Reducing Effects of Whey Protein Hydrolysate on Coloration of Cured Sausages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. WP and WPH EtOH Extracts
2.3. Sausage Model Preparation
2.4. Meat Color Determination
2.5. Fe3⁺-Reducing Activity
2.6. Oxidation–Reduction Potential Measurement
2.7. NO2−-Reducing Activity
2.8. Nitrosyl Myoglobin-Forming Activity
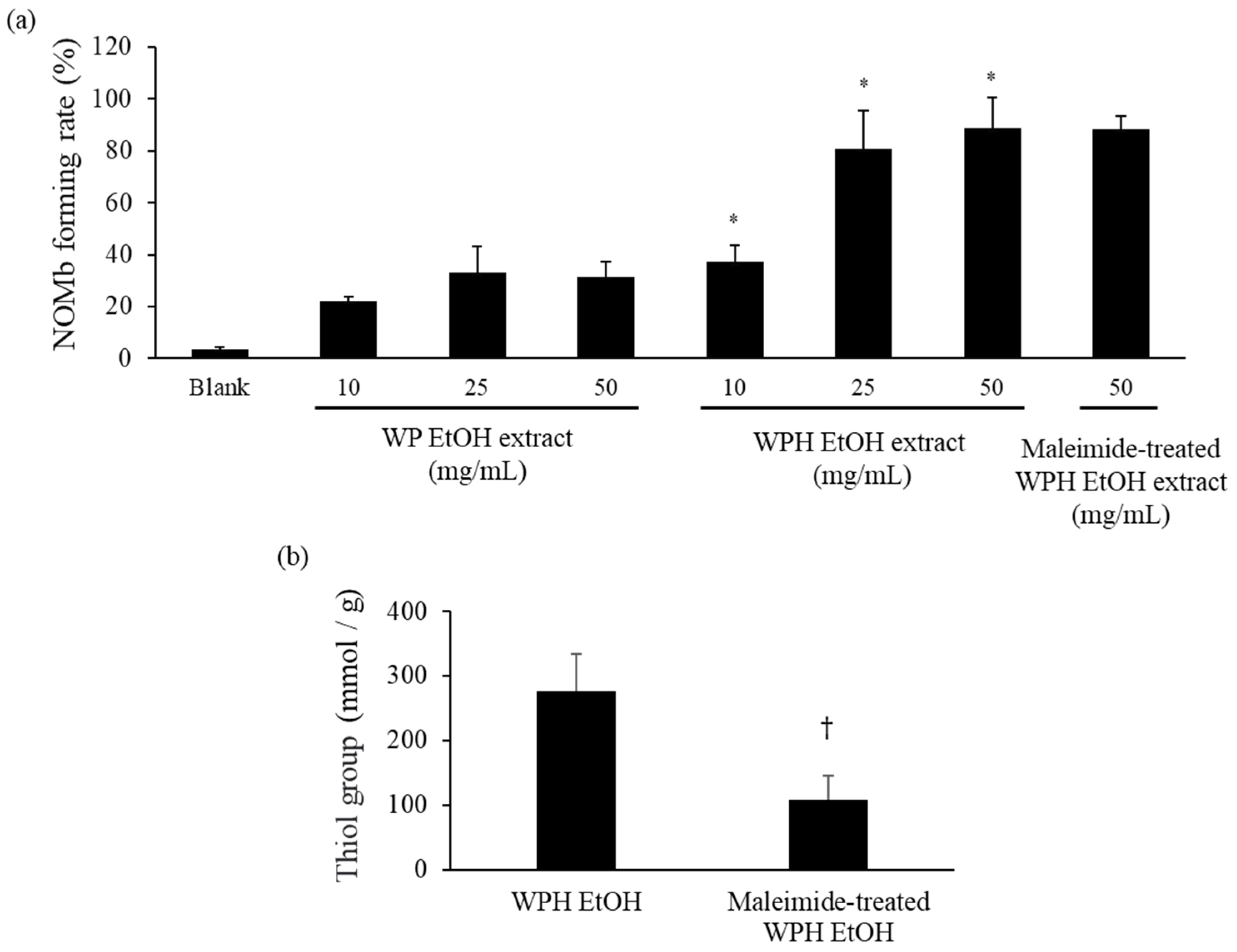
2.9. Maleimide Modification of WPH
2.10. Measurement of Thiol Group Concentration
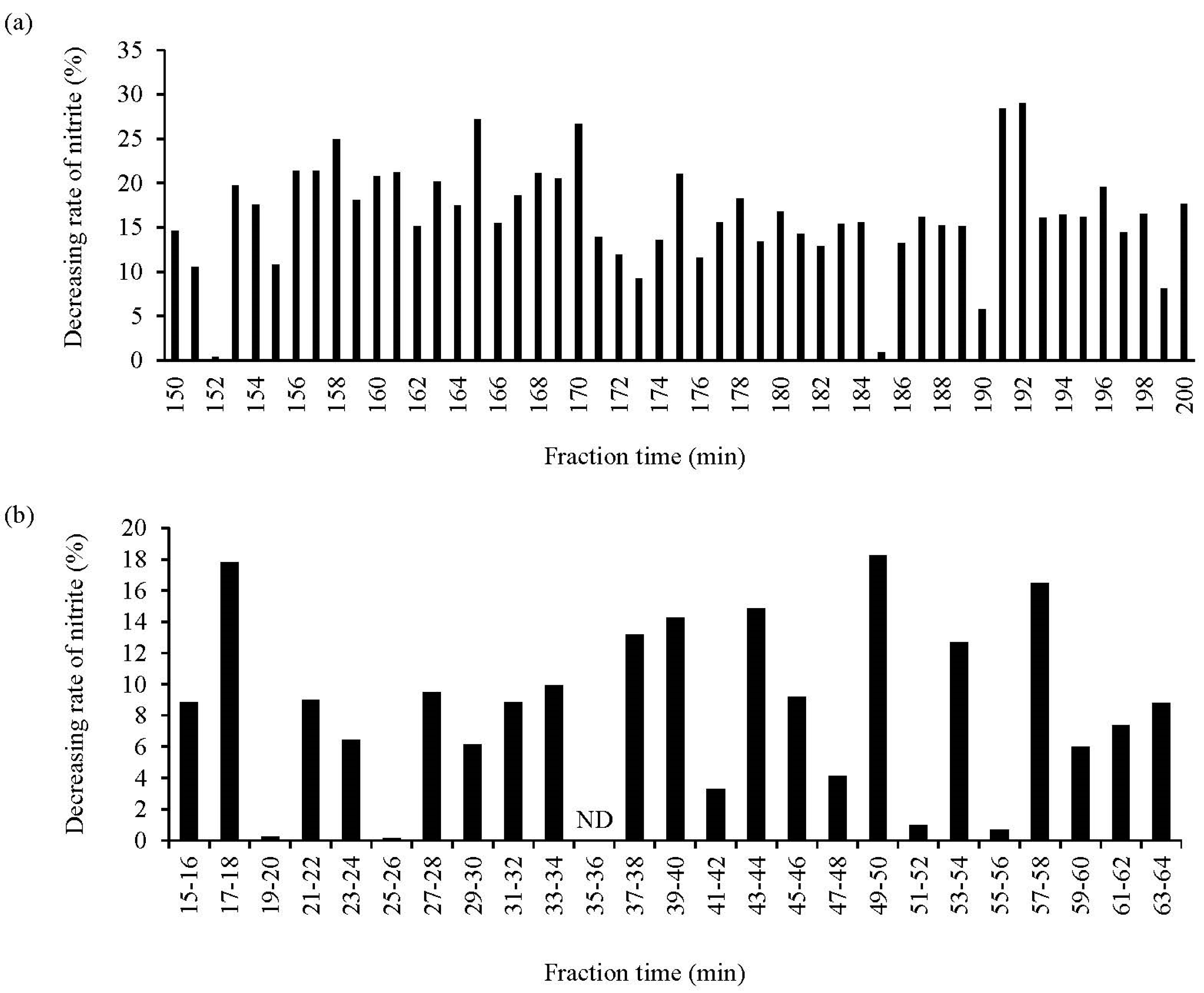
2.11. Isolation and Identification of NO2−-Reducing Peptides
2.12. Statistical Analysis
3. Results
3.1. Meat Color of the Sausage Model with WP and WPH
3.2. Antioxidant and Reducing Activities of WP and WPH
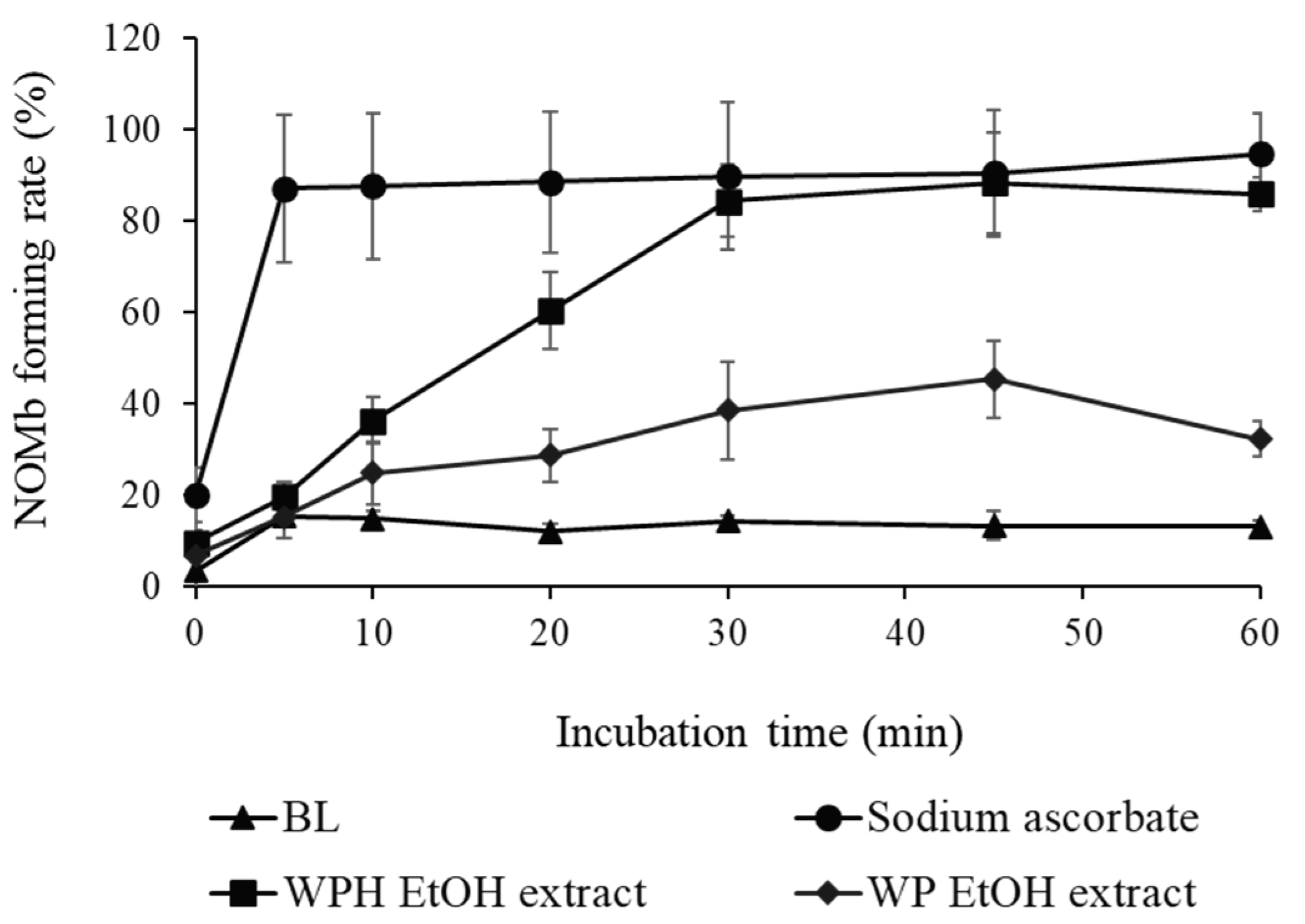
3.3. NOMb-Forming Activity of WP and WPH
3.4. Isolation and Identification of NO2−-Decreasing Peptides from WPH
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Li, Z.; Li, X.; Xin, J.; Wang, Y.; Li, G.; Wu, L.; Shen, Q.W.; Zhang, D. Comparative profiling of sarcoplasmic phosphoproteins in ovine muscle with different color stability. Food Chem. 2018, 240, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, D.K.; Bryan, N.S. Sodium nitrite: The “cure” for nitric oxide insufficiency. Meat Sci. 2012, 92, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.; Faustman, C. Metmyoglobin reducing activity. Meat Sci. 2005, 71, 407–439. [Google Scholar] [CrossRef] [PubMed]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.S.; Adamsen, C.E.; Skibsted, L.H. Spectral characterisation of red pigment in Italian-type dry-cured ham. Increasing lipophilicity during processing and maturation. Eur. Food Res. Technol. 2003, 216, 290–296. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, B.; Xiong, Y.L. Production of cured meat color in nitrite-free Harbin red sausage by Lactobacillus fermentum fermentation. Meat Sci. 2007, 77, 593–598. [Google Scholar] [CrossRef]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Waga, M.; Takeda, S.; Sakata, R. Effect of nitrate on residual nitrite decomposition rate in cooked cured pork. Meat Sci. 2017, 129, 135–139. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Chen, X.; Li, L.; Qiao, W.; Zhang, J. Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N-nitrosamines formation during ripening and storage of dry-cured bacon. LWT-Food Sci. Technol. 2015, 60, 199–206. [Google Scholar] [CrossRef]
- De Wit, J.N. Marschall Rhone-Poulenc Award Lecture. Nutritional and functional characteristics of whey proteins in food products. J. Dairy Sci. 1998, 81, 597–608. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P.; Doucet, D.; McGuffey, M.K. Advances in modifying and understanding whey protein functionality. Trends Food Sci. Technol. 2002, 13, 151–159. [Google Scholar] [CrossRef]
- Jeewanthi, R.K.; Lee, N.K.; Paik, H.D. Improved functional characteristics of whey protein hydrolysates in food industry. Korean J. Food Sci. Anim. Resour. 2015, 35, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, C.; Tang, H.; Yu, P.; Wen, R.; Peng, X.; Xu, X.; Yu, X. Hygroscopicity and antioxidant activity of whey protein hydrolysate and its ability to improve the water holding capacity of pork patties during freeze−thaw cycles. LWT 2023, 182, 114784. [Google Scholar] [CrossRef]
- Peng, X.; Liu, C.; Wang, B.; Kong, L.; Wen, R.; Zhang, H.; Yu, X.; Bai, Y.; Jang, A. Hygroscopic properties of whey protein hydrolysates and their effects on water retention in pork patties during repeated freeze–thaw cycles. LWT 2023, 184, 114984. [Google Scholar] [CrossRef]
- Liu, C.; Kong, L.; Yu, P.; Wen, R.; Yu, X.; Xu, X.; Peng, X. Whey protein hydrolysates improved the oxidative stability and water-holding capacity of pork patties by reducing protein aggregation during repeated freeze-thaw cycles. Foods 2022, 11, 2133. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, P.; Yan, J.; Shi, Y.; Feng, J.; Peng, X. Effects of whey peptides on the quality of pork ball preprepared dishes during repeated freezing-thawing. Foods 2023, 12, 3597. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Morita, H.; Norimatsu, T.; Ito, N. Peptides contribute to colour formation: Accelerating effect of whey protein hydrolysate on colour formation in meat products. Fleischwirtschaft Int. 2004, 19, 113–116. [Google Scholar]
- Sakata, R. Prospects for new technology of meat processing in Japan. Meat Sci. 2010, 86, 243–248. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J. Effect of acid whey on nitrosylmyoglobin concentration in uncured fermented sausage. LWT-Food Sci. Technol. 2015, 64, 713–719. [Google Scholar] [CrossRef]
- Wojciak, K.M.; Keska, P.; Okon, A.; Solska, E.; Libera, J.; Dolatowski, Z.J. The influence of acid whey on the antioxidant peptides generated to reduce oxidation and improve colour stability in uncured roast beef. J. Sci. Food Agric. 2018, 98, 3728–3734. [Google Scholar] [CrossRef]
- Vavrusova, M.; Pindstrup, H.; Johansen, L.B.; Andersen, M.L.; Andersen, H.J.; Skibsted, L.H. Characterisation of a whey protein hydrolysate as antioxidant. Int. Dairy J. 2015, 47, 86–93. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Mirna, A. Verfahren zur gleichzeitigen Bestimmung des Pökelfarbstoffes sowie von Nitrit und Nitrat in Fleischerzeugnissen. Fleischwirtschaft 1972, 52, 1337–1338. [Google Scholar]
- Sakata, R. Studies on physicochemical characteristics of red pigments in meat products. Anim. Sci. J. 2000, 71, 1–16. [Google Scholar] [CrossRef]
- Sakata, R. Absorption Spectrometry Method of Color Tones and Heme Dyes in Meat and Meat Products. In Jpn. J. Meat Sci. Technol; Japan Society for Meat Science and Technology: Tokyo, Japan, 1999; Volume 40, p. 221. [Google Scholar]
- Sakata, R.; Ohso, M.; Nagata, Y. Effect of Porcine Muscle Conditions on the Color of Cooked Cured Meat Products. Agric. Biol. Chem. 1981, 45, 2077–2081. [Google Scholar] [CrossRef]
- Ahhmed, A.M.; Özer, N.; Özcan, C.; Çam, M.; Sağdic, O.; Arıcı, M.; Yılmaz, M.T.; Yetim, H.; Kim, J.-D.; Muguruma, M. Solubility, stability and blood pressure lowering-properties of fresh and cured beef proteins. Act. Sci. Nutr. Health 2019, 3, 16–26. [Google Scholar]
- Takeda, S.; Kaneko, S.; Sogawa, K.; Ahhmed, A.M.; Enomoto, H.; Kawarai, S.; Taira, K.; Mizunoya, W.; Minami, M.; Sakata, R. Isolation, Evaluation, and Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides from Game Meat. Foods 2020, 9, 1168. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kobayashi, K.; Watanabe, T.; Satoh, M.; Nomura, F.; Sogawa, K. Urinary carboxylesterase 5A fragment as an early diagnostic marker of cat chronic kidney disease. J. Electrophor. 2019, 63, 39–45. [Google Scholar] [CrossRef]
- Maeda, H.; Sogawa, K.; Sakaguchi, K.; Abe, S.; Sagizaka, W.; Mochizuki, S.; Horie, W.; Watanabe, T.; Shibata, Y.; Satoh, M.; et al. Urinary albumin and transferrin as early diagnostic markers of chronic kidney disease. J. Vet. Med. Sci. 2015, 77, 937–943. [Google Scholar] [CrossRef]
- Pegg, R.; Shahidi, F. A novel titration methodology for elucidation of the structure of preformed cooked cured-meat pigment by visible spectroscopy. Food Chem. 1996, 56, 105–110. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, G.; Xu, X.; Peng, Z. Studies on the structure and oxidation properties of extracted cooked cured meat pigment by four spectra. Food Chem. 2009, 115, 596–601. [Google Scholar] [CrossRef]
- Peng, X.; Kong, B.; Xia, X.; Liu, Q. Reducing and radical-scavenging activities of whey protein hydrolysates prepared with Alcalase. Int. Dairy J. 2010, 20, 360–365. [Google Scholar] [CrossRef]
- Peng, X.; Xiong, Y.L.; Kong, B. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chem. 2009, 113, 196–201. [Google Scholar] [CrossRef]
- Tinbergen, B. Low-molecular meat fractions active in nitrite reduction. In Proceedings International Symposium on Nitrite in Meat Products; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1974; p. 29. [Google Scholar]
- Imai, T. Health function and utilization of whey protein. Milk Sci. 2007, 55, 227–235. [Google Scholar] [CrossRef]
- Ning, C.; Li, L.; Fang, H.; Ma, F.; Tang, Y.; Zhou, C. l-Lysine/l-arginine/l-cysteine synergistically improves the color of cured sausage with NaNO2 by hindering myoglobin oxidation and promoting nitrosylmyoglobin formation. Food Chem. 2019, 284, 219–226. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Pena-Ramos, E.A.; Xiong, Y.L. Whey and soy protein hydrolysates inhibit lipid oxidation in cooked pork patties. Meat Sci. 2003, 64, 259–263. [Google Scholar] [CrossRef]
- Barrón-Ayala, C.G.; Valenzuela-Melendres, M.; Camou, J.P.; Sebranek, J.G.; Dávila-Ramírez, J.L.; Cumplido-Barbeitia, G. Pork frankfurters prepared with hydrolyzed whey: Preliminary product quality aspects and inhibitory activity of the resulting peptides on angiotensin-converting enzyme. Meat Sci. 2020, 166, 108111. [Google Scholar] [CrossRef]

| Concentration (% w/w) | L* | a* | b* | |
|---|---|---|---|---|
| Blank | — | 63.24 ± 5.66 | 5.72 ± 0.51 | 8.61 ± 1.38 |
| Sodium ascorbate | 0.1 | 61.25 ± 1.63 | 7.76 ± 1.69 | 7.81 ± 0.94 |
| WP | 1.0 | 55.34 ± 0.62 | 6.04 ± 0.99 | 8.11 ± 1.17 |
| 2.5 | 54.45 ± 3.75 | 5.80 ± 1.25 | 7.93 ± 0.80 | |
| 5.0 | 68.46 ± 0.84 | 5.82 ± 0.21 | 10.03 ± 0.58 | |
| WPH | 1.0 | 56.56 ± 0.82 | 6.80 ± 1.04 | 9.46 ± 1.88 |
| 2.5 | 57.24 ± 3.33 | 6.79 ± 2.19 | 8.72 ± 0.04 | |
| 5.0 | 64.47 ± 1.49 † | 6.83 ± 0.39 † | 9.20 ± 0.17 |
| WP EtOH Extract | WPH EtOH Extract | |||||
|---|---|---|---|---|---|---|
| Concentration (mg/mL) | 10 | 25 | 50 | 10 | 25 | 50 |
| Fe3+ reduction Trolox equivalent value (µmol/L) | 11.24 ± 8.74 | 17.56 ± 12.32 | 31.25 ± 25.35 | 27.79 ± 11.98 | 63.05 ± 11.64 * | 104.87 ± 12.32 * |
| ORP value (mV) | 228.0 ± 15.7 | 210.0 ± 5.6 | 206.5 ± 13.9 | 181.3 ± 5.7 * | 168.5 ± 9.3 * | 160.0 ± 15.9 * |
| NO2−-decreasing ratio (%) | 29.86 ± 5.80 | 27.77 ± 7.08 | 22.62 ± 6.29 | 42.08 ± 6.89 * | 40.82 ± 2.03 * | 33.95 ± 5.00 * |
| Fraction | Amino Acid Sequence | Origin of Protein Fragment ※ | NO2−-Decreasing Activity (%) † |
|---|---|---|---|
| FR1 | HIQKEDVPSER | α-casein S1 | 33.7 |
| KEAVALK | Tudor domain containing 15 proteins | 40.48 | |
| FR2 | FFVAPFPEVFGK | α-casein S1 | 60.7 |
| TPEVDDEALEKFDKALK | β-lactoglobulin | 23.5 | |
| FR3 | TPEVDDEALEKFDKALK | β-lactoglobulin | 23.5 |
| EVLENLLR | α-casein S1 | 52.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, S.; Kanda, T.; Ahhmed, A.M.; Sogawa, K.; Umezu, K.; Ogata, M.; Mizunoya, W.; Sakata, R. Reducing Effects of Whey Protein Hydrolysate on Coloration of Cured Sausages. Foods 2024, 13, 13. https://doi.org/10.3390/foods13010013
Takeda S, Kanda T, Ahhmed AM, Sogawa K, Umezu K, Ogata M, Mizunoya W, Sakata R. Reducing Effects of Whey Protein Hydrolysate on Coloration of Cured Sausages. Foods. 2024; 13(1):13. https://doi.org/10.3390/foods13010013
Chicago/Turabian StyleTakeda, Shiro, Teppei Kanda, Abdulatef M. Ahhmed, Kazuki Sogawa, Keitarou Umezu, Masaya Ogata, Wataru Mizunoya, and Ryoichi Sakata. 2024. "Reducing Effects of Whey Protein Hydrolysate on Coloration of Cured Sausages" Foods 13, no. 1: 13. https://doi.org/10.3390/foods13010013
APA StyleTakeda, S., Kanda, T., Ahhmed, A. M., Sogawa, K., Umezu, K., Ogata, M., Mizunoya, W., & Sakata, R. (2024). Reducing Effects of Whey Protein Hydrolysate on Coloration of Cured Sausages. Foods, 13(1), 13. https://doi.org/10.3390/foods13010013






