, S.; Usai, P.; Hummel, T.; Barbarossa, I.T.; Crnjar, R. Olfactory Function in Patients with Inflammatory Bowel Disease (IBD) Is Associated with Their Body Mass Index and Polymorphism in the Odor Binding-Protein (OBPIIa) Gene. Nutrients 2021, 13, 703. [Google Scholar] [CrossRef]
- Steinbach, S.; Proft, F.; Schulze-Koops, H.; Hundt, W.; Heinrich, P.; Schulz, S.; Gruenke, M. Gustatory and olfactory function in rheumatoid arthritis. Scand. J. Rheumatol. 2011, 40, 169–177. [Google Scholar] [CrossRef]
- Steinbach, S.; Reindl, W.; Dempfle, A.; Schuster, A.; Wolf, P.; Hundt, W.; Huber, W. Smell and taste in inflammatory bowel disease. PLoS ONE 2013, 8, e73454. [Google Scholar] [CrossRef]
- Steinbach, S.; Reindl, W.; Kessel, C.; Ott, R.; Zahnert, T.; Hundt, W.; Heinrich, P.; Saur, D.; Huber, W. Olfactory and gustatory function in irritable bowel syndrome. Eur. Arch. Otorhinolaryngol. 2010, 267, 1081–1087. [Google Scholar] [CrossRef]
- Sun, C.; Tang, K.; Wu, J.; Xu, H.; Zhang, W.; Cao, T.; Zhou, Y.; Yu, T.; Li, A. Leptin modulates olfactory discrimination and neural activity in the olfactory bulb. Acta Physiol. 2019, 227, e13319. [Google Scholar] [CrossRef] [PubMed]
- Tekeli, H.; Senol, M.G.; Altundag, A.; Yalcınkaya, E.; Kendirli, M.T.; Yaşar, H.; Salihoglu, M.; Saglam, O.; Cayonu, M.; Cesmeci, E.; et al. Olfactory and gustatory dysfunction in Myasthenia gravis: A study in Turkish patients. J. Neurol. Sci. 2015, 356, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Velluzzi, F.; Deledda, A.; Lombardo, M.; Fosci, M.; Crnjar, R.; Grossi, E.; Sollai, G. Application of Artificial Neural Networks (ANN) to Elucidate the Connections among Smell, Obesity with Related Metabolic Alterations, and Eating Habit in Patients with Weight Excess. Metabolites 2023, 13, 206. [Google Scholar] [CrossRef]
- Velluzzi, F.; Deledda, A.; Onida, M.; Loviselli, A.; Crnjar, R.; Sollai, G. Relationship between Olfactory Function and BMI in Normal Weight Healthy Subjects and Patients with Overweight or Obesity. Nutrients 2022, 14, 1262. [Google Scholar] [CrossRef]
- Walliczek-Dworschak, U.; Wendler, J.; Khan, T.; Aringer, M.; Hähner, A.; Hummel, T. Chemosensory function is decreased in rheumatoid arthritis. Eur. Arch. Otorhinolaryngol. 2020, 277, 1675–1680. [Google Scholar] [CrossRef]
- Wilson, R.S.; Arnold, S.E.; Schneider, J.A.; Boyle, P.A.; Buchman, A.S.; Bennett, D.A. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2009, 1170, 730–735. [Google Scholar] [CrossRef]
- Wilson, R.S.; Schneider, J.A.; Arnold, S.E.; Tang, Y.; Boyle, P.A.; Bennett, D.A. Olfactory Identification and Incidence of Mild Cognitive Impairment in Older Age. Arch. Gen. Psychiatry 2007, 64, 802–808. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- d’Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography-olfactometry in food flavour analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef]
- Dussort, P.; Depretre, N.; Bou-Maroun, E.; Fant, C.; Guichard, E.; Brunerie, P.; Le Fur, Y.Y.; Le Quéré, J.-L. An original approach for gas chromatography-olfactometry detection frequency analysis: Application to gin. Food Res. Int. 2012, 49, 253–262. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography–olfactometry (GC–O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Pollien, P.; Ott, A.; Montigon, F.; Baumgartner, M.; Muñoz-Box, R.; Chaintreau, A. Hyphenated Headspace-Gas Chromatography-Sniffing Technique: Screening of Impact Odorants and Quantitative Aromagram Comparisons. J. Agric. Food Chem. 1997, 45, 2630–2637. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef]
- Fischer, M.; Zopf, Y.; Elm, C.; Pechmann, G.; Hahn, E.G.; Schwab, D.; Kornhuber, J.; Thuerauf, N.J. Subjective and objective olfactory abnormalities in Crohn’s disease. Chem. Senses 2014, 39, 529–538. [Google Scholar] [CrossRef][Green Version]
- Rizzolo, A.; Polesello, A.; Polesello, S. Use of headspace capillary GC to study the development of volatile compounds in fresh fruit. J. High Resolut. Chromatogr. 1992, 15, 472–477. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ohtani, N.; Iwatsuki, K.; Sotoyama, K.; Wada, A.; Tokuno, K.; Iwabuchi, H.; Tanaka, K. Analysis of volatile compounds released during the grinding of roasted coffee beans using solid-phase microextraction. J. Agric. Food Chem. 2003, 51, 1961–1969. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Gloess, A.N.; Yeretzian, C.; Knochenmuss, R.; Groessl, M. On-line analysis of coffee roasting with ion mobility spectrometry–mass spectrometry (IMS–MS). Int. J. Mass Spectrom. 2018, 424, 49–57. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.; Lee, K.-G. Effect of reversed coffee grinding and roasting process on physicochemical properties including volatile compound profiles. Innov. Food Sci. Emerg. Technol. 2017, 44, 97–102. [Google Scholar] [CrossRef]
- López-Galilea, I.; Fournier, N.; Cid, C.; Guichard, E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J. Agric. Food Chem. 2006, 54, 8560–8566. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.A.; Klensporf-Pawlik, D.; Dziadas, M.; Jeleń, H.H. Identification of aroma active compounds of cereal coffee brew and its roasted ingredients. J. Agric. Food Chem. 2013, 61, 2648–2654. [Google Scholar] [CrossRef]
- Sunarharum, W.; Williams, D.; Smyth, H. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef]
- Zapata, J.; Londoño, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of aroma compounds present in an industrial recovery concentrate of coffee flavour. CyTA-J. Food 2018, 16, 367–372. [Google Scholar] [CrossRef]
- Gonzalez-Kristeller, D.C.; do Nascimento, J.B.; Galante, P.A.; Malnic, B. Identification of agonists for a group of human odorant receptors. Front. Pharmacol. 2015, 6, 35. [Google Scholar] [CrossRef]
- Julliard, A.K.; Al Koborssy, D.; Fadool, D.A.; Palouzier-Paulignan, B. Nutrient Sensing: Another Chemosensitivity of the Olfactory System. Front. Physiol. 2017, 8, 468. [Google Scholar] [CrossRef]
- Sollai, G.; Biolchini, M.; Crnjar, R. Taste receptor plasticity in relation to feeding history in two congeneric species of Papilionidae (Lepidoptera). J. Insect Physiol. 2018, 107, 41–56. [Google Scholar] [CrossRef]
- Gaillard, I.; Rouquier, S.; Giorgi, D. Olfactory receptors. Cell. Mol. Life Sci. CMLS 2004, 61, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, I.; Rouquier, S.; Pin, J.P.; Mollard, P.; Richard, S.; Barnabé, C.; Demaille, J.; Giorgi, D. A single olfactory receptor specifically binds a set of odorant molecules. Eur. J. Neurosci. 2002, 15, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Gerkin, R.C.; Castro, J.B. The number of olfactory stimuli that humans can discriminate is still unknown. eLife 2015, 4, e08127. [Google Scholar] [CrossRef] [PubMed]
- Malnic, B.; Godfrey, P.A.; Buck, L.B. The human olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2584. [Google Scholar] [CrossRef]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial receptor codes for odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef]
- Mombaerts, P. Odorant receptor gene choice in olfactory sensory neurons: The one receptor-one neuron hypothesis revisited. Curr. Opin. Neurobiol. 2004, 14, 31–36. [Google Scholar] [CrossRef]
- Mombaerts, P.; Wang, F.; Dulac, C.; Chao, S.K.; Nemes, A.; Mendelsohn, M.; Edmondson, J.; Axel, R. Visualizing an olfactory sensory map. Cell 1996, 87, 675–686. [Google Scholar] [CrossRef]
- Shepherd, G.M. Outline of a theory of olfactory processing and its relevance to humans. Chem. Senses 2005, 30 (Suppl. 1), i3–i5. [Google Scholar] [CrossRef]
- Larsson, M.; Finkel, D.; Pedersen, N.L. Odor identification: Influences of age, gender, cognition, and personality. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2000, 55, P304–P310. [Google Scholar] [CrossRef]
- Cornell Kärnekull, S.; Jönsson, F.U.; Willander, J.; Sikström, S.; Larsson, M. Long-Term Memory for Odors: Influences of Familiarity and Identification Across 64 Days. Chem. Senses 2015, 40, 259–267. [Google Scholar] [CrossRef]
- Schaal, B.; Marlier, L.; Soussignan, R. Olfactory function in the human fetus: Evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behav. Neurosci. 1998, 112, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, Y.; Mercurio, V.; Moio, L.; Blanquet, J.; Meunier, J.M. A new approach to examine the relationships between sensory and gas chromatography-olfactometry data using Generalized Procrustes analysis applied to six French Chardonnay wines. J. Agric. Food Chem. 2003, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- van Ruth, S.M.; O’Connor, C.H. Evaluation of three gas chromatography-olfactometry methods: Comparison of odour intensity-concentration relationships of eight volatile compounds with sensory headspace data. Food Chem. 2001, 74, 341–347. [Google Scholar] [CrossRef]
- Fadool, D.A.; Tucker, K.; Perkins, R.; Fasciani, G.; Thompson, R.N.; Parsons, A.D.; Overton, J.M.; Koni, P.A.; Flavell, R.A.; Kaczmarek, L.K. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 2004, 41, 389–404. [Google Scholar] [CrossRef]
- Guthoff, M.; Tschritter, O.; Berg, D.; Liepelt, I.; Schulte, C.; Machicao, F.; Haering, H.U.; Fritsche, A. Effect of genetic variation in Kv1.3 on olfactory function. Diabetes/Metab. Res. Rev. 2009, 25, 523–527. [Google Scholar] [CrossRef]
- Tucker, K.; Cavallin, M.A.; Jean-Baptiste, P.; Biju, K.C.; Overton, J.M.; Pedarzani, P.; Fadool, D.A. The Olfactory Bulb: A Metabolic Sensor of Brain Insulin and Glucose Concentrations via a Voltage-Gated Potassium Channel. Results Probl. Cell Differ. 2010, 52, 147–157. [Google Scholar]
| N. | Odor-Active Compound | Odor Description | df (F-M) |
|---|---|---|---|
| 1 | Octane, 3,5-dimethyl- | Woody, burnt, unknown | 0-3 |
| 2 | Oxalic acid, isobutyl nonyl ester | Burnt, unknown | 2-1 |
| 3 | Toluene | Coffee, smoked, solvent, roasted, fruit | 14-6 |
| 4 | β-Pinene | Sweet, floral, vanilla, herbs, incense, sulfur, pungent | 6-6 |
| 5 | Ethylbenzene | Petrol | 0-1 |
| 6 | p-Xylene | Vanilla, medicinal, floral, gas, pungent | 4-5 |
| 7 | Oxalic acid, isobutyl pentyl ester | Floral, fruity, vanilla, sweet | 5-3 |
| 8 | Pyridine * | Coffee, smoked, roasted, cheese | 14-3 |
| 9 | D-Limonene * | Sweet, sour, citrus | 1-6 |
| 10 | Furan, 2-pentyl- * | Smoked, plastic, herbs | 3-3 |
| 11 | Pyrazine, methyl- * | Coffee, nutty, roasted, smoke, caramellic | 3-5 |
| 12 | Acetoin | Coffee, sweet, roasted, parfum, woody, caramellic | 10-10 |
| 13 | 2-Propanone, 1-hydroxy- | Sweet, pungent, fish, solvent, wet, feet, medicinal | 7-15 |
| 14 | Pyrazine, 2,5-dimethyl- * | Coffee, citrus, medicinal, sweet, cocoa, shoes | 7-10 |
| 15 | Pyrazine, ethyl- * | Coffee, nutty, egg, pungent, shoes | 4-2 |
| 16 | Pyrazine, 2,3-dimethyl- * | Coffee, burnt, caramellic, fruity | 4-3 |
| 17 | DL-2,3-Butanediol * | Sweet, caramellic, rose, wet | 2-4 |
| 18 | Vinyl butyrate | Floral, parfum, bitter, solvent, pungent, plastic | 5-4 |
| 19 | Hex-4-yn-3-one, 2,2-dimethyl- | Sweet, solvent, pungent | 2-3 |
| 20 | Pyrazine, 2-ethyl-6-methyl- * | Coffee, sweet, smoked, medicinal, solvent, parfum, roasted, balsamic, fruit | 19-25 |
| 21 | Pyrazine, 2-ethyl-3-methyl- * | Coffee, cocoa, solvent, bitter, nutty, roasted, burnt, medicinal, solvent, herbs | 24-22 |
| 22 | Pyrazine, 2-(n-propyl)- * | Green, musty, woody, earthy, wet, herbs, floral, fruit | 18-16 |
| 23 | Pyrazine, 2,6-diethyl- * | Coffee, roasted, earthy, musty, burnt, mushrooms, vegetable | 23-21 |
| 24 | Pyrazine, 3-ethyl-2,5-dimethyl- * | Coffee, nutty, roasted, floral, bitter, woody, solvent, wet | 18-16 |
| 25 | 2-Propanone, 1-(acetyloxy)- | Pungent, parfum, wet | 6-3 |
| 26 | Pyrazine, 2-ethyl-3,5-dimethyl- * | Coffee, musty, roasted, wet, herbs, musty | 17-19 |
| 27 | Furfural * | Coffee, sweet, solvent, floral, pungent | 15-6 |
| 28 | Pyrazine, tetramethyl- | Coffee, roasted, burnt, vanilla, bitter, solvent | 13-13 |
| 29 | Pyrazine, 3,5-diethyl-2-methyl- * | Floral, musty, wet, solvent, fresh | 11-20 |
| 30 | Pyrazine, 2-ethenyl-5-methyl- | Coffee, nutty, bitter, plastic, earthy, musty | 15-11 |
| 31 | Furan, 2-acetyl- * | Parfum | 2-0 |
| 32 | 2,3-Pentanedione * | Floral, earthy, sweat, musk, cheese, pungent, woody | 26-24 |
| 33 | 2-Butanone, 1-(acetyloxy)- | ---------- | 0-0 |
| 34 | 2-Furanmethanol, acetate * | Roasted, fruit, herb, woody, coffee, vegetable, fish | 22-12 |
| 35 | Pyrazine, 2-methyl-6-(2-propenyl)- | Pungent, sour, bitter, herbs, spicy | 8-1 |
| 36 | 2-Cyclopenten-1-one, 2,3-dimethyl- | Sweet, floral, lavender | 3-1 |
| 37 | Acetic acid, diethyl- * | Roasted, solvent, rotten, musty, wet earth, coffee | 20-13 |
| 38 | Pentanoic acid, 4-oxo-, methyl ester | Sweet, nutty | 5-2 |
| 39 | 2-Furancarboxaldehyde, 5-methyl- * | Coffee, sweet, parfum, solvent | 5-4 |
| 40 | 2-Furanmethanol, propanoate * | Coffee, pungent, floral, musty, herb, sweet, burnt, vegetable | 14-9 |
| 41 | Furan, 2,2′-methylenebis- * | Coffee, nutty, popcorn, roasted, fish, sour, plastic, smoke | 22-12 |
| 42 | 2-Furanmethanol * | Coffee, smoke, popcorn, nutty, roasted, sweet | 22-12 |
| 43 | Butanoic acid, 3-methyl- * | Cheese, smoke, stinky feet, acidic, fruity, putrid | 14-19 |
| 44 | Furan, 2-(2-furanylmethyl)-5-methyl- * | Nutty, plastic, unknown | 1-2 |
| 45 | Pyrazine, 2-acetyl-6-methyl | Putrid, musty, cheese, medicinal | 4-6 |
| 46 | 4(H)-Pyridine, N-acetyl- * | Shoes, wet, sweat, plastic, cheese | 9-7 |
| 47 | Octaethylene glycol monododecyl ether | Sweat, acidic | 2-2 |
| 48 | 2-Hexadecanol | Cheese, musty, putrid, plastic, shoes, burnt | 24-23 |
| 49 | N-Furfurylpyrrole * | Solvent, cheese, musty, coffee, caramellic, smoked | 16-20 |
| 50 | 2-Acetylpyrrole * | Coffee, roasted, almond, sweet, burnt, parfum, fresh, popcorn | 25-15 |
| Molecule | Perception Ability | F n (%) | M n (%) | p-Value |
|---|---|---|---|---|
| Toluene | Yes | 14 | 6 | 0.039 |
| No | 20 | 27 | ||
| Pyridine | Yes | 14 | 3 | 0.003 |
| No | 20 | 30 | ||
| D-limonene | Yes | 1 | 6 | 0.041 |
| No | 33 | 27 | ||
| 2-Propanone, 1-hydroxy- | Yes | 7 | 15 | 0.030 |
| No | 27 | 18 | ||
| Furfural | Yes | 15 | 6 | 0.022 |
| No | 19 | 27 | ||
| Pyrazine, 3,5-diethyl-2-methyl- | Yes | 11 | 20 | 0.020 |
| No | 23 | 13 | ||
| 2-Furanmethanol, acetate | Yes | 22 | 12 | 0.020 |
| No | 12 | 21 | ||
| Pyrazine, 2-methyl-6-(2-propenyl)- | Yes | 8 | 1 | 0.014 |
| No | 26 | 32 | ||
| Furan,2,2-methylenebis- | Yes | 22 | 12 | 0.020 |
| No | 12 | 21 | ||
| 2-Furanmethanol | Yes | 22 | 12 | 0.020 |
| No | 12 | 21 | ||
| 2-Acetylpyrrole | Yes | 25 | 15 | 0.019 |
| No | 9 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sollai, G.; Solari, P.; Crnjar, R. Qualitative and Quantitative Sex-Related Differences in the Perception of Single Molecules from Coffee Headspace. Foods 2024, 13, 3239. https://doi.org/10.3390/foods13203239
Sollai G, Solari P, Crnjar R. Qualitative and Quantitative Sex-Related Differences in the Perception of Single Molecules from Coffee Headspace. Foods. 2024; 13(20):3239. https://doi.org/10.3390/foods13203239
Chicago/Turabian StyleSollai, Giorgia, Paolo Solari, and Roberto Crnjar. 2024. "Qualitative and Quantitative Sex-Related Differences in the Perception of Single Molecules from Coffee Headspace" Foods 13, no. 20: 3239. https://doi.org/10.3390/foods13203239
APA StyleSollai, G., Solari, P., & Crnjar, R. (2024). Qualitative and Quantitative Sex-Related Differences in the Perception of Single Molecules from Coffee Headspace. Foods, 13(20), 3239. https://doi.org/10.3390/foods13203239






