Effects of Fish Skin Gelatin Hydrolysates Treated with Alcalase and Savinase on Frozen Dough and Bread Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Productions of Fish Skin Gelatin and Its Hydrolysate and FGH Characteristics
2.2.1. Production of Fish Skin Gelatin
2.2.2. Production of the FGHs
2.2.3. Determination of the Degree of Hydrolysis (DH) of the FGHs
2.2.4. Zeta Potential of the FGHs
2.2.5. Emulsifying Activity and Stability of the FGHs
2.2.6. Foaming Capacity and Stability of the FGHs
2.2.7. Fat-Binding Capacity of FGH
2.3. Preparation of Fresh/Frozen Bread Dough and Baking
2.3.1. Rheological Properties of the Bread Doughs
2.3.2. Determination of Freezable Water Content of the Bread Doughs
2.3.3. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis of the Bread Doughs
2.3.4. Secondary Structure Measurement of Proteins by FTIR of the Bread Doughs
2.3.5. Determination of Specific Volume, Texture and Color Characteristics of the Breads
2.4. Statistical Analysis
3. Results
3.1. Some Characteristics of the FGHs
3.1.1. Degree of Hydrolysis of the FGHs
3.1.2. Zeta Potential of the FGHs
3.1.3. Emulsifying Activity and Stability of the FGHs
3.1.4. Foaming Capacity and Stability of the FGHs
3.1.5. Fat-Binding Capacity of the FGHs
3.2. Some Characteristics of the Bread Dough Produced by Adding FGHs
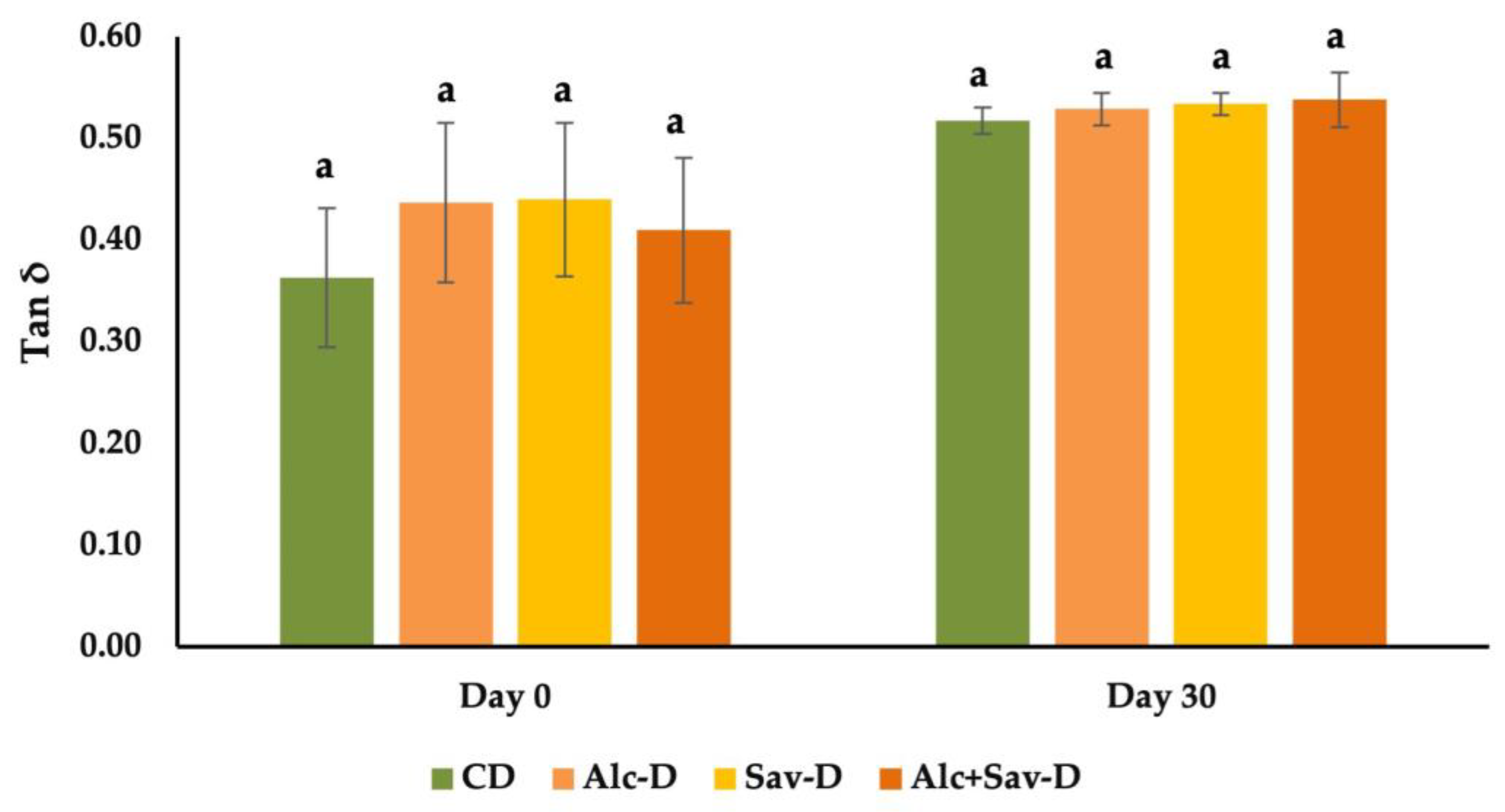
3.2.1. Changes in the Viscoelastic Behavior of the Bread Doughs
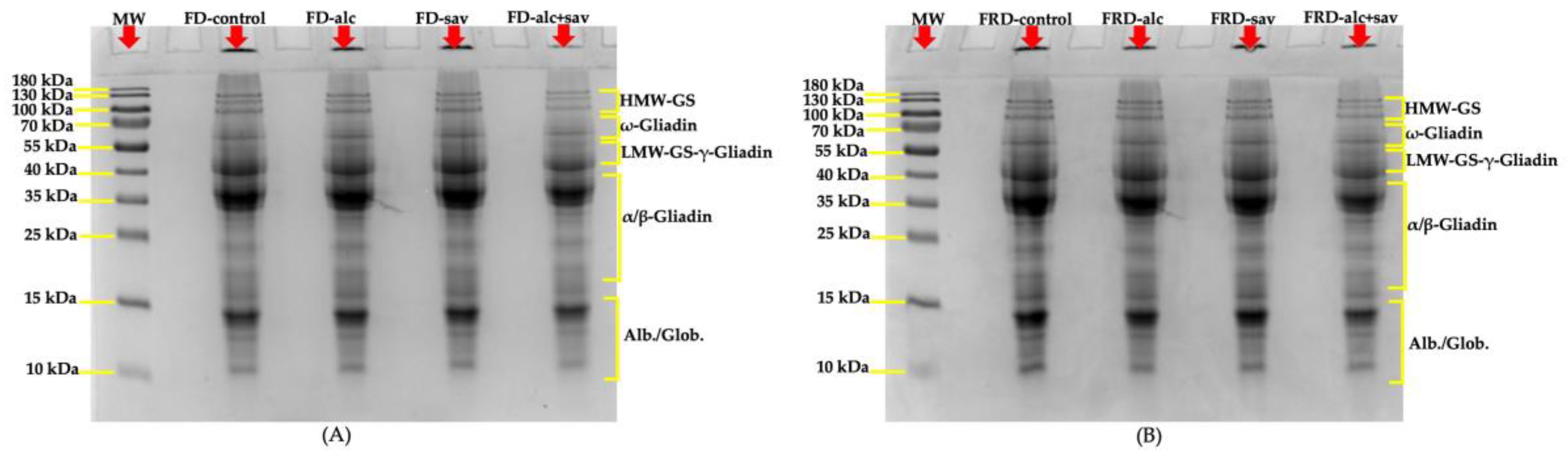
3.2.2. SDS-PAGEs of the Bread Doughs
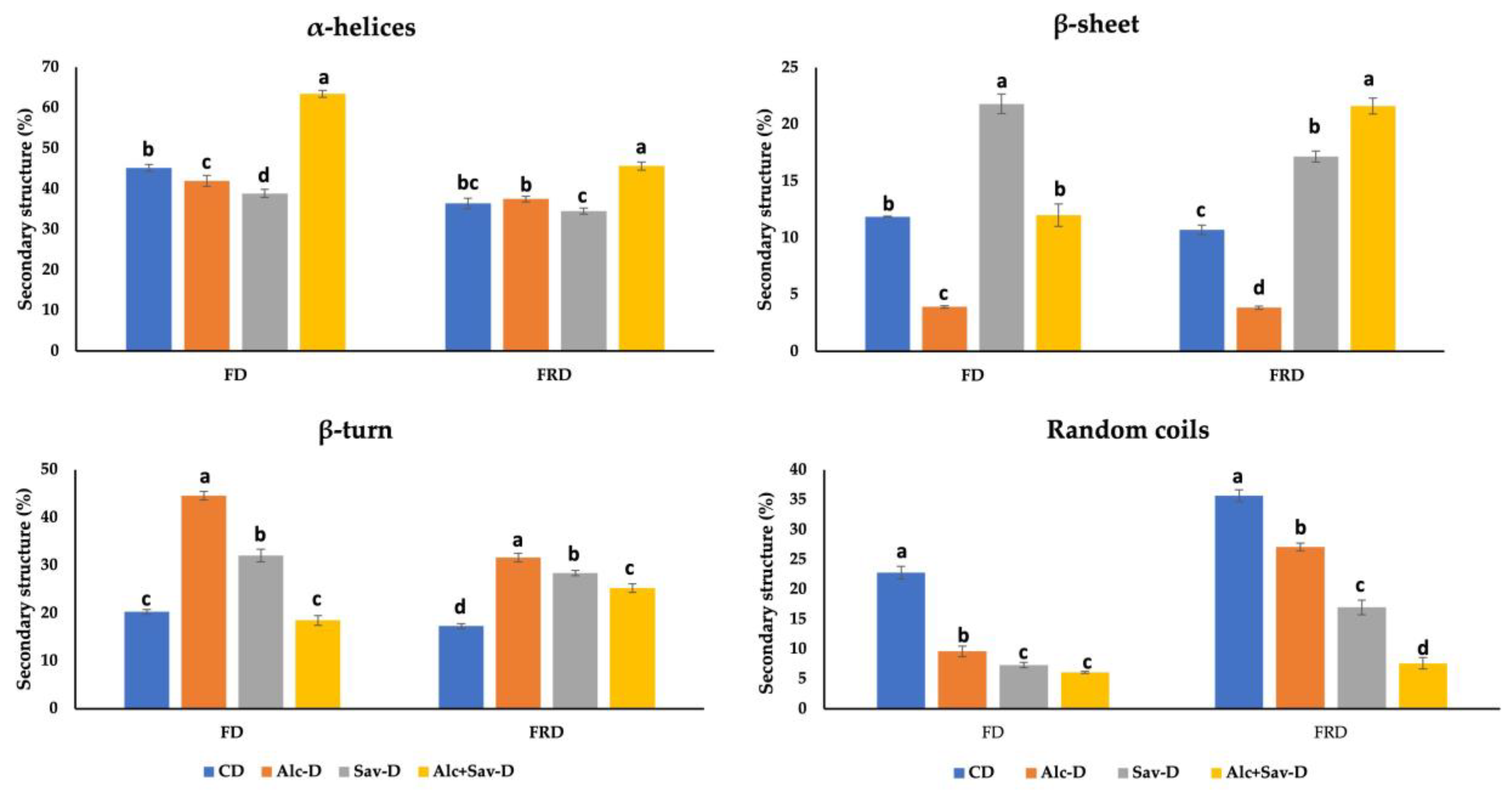
3.2.3. Analysis of Secondary Structures of Protein of the Bread Doughs
3.2.4. Freezable Water of the Bread Doughs
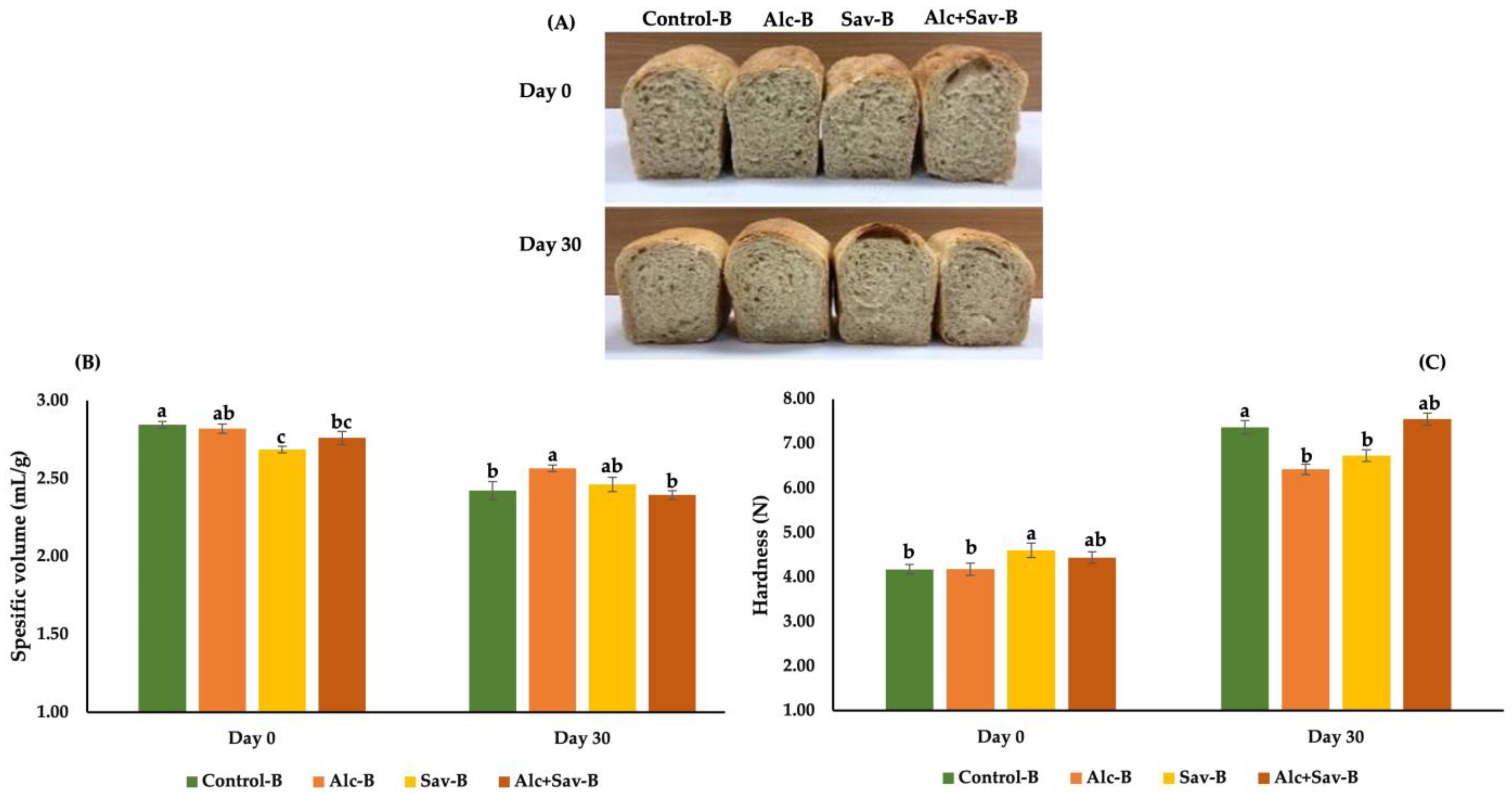
3.3. Some Characteristics of the Breads from the Bread Dough with FGHs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selomulyo, V.O.; Zhou, W. Frozen bread dough: Effects of freezing storage and dough improvers. J. Cereal Sci. 2007, 45, 1–17. [Google Scholar] [CrossRef]
- Rayas-Duarte, P.; Murtini, E.S. Chapter 19—Bread staling. In Breadmaking, 3rd ed.; Cauvain, S.P., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 561–585. [Google Scholar]
- Luo, W.; Sun, D.-W.; Zhu, Z.; Wang, Q.-J. Improving freeze tolerance of yeast and dough properties for enhancing frozen dough quality—A review of effective methods. Trends Food Sci. Technol. 2018, 72, 25–33. [Google Scholar] [CrossRef]
- Steffolani, M.E.; Ribotta, P.D.; Perez, G.T.; Puppo, M.C.; León, A.E. Use of Enzymes to Minimize Dough Freezing Damage. Food Bioprocess Technol. 2012, 5, 2242–2255. [Google Scholar] [CrossRef]
- Matuda, T.G.; Parra, D.F.; Lugão, A.B.; Tadini, C.C. Influence of vegetable shortening and emulsifiers on the unfrozen water content and textural properties of frozen French bread dough. LWT—Food Sci. Technol. 2005, 38, 275–280. [Google Scholar] [CrossRef]
- Wang, P.; Jin, Z.; Xu, X. Physicochemical alterations of wheat gluten proteins upon dough formation and frozen storage—A review from gluten, glutenin and gliadin perspectives. Trends Food Sci. Technol. 2015, 46 Pt A, 189–198. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, J.; Tu, J.; Yu, L.; Niu, L. The alleviative effect of sweet potato protein hydrolysates on the quality deterioration of frozen dough bread in comparison to trehalose. LWT 2023, 175, 114505. [Google Scholar] [CrossRef]
- Xin, C.; Nie, L.; Chen, H.; Li, J.; Li, B. Effect of degree of substitution of carboxymethyl cellulose sodium on the state of water, rheological and baking performance of frozen bread dough. Food Hydrocoll. 2018, 80, 8–14. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, H.; Xu, X.; Xu, D. Deterioration mechanisms and quality improvement methods in frozen dough: An updated review. Trends Food Sci. Technol. 2023, 143, 104251. [Google Scholar] [CrossRef]
- Bekiroglu, H.; Bozkurt, F.; Karadag, A.; Ahhmed, A.M.; Sagdic, O. The effects of different protease treatments on the techno-functional, structural, and bioactive properties of bovine casein. Prep. Biochem. Biotechnol. 2022, 52, 1097–1108. [Google Scholar] [CrossRef]
- Bozkurt, F.; Bekiroglu, H.; Dogan, K.; Karasu, S.; Sagdic, O. Technological and bioactive properties of wheat glutenin hydrolysates prepared with various commercial proteases. LWT 2021, 149, 111787. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, bioactive properties, and potential applications of fish protein hydrolysates: Developments and challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Vv, R.; Brooks, M.S.-L.; Budge, S.M.; Dave, D. Fish Processing Wastes as a Potential Source of Proteins, Amino Acidsand Oils: A Critical Review. J. Microb. Biochem. Technol. 2013, 2, 107–129. [Google Scholar]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Thitipramote, N.; Benjakul, S. Preparation and functional characterisation of fish skin gelatin and comparison with commercial gelatin. Int. J. Food Sci. Technol. 2013, 48, 1093–1102. [Google Scholar] [CrossRef]
- Shiao, W.-C.; Wu, T.-C.; Kuo, C.-H.; Tsai, Y.-H.; Tsai, M.-L.; Hong, Y.-H.; Huang, C.-Y. Physicochemical and Antioxidant Properties of Gelatin and Gelatin Hydrolysates Obtained from Extrusion-Pretreated Fish (Oreochromis sp.) Scales. Mar. Drugs 2021, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Tekle, S.; Bozkurt, F.; Akman, P.K.; Sagdic, O. Bioactive and Functional properties of gelatin peptide fractions obtained from sea bass (Dicentrarchus labrax) skin. Food Sci. Technol. 2022, 42, e60221. [Google Scholar] [CrossRef]
- Bekiroglu, H.; Ozulku, G.; Sagdic, O. Effects of Casein Hydrolysate Prepared with Savinase on the Quality of Bread Made by Frozen Dough. Foods 2023, 12, 3845. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.; Yun, Y.; Shin, M.; Chin, K.B.; Eun, J.-B. Effects of milk proteins and gums on quality of bread made from frozen dough. J. Sci. Food Agric. 2009, 89, 1407–1415. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Zhang, H.; Guo, L.; Hong, T.; Zhang, W.; Jin, Y.; Xu, X. Effect of pigskin gelatin on baking, structural and thermal properties of frozen dough: Comprehensive studies on alteration of gluten network. Food Hydrocoll. 2020, 102, 105591. [Google Scholar] [CrossRef]
- Cao, H.; Zheng, X.; Liu, H.; Yuan, M.; Ye, T.; Wu, X.; Yin, F.; Li, Y.; Yu, J.; Xu, F. Cryo-protective effect of ice-binding peptides derived from collagen hydrolysates on the frozen dough and its ice-binding mechanisms. LWT 2020, 131, 109678. [Google Scholar] [CrossRef]
- Xingxin, G.; Faxiang, W.; Jian, Y.; Xianghong, L.; Jianhui, W.; Yiqun, H.; Yongle, L. Effects of fish enzymatic hydrolysates and fish collagen peptides on the quality of frozen dough. Food Mach. 2018, 34, 16–19. [Google Scholar]
- Sang, S.; Ou, C.; Fu, Y.; Su, X.; Jin, Y.; Xu, X. Complexation of fish skin gelatin with glutentin and its effect on the properties of wheat dough and bread. Food Chem. X 2022, 14, 100319. [Google Scholar] [CrossRef] [PubMed]
- Boran, G.; Regenstein, J.M. Optimization of gelatin extraction from silver carp skin. J. Food Sci. 2009, 74, E432–E441. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vazquez, J.A.; Perez-Martin, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zamorano-Apodaca, J.C.; Garcia-Sifuentes, C.O.; Carvajal-Millan, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Lugo-Sanchez, M.E. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish. Food Chem. 2020, 331, 127350. [Google Scholar] [CrossRef] [PubMed]
- Karoud, W.; Sila, A.; Krichen, F.; Martinez-Alvarez, O.; Bougatef, A. Characterization, Surface Properties and Biological Activities of Protein Hydrolysates Obtained from Hake (Merluccius merluccius) Heads. Waste Biomass Valorization 2019, 10, 287–297. [Google Scholar] [CrossRef]
- AACC. Approved Methods of AACC, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Jia, F.; Jin, H. Characterization of casein hydrolysates derived from enzymatic hydrolysis. Chem. Cent. J. 2013, 7, 62. [Google Scholar] [CrossRef]
- Tamm, F.; Herbst, S.; Brodkorb, A.; Drusch, S. Functional properties of pea protein hydrolysates in emulsions and spray-dried microcapsules. Food Hydrocoll. 2016, 58, 204–214. [Google Scholar] [CrossRef]
- Stefanović, A.B.; Jovanović, J.R.; Balanč, B.D.; Šekuljica, N.Ž.; Tanasković, S.M.J.; Dojčinović, M.B.; Knežević-Jugović, Z.D. Influence of ultrasound probe treatment time and protease type on functional and physicochemical characteristics of egg white protein hydrolysates. Poult. Sci. 2018, 97, 2218–2229. [Google Scholar] [CrossRef] [PubMed]
- Hajfathalian, M.; Ghelichi, S.; García-Moreno, P.J.; Moltke Sørensen, A.-D.; Jacobsen, C. Peptides: Production, bioactivity, functionality, and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 3097–3129. [Google Scholar] [CrossRef] [PubMed]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of Hydrolysis Degree on the Functional Properties of Salmon Byproducts Hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Zhu, T.-w.; Zhang, X.; Li, B.; Wu, H. Effect of interesterified blend-based fast-frozen special fat on the physical properties and microstructure of frozen dough. Food Chem. 2019, 272, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Dai, Y.; Guo, D.; Zhang, J.; Liang, H.; Li, B.; Sun, J.; Wu, J. Effect of desalted egg white and gelatin mixture system on frozen dough. Food Hydrocoll. 2022, 132, 107889. [Google Scholar] [CrossRef]
- Li, W.; Sun, X.; Mariga, A.M.; Yang, Q.; Fang, Y.; Hu, Q.; Pei, F. Effects of ferulic acid on the polymerization behavior of gluten protein and its components. Food Hydrocoll. 2024, 147, 109388. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Guo, X.-N.; Zhu, K.-X. Polymerization of wheat gluten and the changes of glutenin macropolymer (GMP) during the production of Chinese steamed bread. Food Chem. 2016, 201, 275–283. [Google Scholar] [CrossRef]
- Wang, P.; Lee, T.-C.; Xu, X.; Jin, Z. The contribution of glutenin macropolymer depolymerization to the deterioration of frozen steamed bread dough quality. Food Chem. 2016, 211, 27–33. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Mohanad, B.; Xu, L.; Ning, Y.; Xu, J.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of frozen storage on physico-chemistry of wheat gluten proteins: Studies on gluten-, glutenin- and gliadin-rich fractions. Food Hydrocoll. 2014, 39, 187–194. [Google Scholar] [CrossRef]
- Lu, P.; Guo, J.; Fan, J.; Wang, P.; Yan, X. Combined effect of konjac glucomannan addition and ultrasound treatment on the physical and physicochemical properties of frozen dough. Food Chem. 2023, 411, 135516. [Google Scholar] [CrossRef]
- Chen, X.; Su, T.; Yang, H.; Lei, H.; Meng, M.; Luo, X.; Ou, C.; Jia, L.; Sang, S. Effect of fish skin gelatin on characteristics and staling properties of bread made from pre-baked frozen dough. Food Biosci. 2023, 55, 103070. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Zhu, Y.; Qin, S.; Deng, Y.; Zhao, Y. Effect of dietary fiber-rich fractions on texture, thermal, water distribution, and gluten properties of frozen dough during storage. Food Chem. 2019, 297, 124902. [Google Scholar] [CrossRef]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]

| Enzyme | Zeta Potential (mV) | EAI (m2/g) | ESI (min) | Foam Capacity (%) | Foam Stability (%-3 min) | Foam Stability (%-10 min) | Fat-Binding Capacity (mL/g) |
|---|---|---|---|---|---|---|---|
| FGH-alc | −27.28 ± 0.98 a | 34.82 ± 0.9 a | 189.00 ± 1.00 a | 22.49 ± 1.50 a | 15.40 ± 0.79 a | 9.40 ± 0.79 a | 0.81 ± 0.06 a |
| FGH-sav | −21.36 ± 0.24 b | 70.20 ± 0.85 b | 24.42 ± 0.62 b | 27.80 ± 2.69 b | 19.65 ± 1.00 b | 12.74 ± 0.79 b | 1.84 ± 0.03 b |
| Storage Time | Sample | Tm, Onset (°C) | ΔH (J/g) | FW (%) |
|---|---|---|---|---|
| Day 0 | FD-control | −8.22 ± 0.13 c | 36.64 ± 0.56 a | 24.38 ± 0.37 a |
| FD-alc | −6.96 ± 0.29 a | 31.77 ± 0.15 b | 21.90 ± 0.10 b | |
| FD-sav | −7.60 ± 0.22 b | 29.73 ± 0.77 c | 19.73 ± 0.5 c | |
| FD-alc+sav | −6.57 ± 0.19 a | 37.33 ± 1.75 a | 24.56 ± 1.15 a | |
| Day 30 | FRD-control | −6.25 ± 0.37 a | 51.26 ± 2.22 a | 33.96 ± 1.47 a |
| FRD-alc | −7.33 ± 0.28 b | 40.15 ± 1.39 b | 27.47 ± 0.95 b | |
| FRD-sav | −5.86 ± 0.29 a | 36.42 ± 1.43 b | 24.91 ± 0.98 c | |
| FRD-alc+sav | −7.38 ± 0.37 b | 38.59 ± 0.39 ab | 26.87 ± 0.27 b |
| Storage Time | Samples | Crust | Crumb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | L* | a* | b* | ΔE | ||
| Day 0 | Control-B | 62.67 ± 0.95 aA | 9.28 ± 0.63 bBC | 27.68 ± 0.93 aA | - | 65.17 ± 0.91 bC | 0.60 ± 0.20 aA | 14.61 ± 0.84 bC | - |
| Alc-B | 55.00 ± 1.17 bC | 12.65 ± 0.74 aA | 22.67 ± 1.18 cC | 9.79 ± 1.57 aA | 65.59 ± 1.14 bBC | 0.48 ± 0.27 aAB | 14.74 ± 0.96 bC | 1.23 ± 0.85 bB | |
| Sav-B | 55.83 ± 1.13 bC | 12.87 ± 0.67 aA | 24.67 ± 1.36b BC | 8.33 ± 1.59 aA | 68.05 ± 1.06 aA | 0.36 ± 0.17 aABC | 15.75 ± 0.53 abBC | 3.15 ± 1.03 aA | |
| Alc+Sav-B | 55.49 ± 1.82 bC | 13.05 ± 0.37 aA | 27.07 ± 1.39 aA | 8.25 ± 1.74 aA | 67.72 ± 1.02 aAB | 0.28 ± 0.17 aBC | 16.30 ± 0.41 aB | 3.16 ± 0.73 aA | |
| Day 30 | Control-B | 62.61 ± 0.66 aA | 8.44 ± 0.36 bC | 27.08 ± 0.69 aA | 1.30 ± 0.52 bC | 68.33 ± 1.58 aA | 0.16 ± 0.01 aC | 16.89 ± 0.21 abAB | 4.07 ± 0.96 aA |
| Alc-B | 61.51 ± 2.18 aA | 9.98 ± 1.11 abBC | 26.94 ± 0.85 aA | 2.13 ± 2.03 bC | 67.24 ± 1.69 aABC | 0.15 ± 0.08 aC | 16.73 ± 0.37 bAB | 3.17 ± 1.25 aA | |
| Sav-B | 60.83 ± 1.34a AB | 9.40 ± 1.11 abBC | 25.82 ± 0.80 aAB | 2.92 ± 1.27 abBC | 68.26 ± 0.71 aA | 0.23 ± 0.11 aBC | 17.71 ± 0.71 aA | 4.39 ± 0.92 aA | |
| Alc+Sav-B | 58.58 ± 0.70 bB | 10.48 ± 1.31 aB | 26.08 ± 1.29 aAB | 4.81 ± 0.97 aB | 68.59 ± 0.88 aA | 0.11 ± 0.13 aC | 17.54 ± 0.70 abA | 4.58 ± 0.74 aA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekle, S.; Ozulku, G.; Bekiroglu, H.; Sagdic, O. Effects of Fish Skin Gelatin Hydrolysates Treated with Alcalase and Savinase on Frozen Dough and Bread Quality. Foods 2024, 13, 139. https://doi.org/10.3390/foods13010139
Tekle S, Ozulku G, Bekiroglu H, Sagdic O. Effects of Fish Skin Gelatin Hydrolysates Treated with Alcalase and Savinase on Frozen Dough and Bread Quality. Foods. 2024; 13(1):139. https://doi.org/10.3390/foods13010139
Chicago/Turabian StyleTekle, Sefik, Gorkem Ozulku, Hatice Bekiroglu, and Osman Sagdic. 2024. "Effects of Fish Skin Gelatin Hydrolysates Treated with Alcalase and Savinase on Frozen Dough and Bread Quality" Foods 13, no. 1: 139. https://doi.org/10.3390/foods13010139
APA StyleTekle, S., Ozulku, G., Bekiroglu, H., & Sagdic, O. (2024). Effects of Fish Skin Gelatin Hydrolysates Treated with Alcalase and Savinase on Frozen Dough and Bread Quality. Foods, 13(1), 139. https://doi.org/10.3390/foods13010139







