Alleviation of High-Fat Diet-Induced Hyperlipidemia in Mice by Stachys sieboldii Miq. Huangjiu via the Modulation of Gut Microbiota Composition and Metabolic Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals and Experimental Design
2.3. Observation of Basic Physiological Indexes
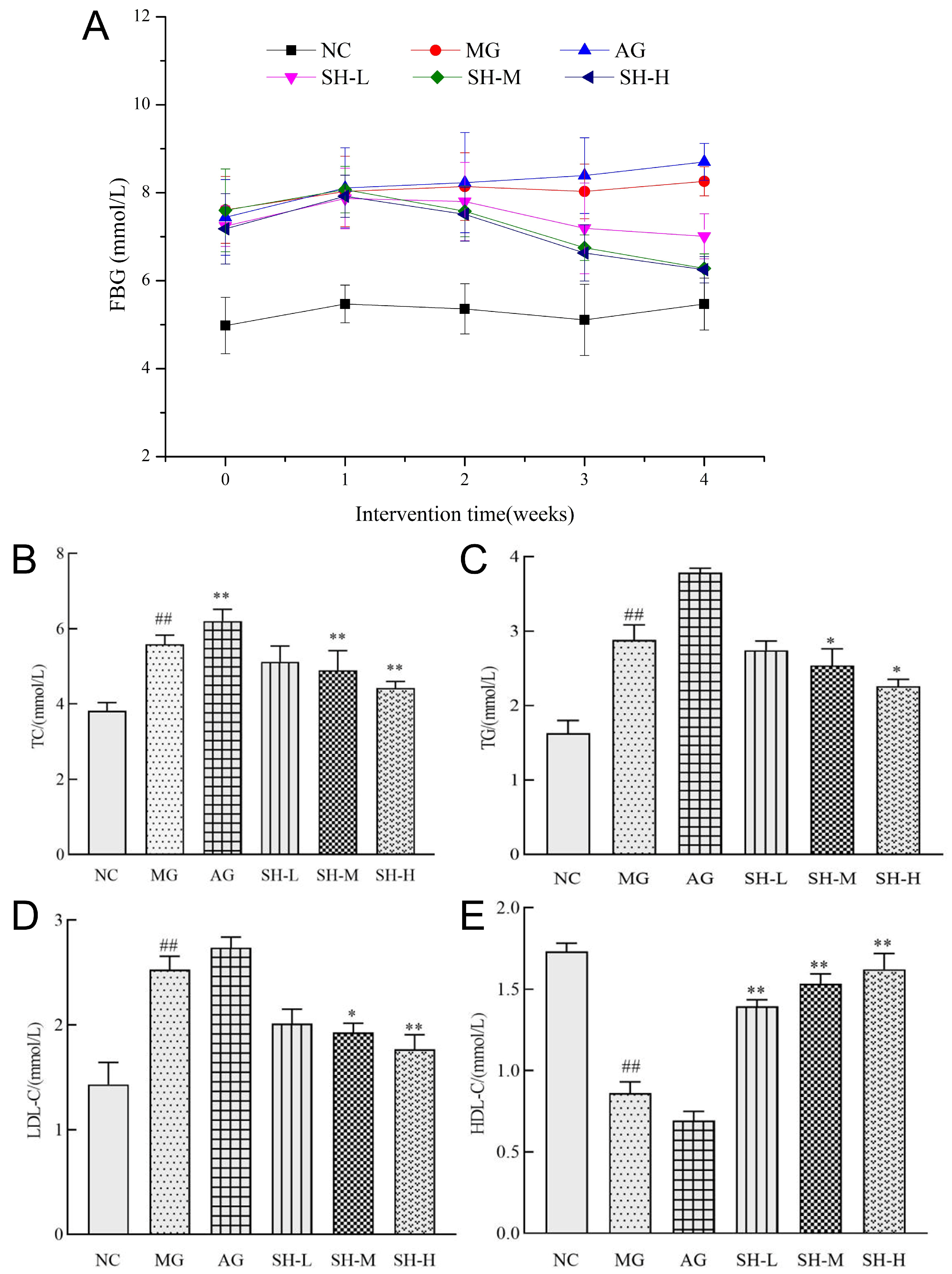
2.4. Measurement of Fasting Blood Glucose Values
2.5. Measurement of Biochemical Indexes
2.5.1. Measurement of Blood Lipid Levels
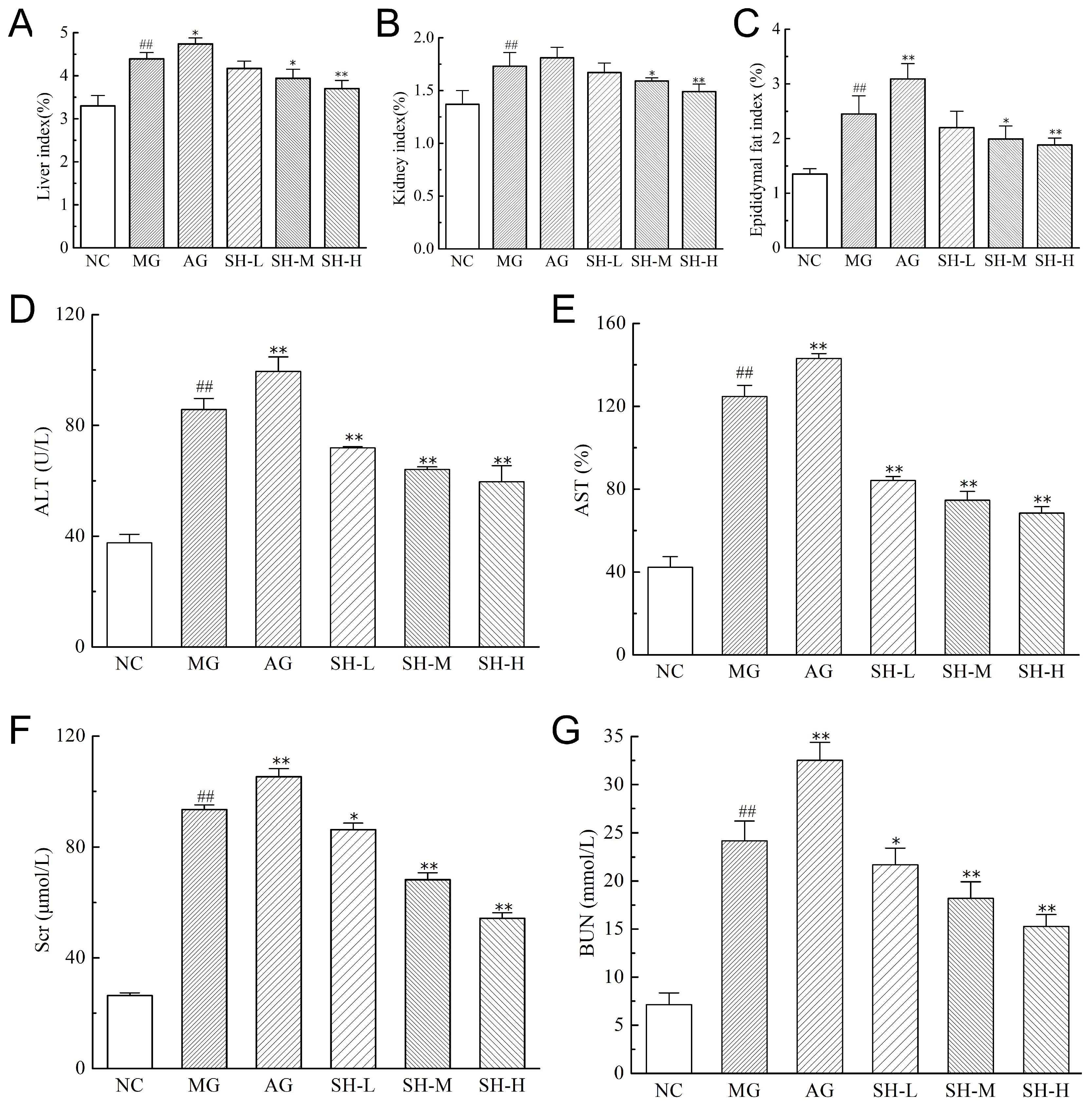
2.5.2. Measurement of the Index of the Liver, Kidney, and Epididymal Fat
2.5.3. Measurement of the Liver and Kidney Function
2.6. Hematoxylin-Eosin (H&E) Staining of Liver and Kidney Tissue Sections
2.7. Measurement of SCFAs in Mice Feces
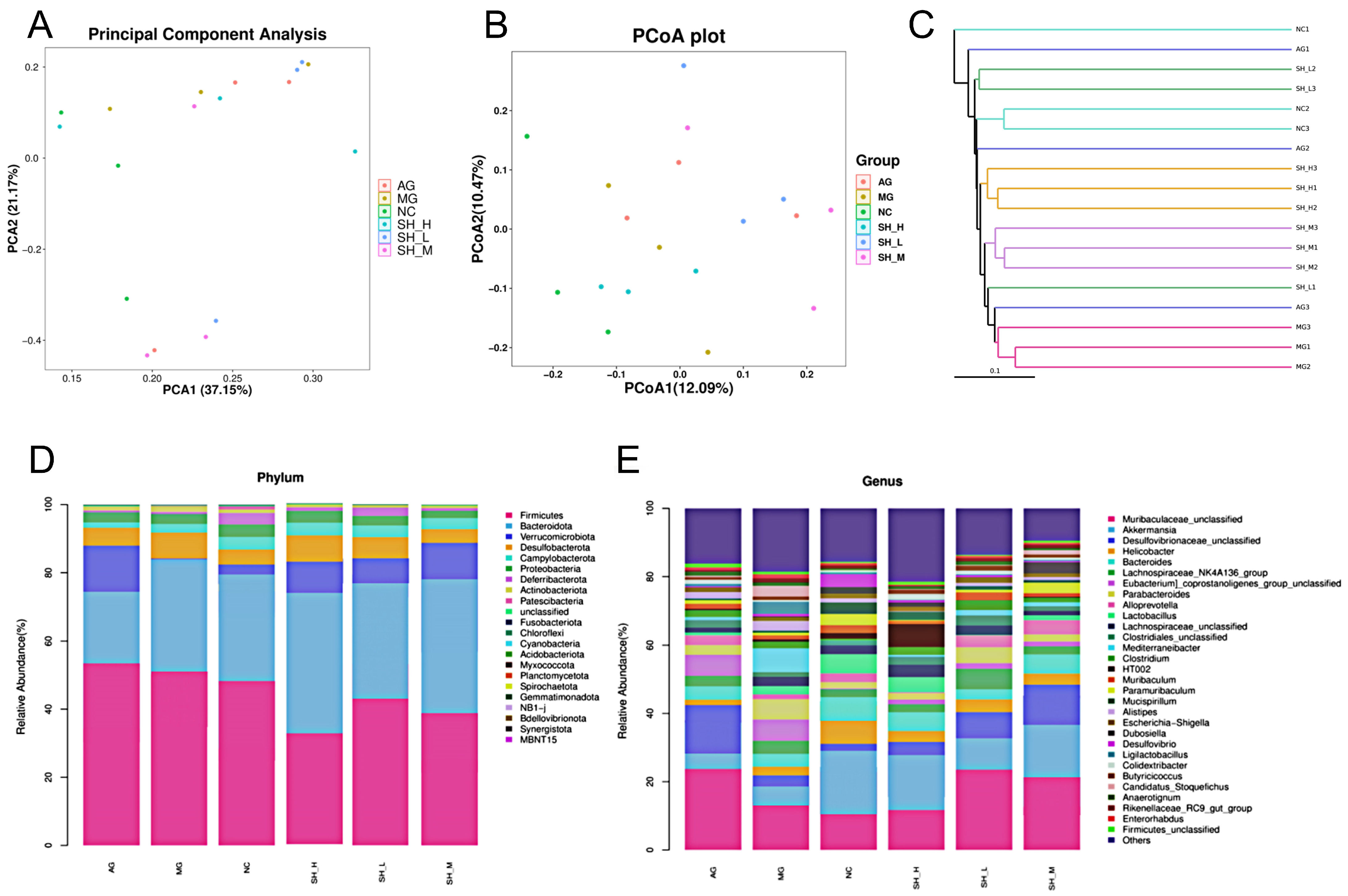
2.8. Analysis of Gut Microbiota in Mice
2.9. Statistical Analysis
3. Results
3.1. The Main Ingredients of CSCHJ
3.2. Establishment of Animal Model of Hyperlipidemia
3.3. Effect of CSCHJ on the Mouse Physiological State
3.4. Effect of CSCHJ on the Glucose and Lipid Metabolism of Mice
3.5. Effect of CSCHJ on the Organs of Mice
3.6. Effect of CSCHJ on Fecal SCFAs Content in Hyperlipidemic Mice
3.7. CSCHJ Reshaped the Gut Microbial Structure in Hyperlipidemic Mice
3.8. Correlation Analysis between HLP-Related Host Parameters and Gut Microbial Genera
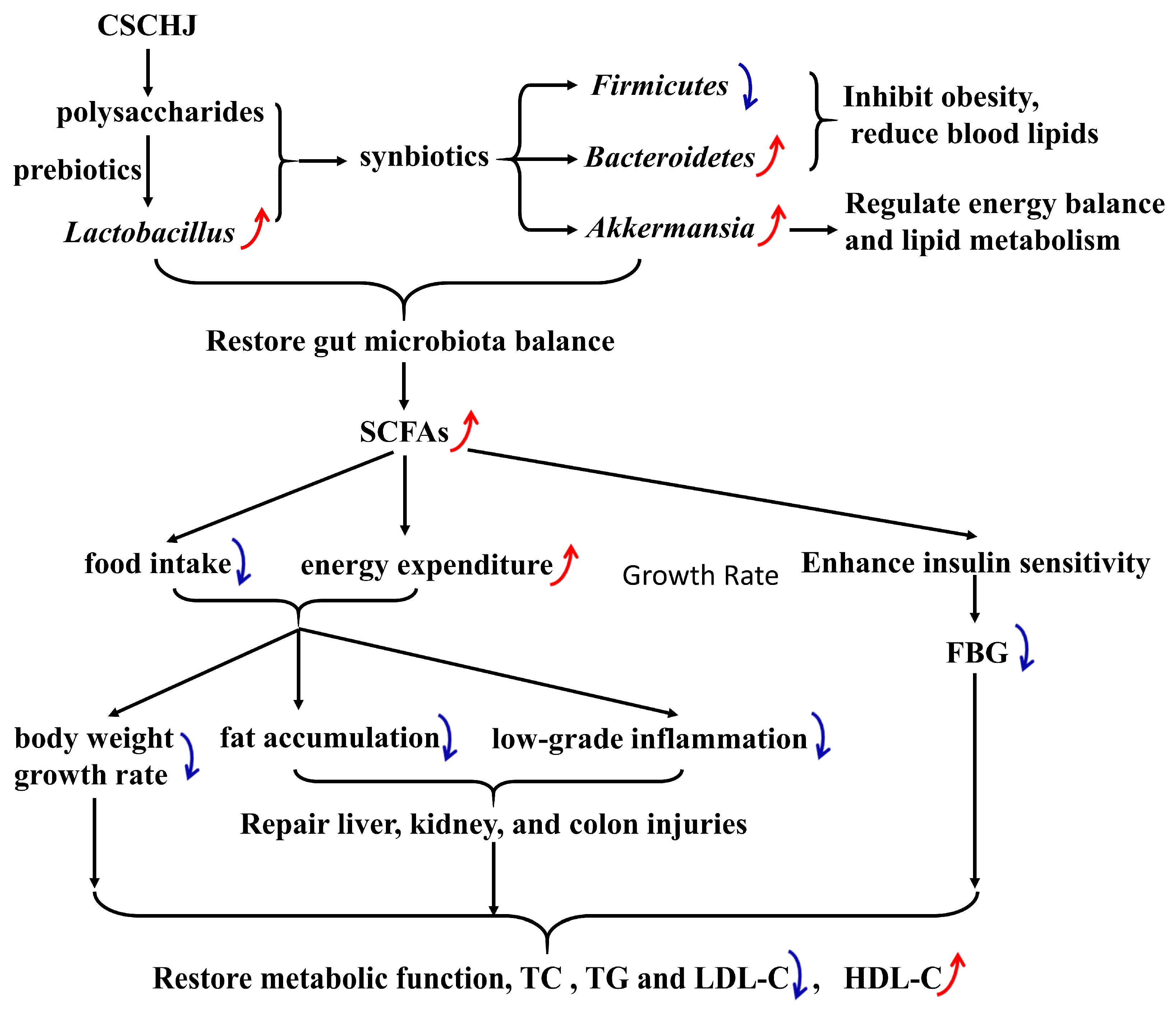
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, H.; Chen, K.; Feng, M.; Shao, W.; Wu, J.; Chen, K.; Liang, T.; Liu, C.; Chen, K. Genipin alleviates high-fat diet-induced hyperlipidemia and hepatic lipid accumulation in mice via miR-142a-5p/SREBP-1c axis. FEBS J. 2018, 285, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Caus, M.; Eritja, À.; Bozic, M. Role of microRNAs in Obesity-Related Kidney Disease. Int. J. Mol. Sci. 2021, 22, 11416. [Google Scholar] [CrossRef]
- Ferguson, D.; Finck, B.N. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A. Cardiotoxicity as a possible side effect of statins. Rev. Cardiovasc. Med. 2023, 24, 22. [Google Scholar] [CrossRef]
- Yamashita, S.; Masuda, D.; Matsuzawa, Y. Pemafibrate, a new selective pparα modulator: Drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr. Atheroscler. Rep. 2020, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Han, J.; Zhang, F.; Chen, W. Red yeast rice: A functional food used to reduce hyperlipidemia. Food Rev. Int. 2023, 39, 4965–4991. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Z.; Liu, Y.; Xu, X.; Xu, Y.; Zhou, W.; Chen, S.; Mao, J. Non-alcoholic components in Huangjiu as potential factors regulating the intestinal barrier and gut microbiota in mouse model of alcoholic liver injury. Foods 2022, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, B.; Wu, Q.; Jiang, X.; Liu, H.; Wang, C.; Huang, M.; Wu, J.; Zhang, J.; Yu, Y. Sensomics-assisted flavor decoding of coarse cereal Huangjiu. Food Chem. 2022, 381, 132296. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, L.; Zhan, P.; Tian, H.; Liu, J. Characterization of the aroma compounds of Millet Huangjiu at different fermentation stages. Food Chem. 2022, 366, 130691. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Xie, J.; Ai, L.; Tian, H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019, 293, 8–14. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Wu, C.; Hu, Z.; Xu, X.; Xie, G.; Wu, D.; Lu, J. Profiling the key metabolites produced during the modern brewing process of Chinese traditional rice wine. Food Res. Int. 2021, 139, 109955. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fu, Z.; Zhang, F.; Mao, Q.; Luan, C.; Han, X.; Xue, J.; Wang, D.; Qin, S.; Hao, F. Effect of yellow rice wine on anti-aging ability in aged mice induced by d-galactose. Food Sci. Hum. Well. 2020, 9, 184–191. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Q.; Zhou, Z.; Li, X.; Ren, D.; Ji, Z.; Mao, J. Structural elucidation of a highly branched α-d-glucan from Huangjiu and its hepatoprotective activity via gut microbiome regulation and intestinal barrier repairment. Carbohyd. Polym. 2024, 324, 121423. [Google Scholar] [CrossRef]
- Peng, L.; Liu, S.; Ji, Z.; Chen, S.; Mao, J. Structure characterisation of polysaccharide isolated from Huangjiu and its anti-inflammatory activity through MAPK signaling. Int. J. Food Sci. Technol. 2019, 54, 1874–1883. [Google Scholar] [CrossRef]
- Chen, G.; Huang, Z.; Wu, L.; Wu, Q.; Guo, W.; Zhao, W.; Liu, B.; Zhang, W.; Rao, P.; Lv, X.; et al. Microbial diversity and flavor of Chinese rice wine (Huangjiu): An overview of currentresearch and future prospects. Curr. Opin. Food Sci. 2021, 42, 37–50. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, L.; Wu, H.; Wang, Y.; Wang, Z.; Zheng, F.; Lu, X.; Xu, J. The Changes of microbial community and flavor compound in the fermentation process of Chinese rice wine using Fagopyrum tataricum grain as feedstock. Sci. Rep. 2019, 9, 3365. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zeng, W.; Fang, F.; Zhou, J.; Du, G. The microbiome of Chinese rice wine (Huangjiu). Curr. Res. Food Sci. 2022, 5, 325–335. [Google Scholar] [CrossRef]
- Shen, C.; Mao, J.; Chen, Y.; Meng, X.; Ji, Z. Extraction optimization of polysaccharides from Chinese rice wine from the Shaoxing region and evaluation of its immunity activities. J. Sci. Food Agric. 2015, 95, 1991–1996. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Zhou, Z.; Ji, Z.; Zhou, W.; Chen, S.; Mao, J. Alleviation of loperamide-induced constipation with sticky rice fermented huangjiu by the regulation of serum neurotransmitters and gut microbiota. J. Sci. Food Agric. 2023, 103, 692–704. [Google Scholar] [CrossRef]
- Jackson, S.; Creo, A.; Kumar, S. Are clinicians aggressive enough in treating diabetes-related hyperlipidemia in youth? Curr. Atheroscler. Rep. 2022, 24, 471–481. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Zhou, Y.; Zheng, X. Black rice anthocyanins alleviate hyperlipidemia, liver steatosis and insulin resistance by regulating lipid metabolism and gut microbiota in obese mice. Food Funct. 2021, 12, 10160. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Feng, R.; Mao, J.; Liu, S.; Zhou, Z.; Ji, Z.; Chen, S.; Mao, J. Structural characterization of peptides from huangjiu and their regulation of hepatic steatosis and gut microbiota dysbiosis in hyperlipidemia mice. Front. Pharmacol. 2021, 12, 689092. [Google Scholar] [CrossRef]
- Danjuma, M.I.; Sajid, J.; Fatima, H.; Elzouki, A.-N. Novel biomarkers for potential risk stratification of drug induced liver injury (DILI): A narrative perspective on current trends. Medicine 2019, 98, e18322. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yuan, Y.; Chen, F.; Zheng, Y.; Li, C.; Cao, J.; Xia, G.; Liu, Z.; Shen, X.; He, Y.; et al. Holothuria leucospilota polysaccharides alleviate hyperlipidemia via alteration of lipid metabolism and inflammation-related gene expression. J. Food Biochem. 2022, 46, e14392. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Zhang, L.; Zhang, T.; Ding, H. Effect of thymoquinone on renal damage induced by hyperlipidemia in LDL receptor-deficient (LDL-R-/-) mice. BioMed Res. Int. 2022, 2022, 709926. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, J.; Wei, X.; Ma, S.; Wang, Q.; Qi, Z.; Duan, Z.; Tan, L.; Tang, H. Preclinical evidence of reno-protective effect of quercetin on acute kidney injury: A meta-analysis of animal studies. Front. Pharmacol. 2023, 14, 1310023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, W.; Sun, Y.; Han, Y.; Chen, X.; Gong, P.; Zhai, P.; Pei, S.; Xie, J.; Ba, Q.; et al. Eucommia bark/leaf extract improves lipid metabolism disorders by affecting intestinal microbiota and microbiome–host interaction in HFD mice. J. Agric. Food Chem. 2023, 71, 3297–3314. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Wu, W.; Chen, Z.; Wen, J.; Jia, R.; Liu, B.; Cao, H.; Zhao, C. Modulation of gut microbiota and lipid metabolism in rats fed high-fat diets by Ganoderma lucidum triterpenoids. Curr. Res. Food Sci. 2023, 6, 100427. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, S.; Hong, B.; Guan, L.; Huang, W.; Feng, J.; Sha, D.; Yuan, D.; Li, B.; Ji, N.; et al. Germinated brown rice relieves hyperlipidemia by alleviating gut microbiota dysbiosis. J. Integr. Agric. 2023, 22, 945–957. [Google Scholar] [CrossRef]
- Yu, Y.; Ji, X.; Song, L.; Cao, Y.; Feng, J.; Zhang, R.; Tao, F.; Zhang, F.; Xue, P. Saponins from Chenopodium quinoa Willd. husks alleviated high-fat-diet-induced hyperlipidemia via modulating the gut microbiota and multiple metabolic pathways. J. Sci. Food Agric. 2024, 104, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; He, Y.; Wei, D.; Fu, P.; Li, Y.; Wang, H.; Yang, D.; Hou, X. Tree peony seed oil alleviates hyperlipidemia and hyperglycemia by modulating gut microbiota and metabolites in high-fat diet mice. Food Sci. Nutr. 2024, 12, 4421–4434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, A.; Li, A.; Song, H.; Luo, P.; Zhan, M.; Zhou, X.; Chen, L.; Zhang, J.; Wang, R. Lactobacillus fermentum CKCC1858 alleviates hyperlipidemia in golden hamsters on a high-fat diet via modulating gut microbiota. Food Funct. 2023, 14, 9580–9590. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Luo, L.; Luo, Q.; Xu, L.; Ma, Q.; Hong, T.; Liu, L.; Liu, Z. Dietary ginger polysaccharides (Gps) improve symptoms in hyperlipidemia rats via alterations in gut microbiota. Heliyon 2023, 9, e17534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nie, J.; Shi, C.; Zheng, W.; Ning, K.; Kang, J.; Sun, J.; Cong, X.; Xie, Q.; Xiang, H. Lysimachia christinae polysaccharide attenuates diet-induced hyperlipidemia via modulating gut microbes-mediated FXR-FGF15 signaling pathway. Int. J. Biol. Macromol. 2023, 248, 125725. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Lei, W.; Tan, P.; Han, M.; Li, H.; Yue, T.; Wang, Z.; Gao, Z. Serum metabolomics combined with 16S rRNA sequencing to reveal the effects of Lycium barbarum polysaccharide on host metabolism and gut microbiota. Food Res. Int. 2023, 165, 112563. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, T.; Li, L.; Liu, F.; Liu, X.; Li, L. The anti-hyperlipidemic effect and underlying mechanisms of barley (Hordeum vulgare L.) grass polysaccharides in mice induced by a high-fat diet. Food Funct. 2023, 14, 7066–7081. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, G.; Wang, S.; Chen, Y. Purification and determination of stachyose in Chinese artichoke (Stachys sieboldii Miq.) by high-performance liquid chromatography with evaporative light scattering detection. Talanta 2006, 70, 208–212. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W.; Pi, X. In Vitro Effects of Stachyose on the Human Gut Microbiota. Starch-Starke 2021, 73, 2100029. [Google Scholar] [CrossRef]
- He, N.; Wang, H.; Yu, S.; Chen, K.; Wu, Z.; Lin, X.; Xiao, L.; Zou, Y.; Li, S. Stachyose ameliorates obesity-related metabolic syndrome via improving intestinal barrier function and remodeling gut microbiota. J. Funct. Foods 2024, 15, 106106. [Google Scholar] [CrossRef]
- Na, E.; Moon, K.H.; Lim, S.Y. The effect of Stachy sieboldii miq. supplementation on modulating gut microflora and cytokine expression in mice. Comb. Chem. High Throughput Scr. 2021, 24, 177–186. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for gastrointestinal health: Current and future perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Ren, D.; Ding, M.; Su, J.; Ye, J.; He, X.; Zhang, Y.; Shang, X. Stachyose in combination with L. rhamnosus GG ameliorates acute hypobaric hypoxia-induced intestinal barrier dysfunction through alleviating inflammatory response and oxidative stress. Free Radical Bio. Med. 2024, 212, 505–519. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; He, J.; He, J.; Shi, S.; Sun, M.; Niu, X.; Zeng, Z.; Zhao, Y.; Zhang, Y.; et al. Effect of Lacticaseibacillus paracasei K56 with galactooligosaccharide synbiotics on obese individuals: An in vitro fermentation model. J. Sci. Food Agric. 2024, 104, 5042–5051. [Google Scholar] [CrossRef] [PubMed]
- He, W.S.; Li, L.; Rui, J.; Li, J.; Sun, Y.; Cui, D.; Xu, B. Tomato seed oil attenuates hyperlipidemia and modulates gut microbiota in C57BL/6J mice. Food Funct. 2020, 11, 4275–4290. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; He, Z.; Guo, R.; Zheng, B.; Li, T.; Liu, R.H. Highland barley whole grain (Hordeum vulgare L.) ameliorates hyperlipidemia by modulating cecal microbiota, miRNAs, and AMPK pathways in leptin receptor-deficient db/db mice. J. Agric. Food Chem. 2020, 68, 11735–11746. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Li, X.; Guo, X.; Sheng, Y.; Xu, G.; Han, X.; An, L.; Du, P. Effects of Agaricus blazei Murrill polysaccharides on hyperlipidemic rats by regulation of intestinal microflora. Food Sci. Nutr. 2020, 8, 2758–2772. [Google Scholar] [CrossRef]
- Zafara, H.; Saier, M.H. Gut bacteroides species in health and disease. Gut Microbes. 2021, 13, e1848158. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Yu, J.; Shi, X.; Zhu, J.; Gao, X.; Liu, W. Polysaccharides catabolism by the human gut bacterium-Bacteroides thetaiotaomicron: Advances and perspectives. Crit. Rev. Food Sci. 2021, 61, 3569–3588. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhou, M.; Zogona, D.; Xing, Z.; Wu, T.; Chen, R.; Cui, D.; Liang, F.; Xu, X. Akkermansia muciniphila: A potential candidate for ameliorating metabolic diseases. Front. Immunol. 2024, 15, 1370658. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, J.; Hao, Y.; Zhou, H.; Hu, Y.; Zhang, C.; Zheng, H.; Wang, X.; Zeng, F.; Hu, J.; et al. Akkermansia muciniphila plays critical roles in host health. Crit. Rev. Microbiol. 2023, 49, 82–100. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.; Zhang, Y.; Li, Y.; Gu, L.; Sun, H.; Liu, M.; Zhou, H.; Wang, Y.; Xu, Z. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Hoch, T.; Pischetsrieder, M.; Hess, A. Snack food intake in ad libitum fed rats is triggered by the combination of fat and carbohydrates. Front. Psychol. 2014, 5, 250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilkinson, K.R.; Tucker, L.A.; Davidson, L.E.; Bailey, B.W. Milk-fat intake and differences in abdominal adiposity and bmi: Evidence based on 13,544 randomly-selected adults. Nutrients 2021, 13, 1832. [Google Scholar] [CrossRef]
- Firat, Y.Y.; Inanc, N.; Soylu, M.; Basmisirli, E.; Capar, A.G.; Aykemat, Y. Relationship between dairy consumption and abdominal obesity. J. Am. Nutr. Assoc. 2022, 41, 569–576. [Google Scholar]
- Kim, D.; Kim, J. Dairy consumption is associated with a lower incidence of the metabolic syndrome in middle-aged and older Korean adults: The Korean Genome and Epidemiology Study (KoGES). Br. J. Nutr. 2017, 117, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zeng, F.; Li, S.; Liu, Y.; Gong, S.; Lv, X.; Zhang, J.; Liu, B. Spirulina active substance mediated gut microbes improve lipid metabolism in high-fat diet fed rats. J. Funct. Foods 2019, 59, 215–222. [Google Scholar] [CrossRef]
- Guarner, F. Decade in review-gut microbiota: The gut microbiota era marches on. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 647–649. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Li, T.; Teng, H.; An, F.; Huang, Q.; Chen, L.; Song, H. The beneficial effects of purple yam (Dioscorea alata L.) resistant starch on hyperlipidemia in high-fat-fed hamsters. Food Funct. 2019, 10, 2642–2650. [Google Scholar] [CrossRef]
- Hu, L.; Tian, K.; Zhang, T.; Fan, C.; Zhou, P.; Zeng, D.; Zhao, S.; Li, L.; Smith, H.S.; Li, J.; et al. Cyanate induces oxidative stress injury and abnormal lipid metabolism in liver through Nrf2/HO-1. Molecules 2019, 24, 3231. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, Y.; Cai, M.; Gu, R.; Pan, X.; Du, J. Perilla peptides delay the progression of kidney disease by improving kidney apoptotic injury and oxidative stress and maintaining intestinal barrier function. Food Biosci. 2021, 43, 101333. [Google Scholar] [CrossRef]
- Namaei, P.; Ghadiri, F.; Jamali, R.; Azimi, A.R.; Shabestari, H.R.F.; Vahabizad, F. Evaluation of liver injury in multiple sclerosis patients receiving pulsed steroid therapy. Mult. Scler. Relat. Dis. 2022, 65, 103968. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.P.; Houdek, H.M.; Watt, J.A.; Rosenberger, T.A. Acetate supplementation increases brain phosphocreatine and reduces AMP levels with no effect on mitochondrial biogenesis. Neurochem. Int. 2013, 62, 296–305. [Google Scholar] [CrossRef]
- Jiang, L.; Krystal, J.H.; Mason, G.F.; Jiang, L.; Gulanski, B.I.; Feyter, H.M.D.; Weinzimer, S.A.; Pittman, B.; Guidone, E.; Koretski, J.; et al. Increased brain uptake and oxidation of acetate in heavy drinkers find the latest version: Increased brain uptake and oxidation of acetate in heavy drinkers. J. Clin. Investig. 2013, 123, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obesity 2014, 39, 424–429. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obesity 2016, 40, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41. Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Bellahcene, M.; O’Dowd, J.F.; Wargent, E.T.; Zaibi, M.S.; Hislop, D.C.; Ngala, R.A.; Smith, D.M.; Cawthorne, M.A.; Stocker, C.J.; Arch, J.R. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br. J. Nutr. 2013, 109, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.F.; Almeida, D.C.d.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.D.; et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Vadder, F.D.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Mithieux, G. Microbiota-generated metabolites promote meta-bolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Vily-Petit, J.; Soty, M.; Silva, M.; Micoud, M.; Bron, C.; Guérin-Deremaux, L.; Mithieux, G. Improvement of energy metabolism associated with NUTRIOSE® soluble fiber, a dietary ingredient exhibiting prebiotic properties, requires intestinal gluconeogenesis. Food Res. Int. 2023, 167, 112723. [Google Scholar] [CrossRef]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology 2016, 31, 283. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Gan, R.; Huang, S.; Zhao, C.; Shang, A.; Xu, X.; Li, H. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Cai, M.; Chen, S.; Ma, Q.H.; Yang, K.; Sun, P.L. Isolation of crude oligosaccharides from Hericium erinaceus by integrated membrane technology and its proliferative activity. Food Hydrocoll. 2019, 95, 426–431. [Google Scholar] [CrossRef]
- Das, B.; Sahoo, R.N.; Pargal, S.; Krishna, G.; Verma, R.; Chinnusamy, V.; Sehgal, V.K.; Gupta, V.K.; Dash, S.K.; Swain, P. Quantitative monitoring of sucrose, reducing sugar and total sugar dynamics for phenotyping of water-deficit stress tolerance in rice through spectroscopy and chemometrics. Spectrochim. Acta A 2018, 192, 41–51. [Google Scholar] [CrossRef]




| Ingredients | Alcohol (20 °C, %) | Polysaccharides (g/L) | Total Acids (g/L) | Amino Nitrogen (g/L) |
|---|---|---|---|---|
| Content | 17.56 ± 0.44 | 15.54 ± 1.30 | 3.65 ± 0.28 | 0.34 ± 0.01 |
| Groups | TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) |
|---|---|---|---|---|
| Normal control group | 2.82 ± 0.22 | 1.13 ± 0.35 | 1.08 ± 0.38 | 1.69 ± 0.28 |
| Model group | 4.40 ± 0.48 ** | 2.24 ± 0.31 * | 2.02 ± 0.14 * | 1.96 ± 0.22 * |
| Groups | Observed Otus | Shannon | Simpson | Chao1 | Goods Coverage | Pielou |
|---|---|---|---|---|---|---|
| NC | 828.67 | 6.68 | 0.97 | 829.61 | 1.00 | 0.72 |
| MG | 629.67 | 6.67 | 0.95 | 630.66 | 1.00 | 0.69 |
| AG | 655.00 | 6.67 | 0.73 | 755.51 | 1.00 | 0.70 |
| SH-L | 732.33 | 6.38 | 0.94 | 712.56 | 1.00 | 0.69 |
| SH-M | 762.33 | 6.66 | 0.94 | 732.73 | 1.00 | 0.69 |
| SH-H | 764.67 | 6.80 | 0.96 | 765.47 | 1.00 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, J.; Wu, Y.; Tian, H.; Dong, J. Alleviation of High-Fat Diet-Induced Hyperlipidemia in Mice by Stachys sieboldii Miq. Huangjiu via the Modulation of Gut Microbiota Composition and Metabolic Function. Foods 2024, 13, 2360. https://doi.org/10.3390/foods13152360
Geng J, Wu Y, Tian H, Dong J. Alleviation of High-Fat Diet-Induced Hyperlipidemia in Mice by Stachys sieboldii Miq. Huangjiu via the Modulation of Gut Microbiota Composition and Metabolic Function. Foods. 2024; 13(15):2360. https://doi.org/10.3390/foods13152360
Chicago/Turabian StyleGeng, Jingzhang, Yunxia Wu, Honglei Tian, and Jianwei Dong. 2024. "Alleviation of High-Fat Diet-Induced Hyperlipidemia in Mice by Stachys sieboldii Miq. Huangjiu via the Modulation of Gut Microbiota Composition and Metabolic Function" Foods 13, no. 15: 2360. https://doi.org/10.3390/foods13152360
APA StyleGeng, J., Wu, Y., Tian, H., & Dong, J. (2024). Alleviation of High-Fat Diet-Induced Hyperlipidemia in Mice by Stachys sieboldii Miq. Huangjiu via the Modulation of Gut Microbiota Composition and Metabolic Function. Foods, 13(15), 2360. https://doi.org/10.3390/foods13152360







