Study of Factors Influencing the Oral Bioaccessibility of Commonly Used and Detected Pesticides in Bananas and Mangoes Based on in vitro Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. In vitro Digestion Model
2.3. Influencing Factors
2.3.1. Digestion Conditions
2.3.2. Dietary Components
2.3.3. Digestion Fluids in Four Populations
2.3.4. Food Matrix and Initial Pesticide Concentrations
2.4. Chemical Analyses
2.5. Method Accuracy
2.6. Data Analysis
3. Results and Discussion
3.1. Effect of Digestion Conditions on Bioaccessibility
3.2. Effect of Dietary Components on Bioaccessibility
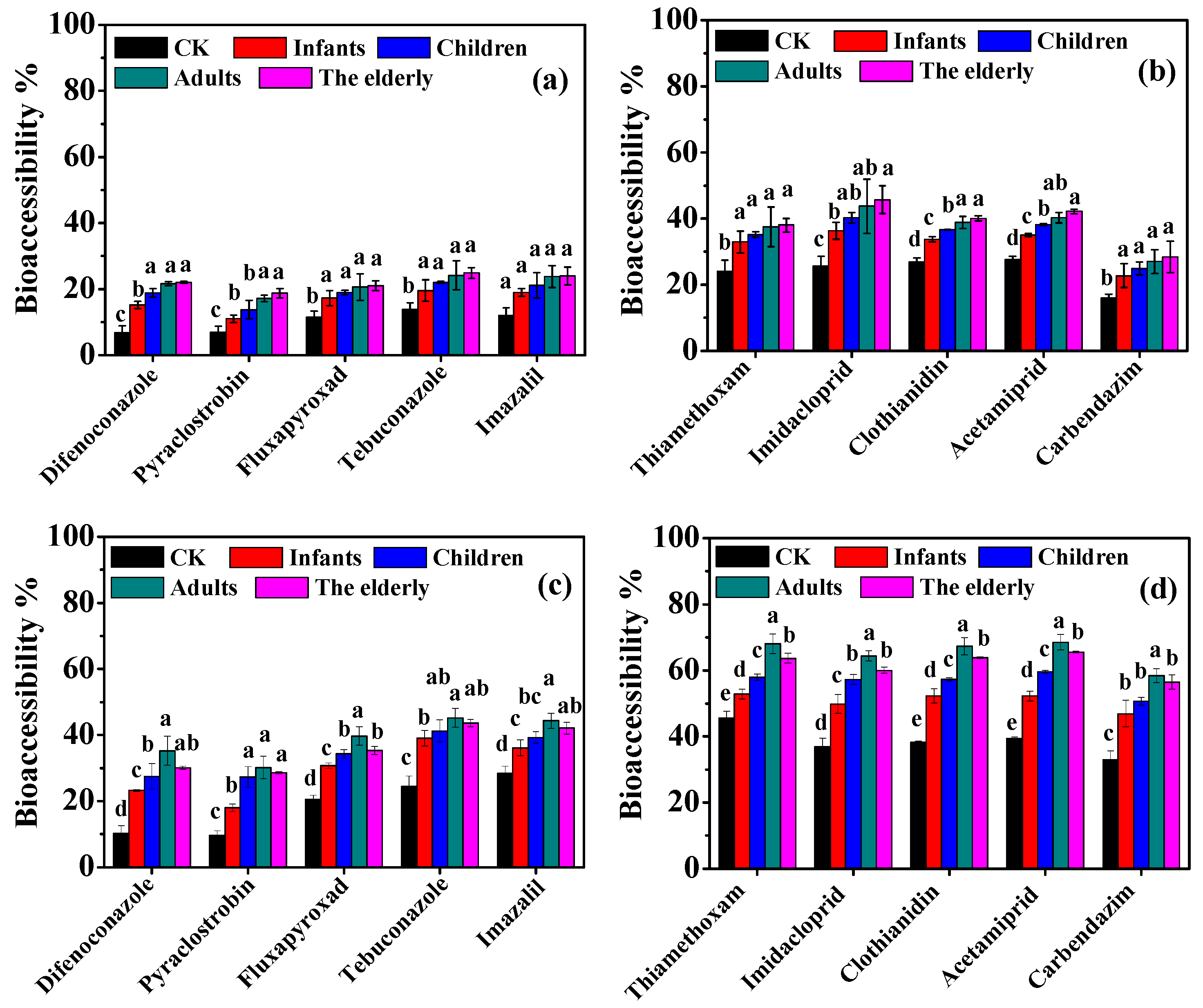
3.3. Effect of Digestion Fluids in Different Populations on Bioaccessibility
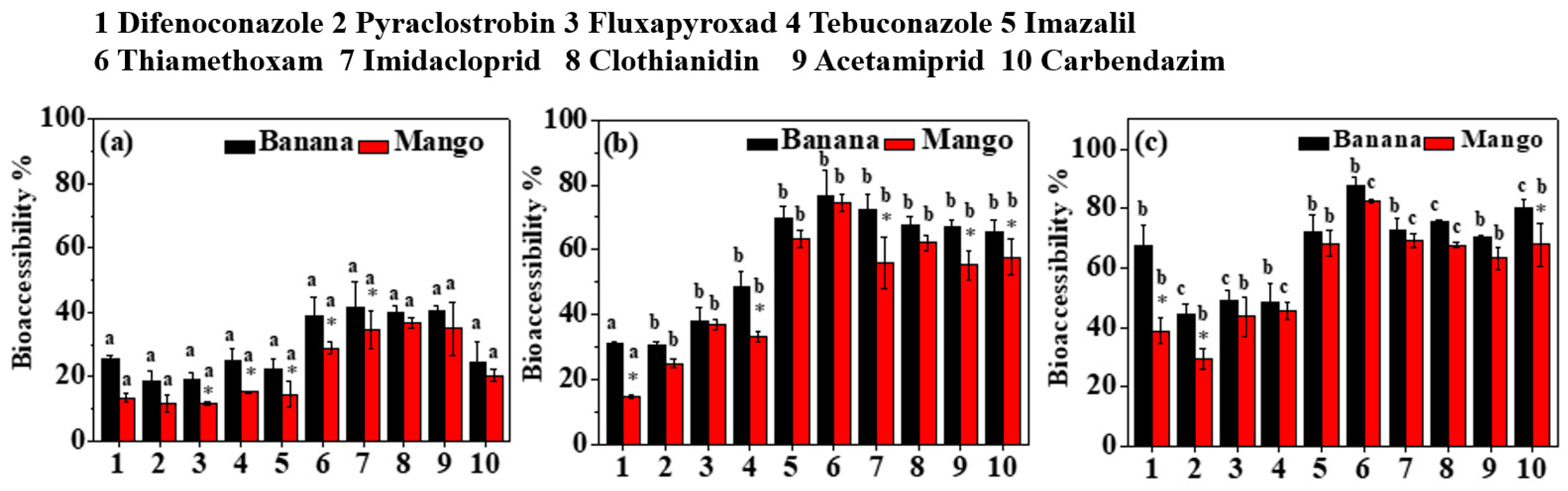
3.4. Effect of Food Matrix on Bioaccessibility
3.5. Effect of Initial Pesticide Concentration on Bioaccessibility
3.6. Dietary Risk Assessment of Pesticides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Dai, T.; Chen, M.; Liang, R.; Liu, W.; Liu, C.; Sun, J.; Chen, J.; Deng, L. Role of maturity status on the quality and volatile properties of mango fruits dried by infrared radiation. Food Biosci. 2023, 52, 102497. [Google Scholar] [CrossRef]
- Tan, L.; He, Y.; Li, S.; Deng, J.; Avula, B.; Zhang, J.; Pugh, N.D. Proximate composition and nutritional analysis of selected bananas cultivated in Hainan, China. J. Food Compos. Anal. 2024, 125, 105798. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Lv, D.; Fan, Q.; Wu, X.; Xu, X. Multiresidue analysis and dietary intake risk assessment of 29 pesticides in banana from five provinces of southern China. J. Food Compos. Anal. 2024, 125, 105819. [Google Scholar] [CrossRef]
- Vss, T.; Patil, P.Y.; Kannan, K. Pesticide Residue in Mango Orchards and Health Risk. Acta Sci. Microbiol. 2020, 3, 8–14. [Google Scholar] [CrossRef]
- Su, Y.; Lu, J.; Liu, J.; Li, F.; Wang, N.; Lei, H.; Shen, X. Optimization of a QuEChERS-LC-MS/MS method for 51 pesticide residues followed by determination of the residue levels and dietary intake risk assessment in foodstuffs. Food Chem. 2024, 434, 137467. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Pandey, A.; Arif, S.; Singh, S.P. Farm to fork strategy for evaluating the qualitative and quantitative health risks to the Nepalese population associated with pesticide residues in apple and banana. Food Control 2024, 163, 110526. [Google Scholar] [CrossRef]
- Xiao, J.; Li, M.; Zhang, M.; Dai, K.; Ju, X.; Liu, Y.; Liu, Z.; Cao, H.; Shi, Y. Transport and interaction mechanism of four pesticide residues from Chaenomeles speciosa across Caco-2 cells. Food Chem. 2024, 431, 137156. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, H.; Li, L.; Wu, Y.; Yang, X.; Jiang, A.; Wu, W. The bioaccessibility and bioavailability of pentachlorophenol in five animal-derived foods measured by simulated gastrointestinal digestion. Foods 2024, 13, 1254. [Google Scholar] [CrossRef]
- Shi, Y.H.; Xiao, J.J.; Liu, Y.Y.; Fu, Y.Y.; Ye, Z.; Liao, M.; Cao, H.Q. Interactions of food matrix and dietary components on neonicotinoid bioaccessibility in raw fruit and vegetables. Food Funct. 2019, 10, 289–295. [Google Scholar] [CrossRef]
- Chen, A.S.; Liu, D.H.; Hou, H.N.; Yao, J.N.; Xiao, S.C.; Ma, X.R.; Li, P.Z.; Cao, Q.; Liu, X.K.; Zhou, Z.Q.; et al. Dietary pattern interfered with the impacts of pesticide exposure by regulating the bioavailability and gut microbiota. Sci. Total Environ. 2023, 858, 159936. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Xiang, Y.; Lin, F.; Yue, X.; Li, M.; Xiao, J.; Cao, H.; Shi, Y. In vivo-in vitro correlations (IVIVC) for the assessment of pyrethroid bioavailability in honey. Food Chem. 2023, 429, 136873. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; Xiao, O.; Cui, W.; Chen, J.; Dai, X.; Li, M.; Kong, Z. Bioaccessibility and intestinal transport of tebuconazole in table grape by using in vitro digestion models. Foods 2022, 11, 3926. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Xiao, J.J.; Feng, R.P.; Liu, Y.Y.; Liao, M.; Wu, X.W.; Hua, R.M.; Cao, H.Q. In-vitro bioaccessibility of five pyrethroids after human ingestion and the corresponding gastrointestinal digestion parameters: A contribution for human exposure assessments. Chemosphere 2017, 182, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, G.; Yang, Y.; Yu, Y. A review on in-vitro oral bioaccessibility of organic pollutants and its application in human exposure assessment. Sci. Total Environ. 2020, 752, 142001. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Sanchez, B.M.; Sendon, R.; de Quiros, A.R.B.; Barbosa-Pereira, L. Study on the chemical behaviour of Bisphenol S during the in vitro gastrointestinal digestion and its bioaccessibility. Food Chem. 2022, 367, 130758. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Yang, Y.; Lei, B.; Ma, S.; Yu, Y. Influence of nutrients on the bioaccessibility and transepithelial transport of polybrominated diphenyl ethers measured using an in vitro method and Caco-2 cell monolayers. Ecotoxicol. Environ. Saf. 2021, 208, 111569. [Google Scholar] [CrossRef]
- Faria, M.A.; Melo, A.; Ferreira, I.I.M.P.L.V.O. Influence of dietary patterns on contaminants bioaccessibility and intestinal transport by in vitro assays. Food Res. Int. 2020, 137, 109358. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; He, S.X.; Li, X.Y.; Zeng, J.Y.; Li, M.Y.; Guan, D.X.; Ma, L.Q. Chromium contents, distribution and bioaccessibility in cultivated mushrooms from market: Health implications for human consumption. J. Hazard. Mater. 2024, 461, 132643. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunçao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zhang, R.R.; Zhang, Q.; Ma, L.Q.; Cui, X.Y. Effects of food constituents on absorption and bioaccessibility of dietary synthetic phenolic antioxidant by Caco-2 cells. J. Agric. Food Chem. 2020, 68, 4670–4677. [Google Scholar] [CrossRef]
- USEPA ECOTOX Database. Available online: http://cfpub.epa.gov/ecotox/ (accessed on 3 April 2024).
- National Food Safety Standard. Maximum Residue Limits for Pesticides in Food; National Health Commission, Ministry of Agriculture and Rural Affairs and State Administration for Market Regulation: Beijing, China, 2021.
- WHO. Food Safety Collaborative Platform. Available online: https://apps.who.int/foscollab/Download/DownloadConso (accessed on 3 April 2024).
- Inventory of Evaluations Performed by the Joint Meeting on Pesticide Residue (JMPR). Available online: https://apps.who.int/pesticide-residues-jmpr-database/Home/Range/All (accessed on 3 April 2024).
- Shi, Y.H.; Xiao, J.J.; Feng, R.P.; Liu, Y.Y.; Liao, M.; Wu, X.W.; Hua, R.M.; Cao, H.Q. Factors affecting the bioaccessibility and intestinal transport of difenoconazole, hexaconazole, and spirodiclofen in human Caco-2 cells following in vitro digestion. J. Agric. Food Chem. 2017, 65, 9139–9146. [Google Scholar] [CrossRef]
- Xiao, J.J.; Fu, Y.Y.; Ye, Z.; Liu, Y.Y.; Shi, Y.H.; Liao, M.; Cao, H.Q. Analysis of the pesticide behavior in Chaenomelis speciosa and the role of digestive enzyme in vitro oral bioaccessibility. Chemosphere 2019, 231, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; McClements, D.J. Establishing the impact of food matrix effects on the bioaccessibility of nutraceuticals and pesticides using a standardized food model. Food Funct. 2019, 10, 1375–1385. [Google Scholar] [CrossRef]
- Milini, D.D.; Vojinovi, U.D.; Kosti, A.Z.; Pesica, M.B.; Spirovic Trifunovic, B.D.; Brkic, D.V.; Stevic, M.Z.; Kojic, M.O.; Stanisavljevic, N.S. In vitro assessment of pesticide residues bioaccessibility in conventionally grown blueberries as affected by complex food matrix. Chemosphere 2020, 252, 126568. [Google Scholar] [CrossRef]
- Yu, Y.X.; Chen, L.; Yang, D.; Pang, Y.P.; Zhang, S.H.; Zhang, X.Y.; Yu, Z.Q.; Wu, M.H.; Fu, J.M. Polycyclic aromatic hydrocarbons in animal-based foods from Shanghai: Bioaccessibility and dietary exposure. Food Addit. Contamin. Part A 2012, 29, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, C.; Yu, N.Y.; Juhasz, A.L.; Cui, X.Y.; Ma, L.Q. In vivo bioavailability and in vitro bioaccessibility of perfluorooctanoic acid (PFOA) in food matrices: Correlation analysis and method development. Environ. Sci. Technol. 2015, 49, 150–158. [Google Scholar] [CrossRef]
- Chen, Y.; Juhasz, A.; Li, H.B.; Li, C.; Ma, L.Q.; Cui, X.Y. The influence of food on the in vivo bioavailability of DDT and its metabolites in soil. Environ. Sci. Technol. 2020, 54, 5003–5010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Z.; Li, R.; Tan, Y.; Lv, S.; McClements, D.J. Impact of pesticide type and emulsion fat content on the bioaccessibility of pesticides in natural products. Molecules 2020, 25, 1466. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xiao, J.J.; Fu, Y.Y.; Liao, M.; Cao, H.Q.; Shi, Y.H. Study of factors influencing the bioaccessibility of triazolone in cherry tomatoes using a static SHIME model. Int. J. Environ. Res. Public Health 2018, 15, 993. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Li, R.; Tan, Y.; Lv, S.; McClements, D.J. Impact of pesticide polarity and lipid phase dimensions on the bioaccessibility of pesticides in agricultural produce consumed with model fatty foods. Food Funct. 2020, 11, 6028–6037. [Google Scholar] [CrossRef]
- Wang, J.; Lin, K.; Taylor, A.; Gan, J. In vitro assessment of pyrethroid bioaccessibility via particle ingestion. Environ. Int. 2018, 119, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ertl, H.; Butte, W. Bioaccessibility of pesticides and polychlorinated biphenyls from house dust: In vitro methods and human exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.X.; Li, J.L.; Zhang, X.Y.; Yu, Z.Q.; Van de Wiele, T.; Han, S.Y.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Assessment of the bioaccessibility of polybrominated diphenyl ethers in foods and the correlations of the bioaccessibility with nutrient contents. J. Agric. Food Chem. 2010, 58, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Fan, Y.; Yan, X.; Zheng, J.; Chen, S.J.; Mai, B.X. In vitro oral and inhalation bioaccessibility of hydrophobic organic contaminants (HOCs) in airborne particles and influence of relevant parameters. Environ. Res. 2019, 170, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Baima, C.; Jiang, J.; Liu, Z.; Wang, J.; Chen, X.D.; Wu, P. In vitro gastric digestion and emptying of tsampa under simulated elderly and young adult digestive conditions using a dynamic stomach system. J. Food Eng. 2022, 327, 111054. [Google Scholar] [CrossRef]
- Sundhar, S.; Shakila, R.J.; Shalini, R.; Aanand, S.; Jeyasekaran, G.; Jayakumar, N. In-vitro bioaccessibility of pesticide residues in edible seaweeds: Exposure and health risk assessment. J. Food Compos. Anal. 2023, 123, 105574. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Gao, M.; Liu, W.; Yu, Y. Bioaccessibility dependence of dietary exposure to dichlorodiphenyltrichloroethane and its metabolites and hexachlorocyclohexane isomers and their induced health risk: A case study in Beijing City, China. Environ. Pollut. 2021, 281, 117065. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.J.; Shi, Y.H.; Deng, Y.; Liu, Y.; Feng, W.; Liao, M.; Cao, H.Q. Incorporating tenax into the in vitro method to improve the predictive capability of bioaccessibility of triazole fungicides in grape. Food Chem. 2022, 396, 133740. [Google Scholar] [CrossRef]
- Oomen, A.G.; Sips, A.J.; Groten, J.P.; Sijm, D.T.; Tolls, J. Mobilization of PCBs and lindane from soil during in vitro digestion and their distribution among bile salt micelles and proteins of human digestive fluid and the soil. Environ. Sci. Technol. 2000, 34, 297–303. [Google Scholar] [CrossRef]
- Yu, Y.X.; Han, S.Y.; Zhang, D.P.; Van de Wiele, T.; Lu, M.; Wang, D.Q.; Yu, Z.Q.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Factors affecting the bioaccessibility of polybrominated diphenyl ethers in an in vitro digestion method. J. Agric. Food Chem. 2009, 57, 133–139. [Google Scholar] [CrossRef]
- Siedlikowski, M.; Bradley, M.; Kubow, S.; Goodrich, J.M.; Franzblau, A.; Basu, N. Bioaccessibility and bioavailability of methylmercury from seafood commonly consumed in North America: In vitro and epidemiological studies. Environ. Res. 2016, 149, 266–273. [Google Scholar] [CrossRef] [PubMed]
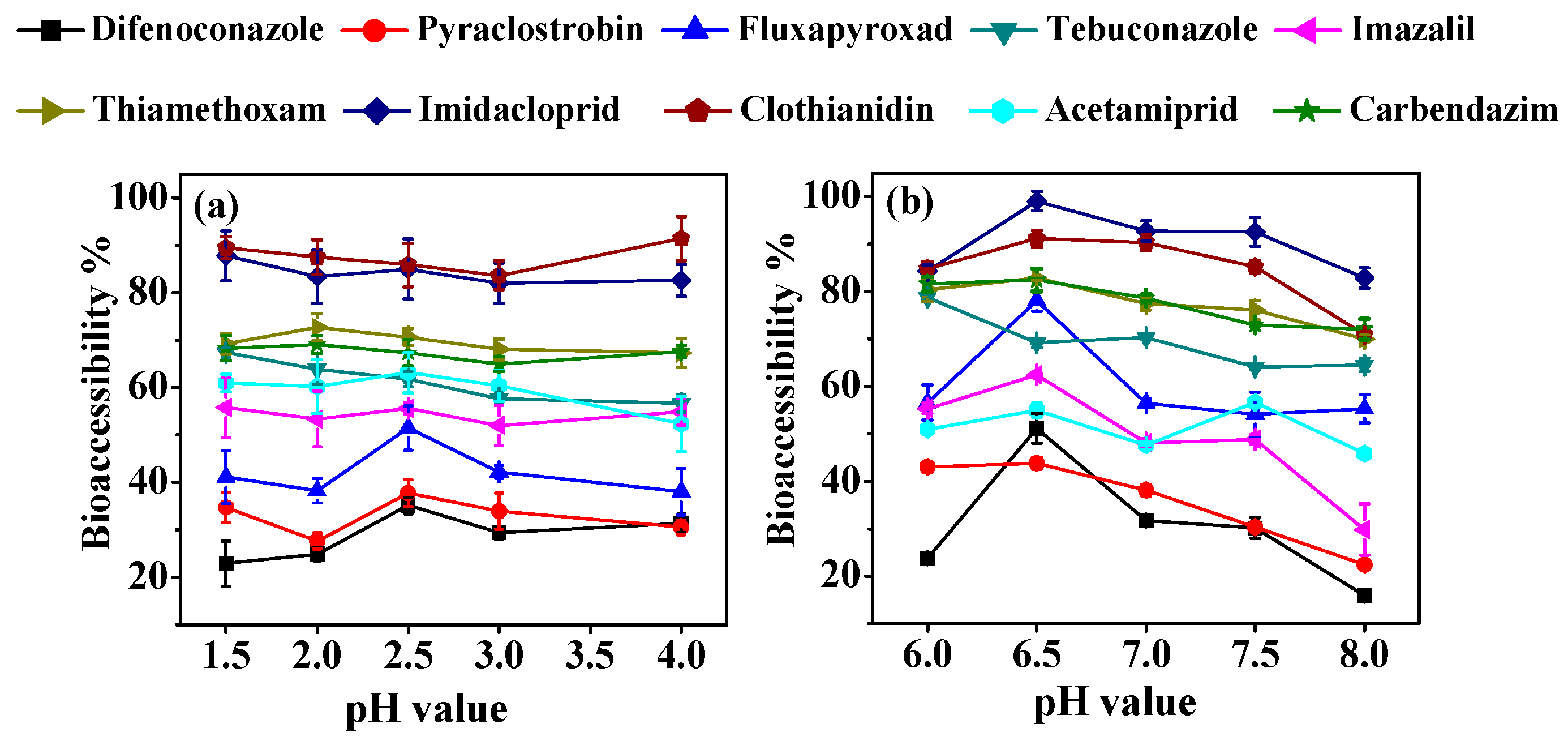
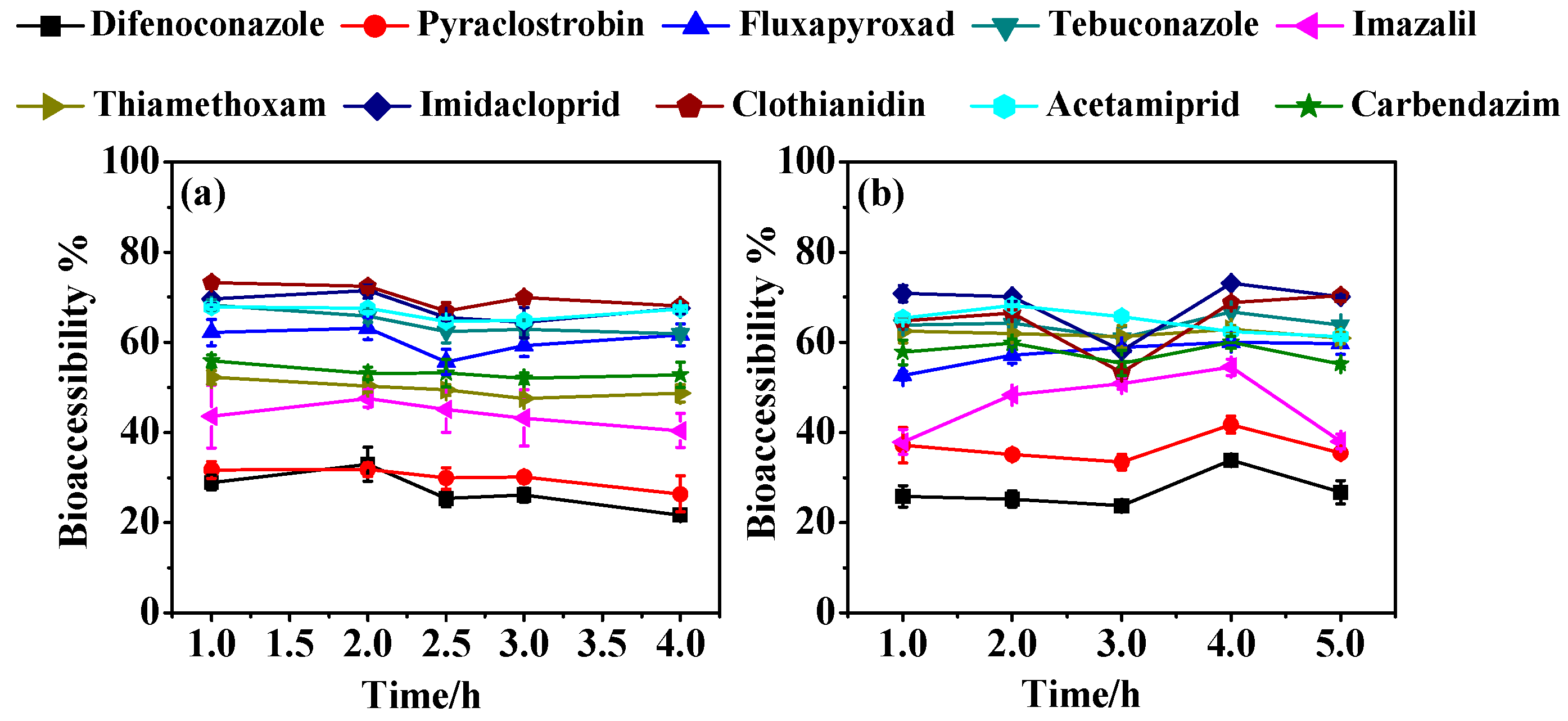
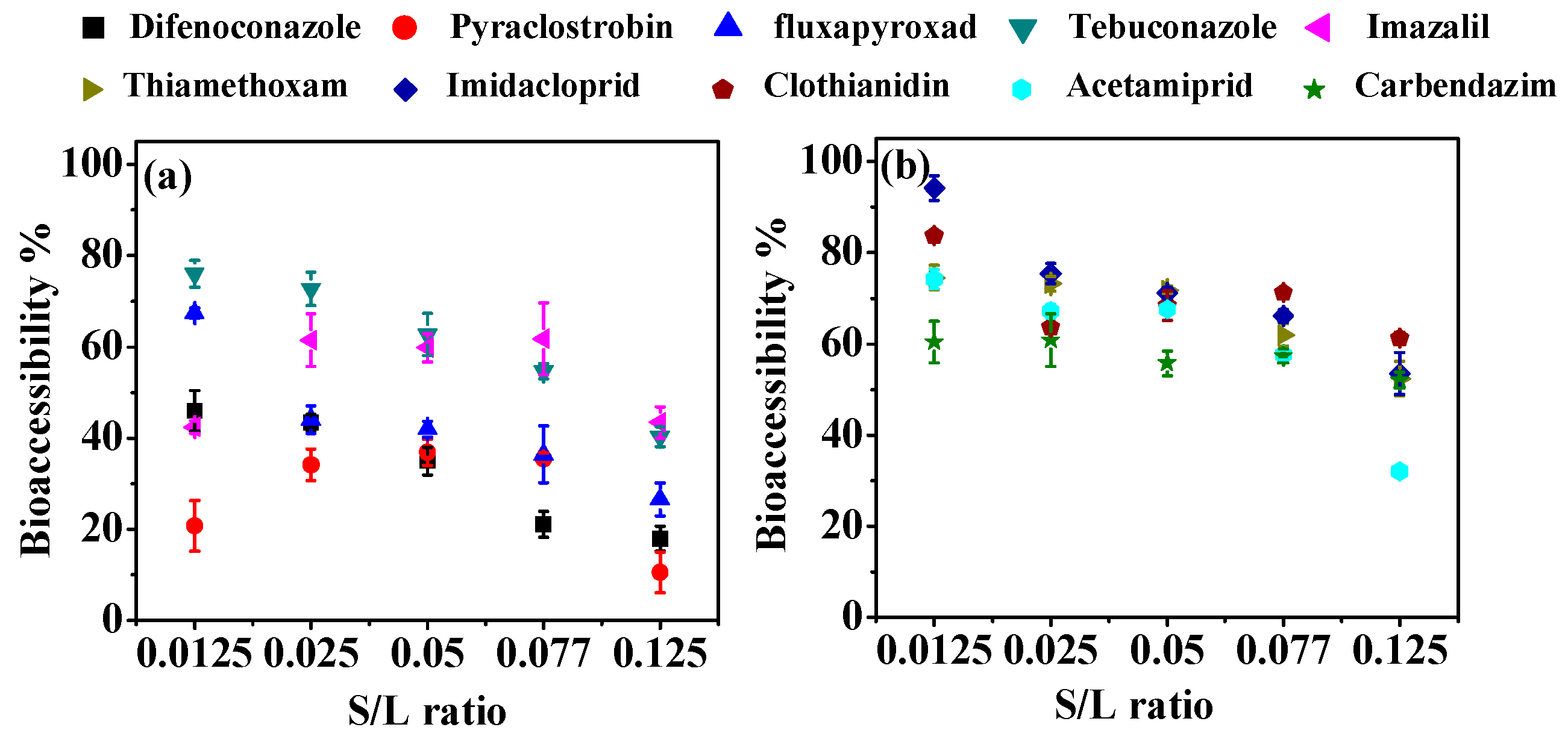
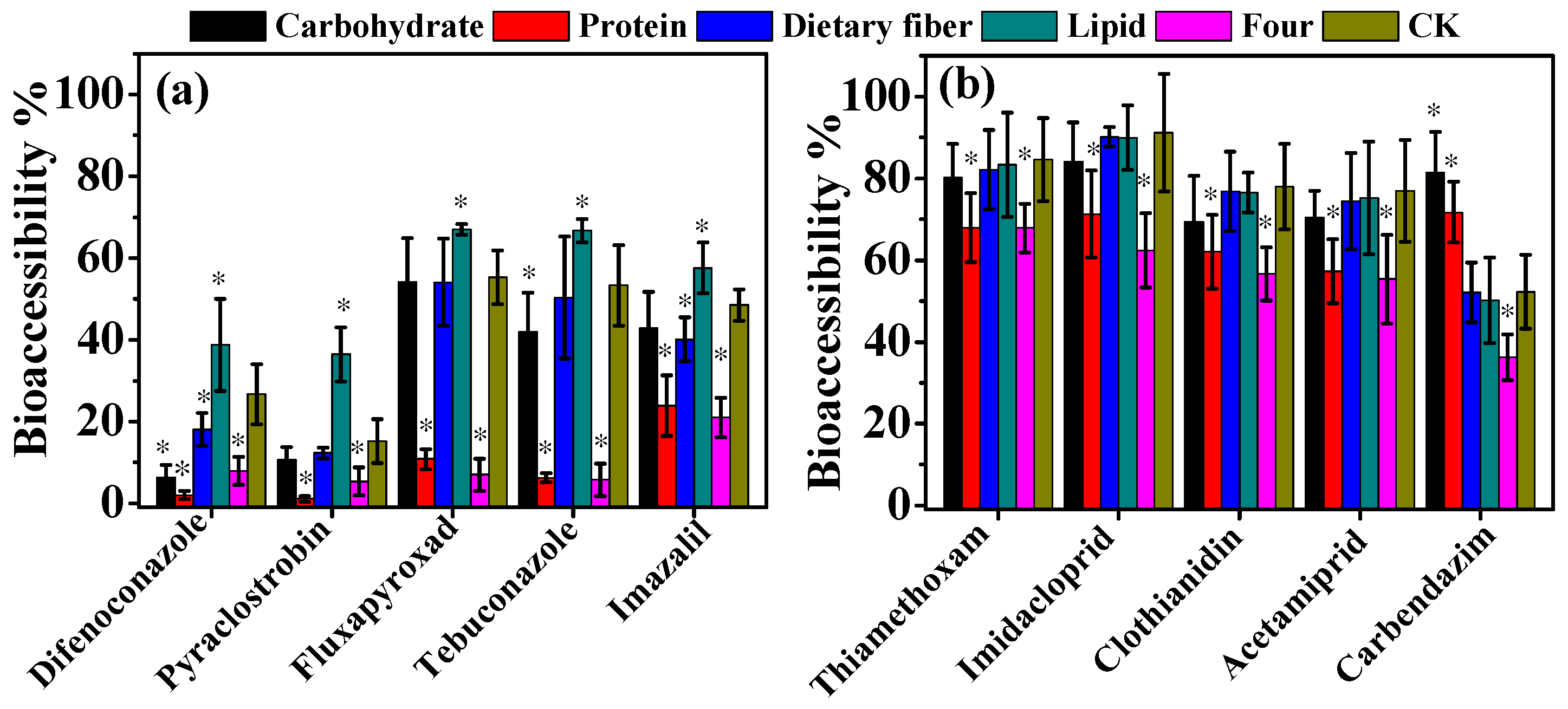
| Pesticides | Fitted Equation | |||||
|---|---|---|---|---|---|---|
| Linear | R2 | Exponential | R2 | Logarithmic | R2 | |
| Difenoconazole | y = −270.3x + 48.316 | 0.9130 | y = 51.823e−9.156x | 0.9309 | y = −13.34ln(x) − 9.263 | 0.9155 |
| Fluxapyroxad | y = −292.9x + 60.226 | 0.7606 | y = 61.990e−7.009x | 0.8703 | y = −15.74ln(x) − 6.187 | 0.9036 |
| Tebuconazole | y = −320.72x + 79.838 | 0.9970 | y = 83.059e−5.683x | 0.9965 | y = −15.18ln(x) + 13.567 | 0.9191 |
| Thiamethoxam | y = −205.76x + 78.693 | 0.9571 | y = 79.948e−3.258x | 0.9529 | y = −9.181ln(x) + 37.932 | 0.7840 |
| Imidacloprid | y = −305.38x + 89.74 | 0.8564 | y = 91.195e−4.358x | 0.9100 | y = −15.81ln(x) + 22.395 | 0.9438 |
| Acetamiprid | y = −236.23x + 53.497 | 0.9242 | y = 57.629e−7.076x | 0.8852 | y = −10.33ln(x) + 7.3547 | 0.7273 |
| Carbendazim | y = −74.264x + 61.581 | 0.8486 | y = 61.721e−1.317x | 0.8520 | y = −3.503ln(x) + 46.275 | 0.7768 |
| Clothianidin | Polynomial y = −180618x3 + 37621x2 − 2220.4x + 103.51 R2 = 0.8237 | |||||
| Pyraclostrobin | Polynomial y = −6630.7x2 + 794.94x + 14.50 R2 = 0.9549 | |||||
| Imazalil | Polynomial y = −8707.1x2 + 1140.5x + 57.86 R2 =0.7843 | |||||
| Pesticides | Initial Concentration (μg/kg) | Bioaccessibility (%) | Bioaccessible Concentration (μg/kg) | CHQ a (%) | cHQ-BA b (%) | aHQ a (%) | aHQ-BA b (%) |
|---|---|---|---|---|---|---|---|
| Spiked sample | |||||||
| Difenoconazole | 1040.00 ± 6.32 | 38.81 ± 4.38 | 403.81 ± 48.01 | 1.20 × 10−1 | 4.64 × 10−2 | 3.96 × 10−2 | 1.54 × 10−2 |
| Pyraclostrobin | 1052.00 ± 24.51 | 29.55 ± 2.32 | 311.25 ± 31.65 | 4.03 × 10−2 | 1.19 × 10−2 | 2.41 × 10−1 | 7.12 × 10−2 |
| Fluxapyroxad | 826.90 ± 1.18 | 43.57 ± 4.83 | 360.32 ± 40.45 | 4.75 × 10−2 | 2.07 × 10−2 | 3.15 × 10−2 | 1.37 × 10−2 |
| Tebuconazole | 1092.80 ± 19.97 | 45.69 ± 3.81 | 499.81 ± 50.76 | 4.19 × 10−2 | 1.92 × 10−2 | 4.17 × 10−2 | 1.91 × 10−2 |
| Imazalil | 931.00 ± 26.56 | 48.36 ± 4.27 | 450.99 ± 52.60 | 3.57 × 10−2 | 1.73 × 10−2 | 2.13 × 10−1 | 1.03 × 10−1 |
| Thiamethoxam | 1001.13 ± 13.41 | 82.57 ± 0.69 | 826.69 ± 17.98 | 1.44 × 10−2 | 1.19 × 10−2 | 1.14 × 10−2 | 9.45 × 10−3 |
| Imidacloprid | 962.29 ± 25.88 | 69.18 ± 2.50 | 666.14 ± 41.96 | 1.84 × 10−2 | 1.28 × 10−2 | 2.75 × 10−2 | 1.90 × 10−2 |
| Clothianidin | 1048.40 ± 6.80 | 67.74 ± 0.89 | 710.23 ± 13.94 | 1.48 × 10−2 | 9.34 × 10−3 | 1.03 × 10−1 | 6.51 × 10−2 |
| Acetamiprid | 898.30 ± 2.50 | 63.32 ± 3.86 | 568.87 ± 36.26 | 1.21 × 10−2 | 8.17 × 10−3 | 2.00 × 10−2 | 1.35 × 10−2 |
| Carbendazim | 1096.40 ± 9.00 | 57.91 ± 5.08 | 635.23 ± 60.91 | 4.20 × 10−2 | 2.43 × 10−2 | 1.57 × 10−1 | 9.08 × 10−2 |
| Commercial sample 1 | |||||||
| Difenoconazole | 93.03 ± 6.33 | 23.27 ± 0.41 | 21.67 ± 1.85 | 1.07 × 10−2 | 2.50 × 10−3 | 3.60 × 10−3 | 8.00 × 10−4 |
| Pyraclostrobin | 141.05 ± 8.54 | 31.31 ± 0.89 | 44.21 ± 3.93 | 5.40 × 10−3 | 1.70 × 10−3 | 3.23 × 10−2 | 1.01 × 10−2 |
| Fluxapyroxad | 106.45 ± 4.38 | 48.88 ± 0.64 | 52.05 ± 2.82 | 6.10 × 10−3 | 3.00 × 10−3 | 4.10 × 10−3 | 2.00 × 10−3 |
| Tebuconazole | 32.89 ± 2.45 | 54.54 ± 0.11 | 17.94 ± 1.37 | 1.30 × 10−3 | 7.00 × 10−4 | 1.30 × 10−3 | 7.00 × 10−4 |
| Imidacloprid | 19.89 ± 0.91 | 78.72 ± 0.39 | 15.66 ± 0.79 | 4.00 × 10−4 | 3.00 × 10−4 | 6.00 × 10−4 | 5.00 × 10−4 |
| Commercial sample 2 | |||||||
| Difenoconazole | 180.80 ± 8.44 | 20.29 ± 4.52 | 36.94 ± 9.89 | 2.08 × 10−2 | 4.20 × 10−3 | 6.90 × 10−3 | 1.40 × 10−3 |
| Pyraclostrobin | 80.48 ± 4.49 | 40.33 ± 3.37 | 32.56 ± 4.52 | 3.10 × 10−3 | 1.20 × 10−3 | 1.84 × 10−2 | 7.40 × 10−3 |
| Clothianidin | 168.92 ± 2.32 | 88.13 ± 2.50 | 148.91 ± 6.27 | 1.90 × 10−3 | 1.70 × 10−3 | 3.20 × 10−3 | 2.80 × 10−3 |
| Commercial sample 3 | |||||||
| Difenoconazole | 134.33 ± 16.44 | 43.12 ± 2.04 | 58.15 ± 9.83 | 1.54 × 10−2 | 6.70 × 10−3 | 5.10 × 10−3 | 2.20 × 10−3 |
| Pyraclostrobin | 284.87 ± 17.89 | 32.55 ± 1.82 | 92.94 ± 11.01 | 1.09 × 10−2 | 3.60 × 10−3 | 6.51 × 10−2 | 2.13 × 10−2 |
| Imidacloprid | 370.28 ± 16.63 | 79.06 ± 0.53 | 292.80 ± 15.11 | 7.10 × 10−3 | 5.60 × 10−3 | 1.06 × 10−2 | 8.40 × 10−3 |
| Carbendazim | 135.38 ± 4.53 | 55.03 ± 0.83 | 74.52 ± 3.62 | 5.20 × 10−3 | 2.90 × 10−3 | 1.94 × 10−2 | 1.07 × 10−2 |
| Commercial sample 4 | |||||||
| Pyraclostrobin | 168.54 ± 1.88 | 30.76 ± 0.65 | 51.85 ± 1.67 | 6.50 × 10−3 | 2.00 × 10−3 | 3.85 × 10−2 | 1.19 × 10−2 |
| Fluxapyroxad | 140.81 ± 3.75 | 42.31 ± 0.35 | 59.59 ± 2.08 | 8.10 × 10−3 | 3.40 × 10−3 | 5.40 × 10−3 | 2.30 × 10−3 |
| Thiamethoxam | 17.36 ± 0.70 | 65.21 ± 1.22 | 11.33 ± 0.67 | 2.00 × 10−4 | 2.00 × 10−4 | 2.00 × 10−4 | 1.00 × 10−4 |
| Imidacloprid | 20.01 ± 0.34 | 57.68 ± 0.24 | 11.54 ± 0.24 | 4.00 × 10−4 | 2.00 × 10−4 | 6.00 × 10−4 | 3.00 × 10−4 |
| Clothianidin | 132.54 ± 2.58 | 72.20 ± 0.16 | 95.70 ± 2.07 | 1.50 × 10−3 | 1.10 × 10−3 | 2.50 × 10−3 | 1.80 × 10−3 |
| Acetamiprid | 32.60 ± 0.91 | 75.28 ± 0.13 | 24.54 ± 0.73 | 5.00 × 10−4 | 4.00 × 10−4 | 3.70 × 10−3 | 2.80 × 10−3 |
| Carbendazim | 493.86 ± 7.24 | 54.32 ± 0.54 | 268.29 ± 6.60 | 1.89 × 10−2 | 1.03 × 10−2 | 7.06 × 10−2 | 3.83 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zhang, Q.; Lv, D.-Z.; Song, J.; Fan, Q.; Tian, H.; Wang, M.-Y. Study of Factors Influencing the Oral Bioaccessibility of Commonly Used and Detected Pesticides in Bananas and Mangoes Based on in vitro Methods. Foods 2024, 13, 2019. https://doi.org/10.3390/foods13132019
Ma C, Zhang Q, Lv D-Z, Song J, Fan Q, Tian H, Wang M-Y. Study of Factors Influencing the Oral Bioaccessibility of Commonly Used and Detected Pesticides in Bananas and Mangoes Based on in vitro Methods. Foods. 2024; 13(13):2019. https://doi.org/10.3390/foods13132019
Chicago/Turabian StyleMa, Chen, Qun Zhang, Dai-Zhu Lv, Jia Song, Qiong Fan, Hai Tian, and Ming-Yue Wang. 2024. "Study of Factors Influencing the Oral Bioaccessibility of Commonly Used and Detected Pesticides in Bananas and Mangoes Based on in vitro Methods" Foods 13, no. 13: 2019. https://doi.org/10.3390/foods13132019
APA StyleMa, C., Zhang, Q., Lv, D.-Z., Song, J., Fan, Q., Tian, H., & Wang, M.-Y. (2024). Study of Factors Influencing the Oral Bioaccessibility of Commonly Used and Detected Pesticides in Bananas and Mangoes Based on in vitro Methods. Foods, 13(13), 2019. https://doi.org/10.3390/foods13132019





