Effect of Nano- and Microzinc Supplementation on the Mineral Composition of Bones of Rats with Induced Mammary Gland Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Cancer Induction
2.3. Determination of Levels of Elements
2.4. Sampling
2.5. Instrumental Analysis
2.6. Statistical and Bioinformatic Analysis
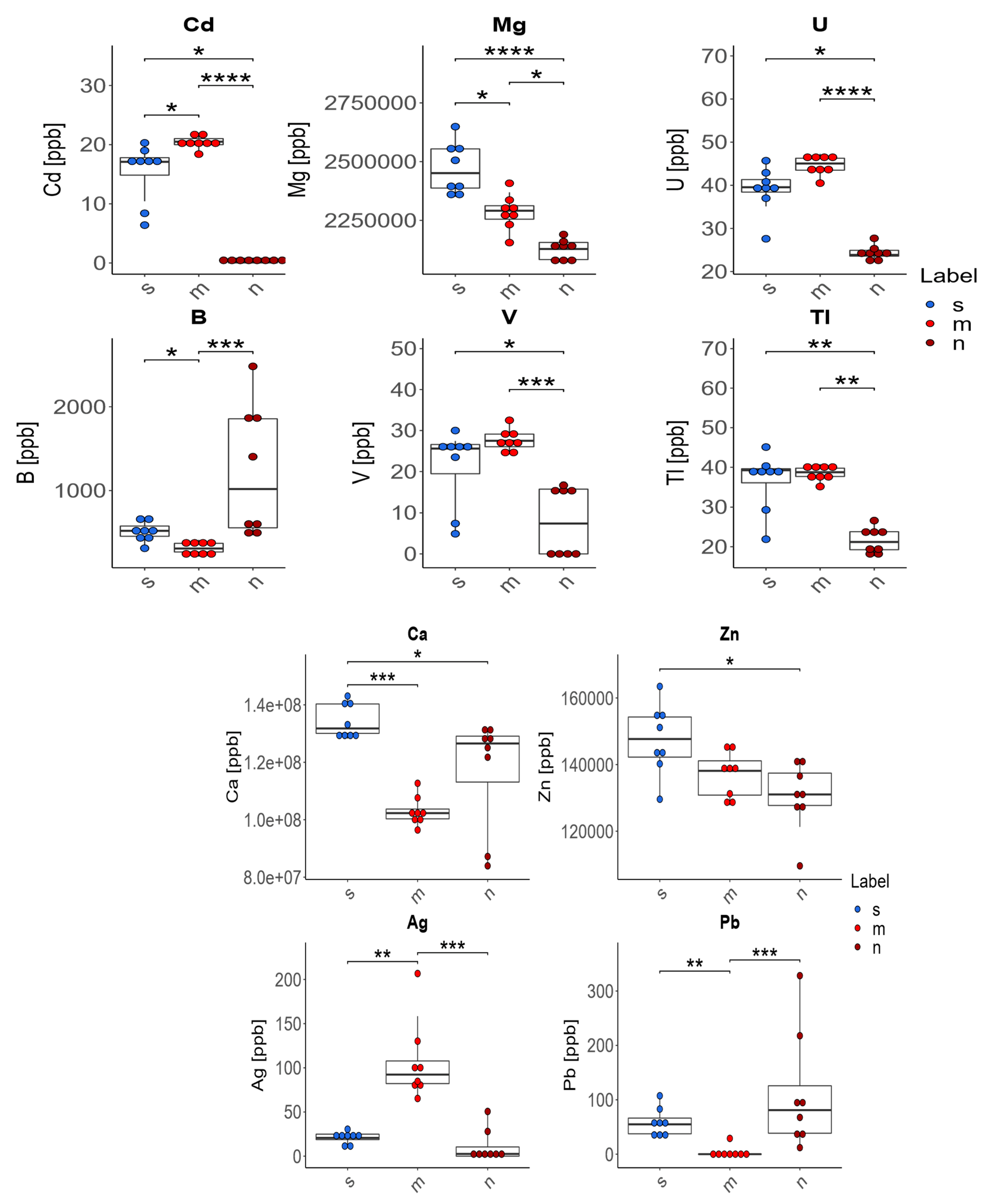
3. Results
- −
- In the case of four of the elements tested (Ag, Cd, Pb, B), supplementation of the diet of rats with zinc microparticles (m) caused significant changes in their concentration in comparison to both the control group on a standard diet (s) and the group receiving zinc nanoparticles (n). The content of Ag and Cd in the bones of these rats was higher and the content of Pb and B was lower than in rats receiving a diet with no supplement or with zinc nanoparticles.
- −
- The supplementation of the diet of rats with zinc microparticles (m) caused a significant decrease in the concentrations of Mg, B, and Ca and an increase for Cd and Ag only in comparison with the standard diet (s).
- −
- The supplementation of the diet of rats with zinc nanoparticles (n) caused significant changes in the distribution of elements in comparison to the standard diet (s)—a decrease for Ca, Mg, Zn, U, V, Cd, and Tl.
- −
- The supplementation of the diet of rats with zinc microparticles (m) caused a significant increase for V, Tl, and U only in comparison with the group receiving zinc nanoparticles (n).
4. Discussion
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J.; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25, iii124–iii137. [Google Scholar] [CrossRef] [PubMed]
- Handforth, C.; D’Oronzo, S.; Coleman, R.; Brown, J. Cancer Treatment and Bone Health. Calcif. Tissue Int. 2018, 102, 251–264. [Google Scholar] [CrossRef]
- Castañeda, S.; Casas, A.; González-Del-Alba, A.; Martínez-Díaz-Guerra, G.; Nogués, X.; Thies, C.O.; Suau, T.; Rodríguez-Lescure, Á. Bone loss induced by cancer treatments in breast and prostate cancer patients. Clin. Transl. Oncol. 2022, 24, 2090–2106. [Google Scholar] [CrossRef]
- Raju, G.N.; Sarita, P.; Kumar, M.R.; Murty, G.R.; Reddy, B.S.; Lakshminarayana, S.; Vijayan, V.; Lakshmi, P.R.; Gavarasana, S.; Reddy, S.B. Trace elemental correlation study in malignant and normal breast tissue by PIXE technique. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 247, 361–367. [Google Scholar] [CrossRef]
- Pasha, Q.; Malik, S.A.; Iqbal, J.; Shaheen, N.; Shah, M.H. Comparative Evaluation of Trace Metal Distribution and Correlation in Human Malignant and Benign Breast Tissues. Biol. Trace Elem. Res. 2008, 125, 30–40. [Google Scholar] [CrossRef]
- Kubala-Kukus, A.; Banaś, D.; Braziewicz, J.; Gózd, S.; Majewska, U.; Pajek, M. Analysis of elemental concentration censored distributions in breast malignant and breast benign neoplasm tissues. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 695–701. [Google Scholar] [CrossRef]
- Naidu, B.G.; Srikanth, S.; Raju, G.J.N.; Sarita, P. PIXE analysis of blood serum of breast cancer patients undergoing successive chemotherapy. J. Radioanal. Nucl. Chem. 2020, 323, 1307–1316. [Google Scholar] [CrossRef]
- Al-Ebraheem, A.; Farquharson, M.; Ryan, E. The evaluation of biologically important trace metals in liver, kidney and breast tissue. Appl. Radiat. Isot. 2009, 67, 470–474. [Google Scholar] [CrossRef]
- Park, K.H.; Park, B.; Yoon, D.S.; Kwon, S.-H.; Shin, D.M.; Lee, J.W.; Lee, H.G.; Shim, J.-H.; Park, J.H.; Lee, J.M. Zinc inhibits osteoclast differentiation by suppression of Ca2+-Calcineurin-NFATc1 signaling pathway. Cell Commun. Signal. 2013, 11, 1–12. [Google Scholar] [CrossRef]
- Hu, D.; Li, K.; Xie, Y.; Pan, H.; Zhao, J.; Huang, L.; Zheng, X. Different response of osteoblastic cells to Mg2+, Zn2+ and Sr2+ doped calcium silicate coatings. J. Mater. Sci. Mater. Med. 2016, 27, 56. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, Y.; Yoon, D.S.; Lee, K.-M.; Kim, D.; Lee, J.W. Zinc Promotes Osteoblast Differentiation in Human Mesenchymal Stem Cells Via Activation of the cAMP-PKA-CREB Signaling Pathway. Stem Cells Dev. 2018, 27, 1125–1135. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Alcantara, E.H.; Lomeda, R.-A.R.; Feldmann, J.; Nixon, G.F.; Beattie, J.H.; Kwun, I.-S. Zinc deprivation inhibits extracellular matrix calcification through decreased synthesis of matrix proteins in osteoblasts. Mol. Nutr. Food Res. 2011, 55, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.J.; Yamaguchi, M. Role of endogenous zinc in the enhancement of bone protein synthesis associated with bone growth of newborn rats. J. Bone Miner. Metab. 2001, 19, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Roshan, M.; Ebrahimi, M.; Ebrahimi, A. Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin. Cases Miner. Bone Metab. 2015, 12, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.; Meunier, N.; Andriollo-Sanchez, M.; Ciarapica, D.; Hininger-Favier, I.; Polito, A.; O’Connor, J.M.; Coudray, C.; Cashman, K.D. The relationship between the zinc nutritive status and biochemical markers of bone turnover in older European adults: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59, S73–S78. [Google Scholar] [CrossRef]
- Horiuchi, S.; Hiasa, M.; Yasue, A.; Sekine, K.; Hamada, K.; Asaoka, K.; Tanaka, E. Fabrications of zinc-releasing biocement combining zinc calcium phosphate to calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2014, 29, 151–160. [Google Scholar] [CrossRef]
- Hinton, R.; Jing, Y.; Jing, J.; Feng, J. Roles of Chondrocytes in Endochondral Bone Formation and Fracture Repair. J. Dent. Res. 2017, 96, 23–30. [Google Scholar] [CrossRef]
- Hie, M.; Iitsuka, N.; Otsuka, T.; Nakanishi, A.; Tsukamoto, I. Zinc deficiency decreases osteoblasts and osteoclasts associated with the reduced expression of Runx2 and RANK. Bone 2011, 49, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-E.; Li, Z.-H.; Zheng, W.; Zhao, Y.-F.; Jin, Y.-F.; Tang, Z.-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. Part A 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef]
- Nadeem, J.; Dirk, L. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotech. 2022, 20, 262. [Google Scholar]
- Roy, R.; Kumar, S.; Tripathi, A.; Das, M.; Dwivedi, P.D. Interactive threats of nanoparticles to the biological system. Immunol Lett. 2014, 158, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wingett, D.; Engelhard, M.; Feris, K.; Reddy, K.M.; Turner, P.; Layne, J.; Hanley, C.; Bell, J.; Tenne, D.; et al. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J. Mater. Sci. Mater. Med. 2009, 20, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Hong, S.; Mecke, A.; Baker, J.R.; Orr, B.G.; Holl, M.M.B. Nanoparticle Interaction with Biological Membranes: Does Nanotechnology Present a Janus Face? Acc. Chem. Res. 2007, 40, 335–342. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.-S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surfaces B Biointerfaces 2014, 117, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.P.; Valentine, R.J.; Moulton, C.J.; Johnson, A.J.W.; Evans, E.M.; Layman, D.K. Breast tumors induced by N-methyl-N-nitrosourea are damaging to bone strength, structure, and mineralization in the absence of metastasis in rats. J. Bone Miner. Res. 2011, 26, 769–776. [Google Scholar] [CrossRef]
- Bobrowska-Korczak, B.; Gątarek, P.; Skrajnowska, D.; Bielecki, W.; Wyrebiak, R.; Kovalczuk, T.; Wrzesień, R.; Kałużna-Czaplińska, J. Effect of Zinc Supplementation on the Serum Metabolites Profile at the Early Stage of Breast Cancer in Rats. Nutrients 2020, 12, 3457. [Google Scholar] [CrossRef] [PubMed]
- Hubert, M.; Vandervieren, E. An adjusted boxplot for skewed distributions. Comput. Stat. Data Anal. 2008, 52, 5186–5201. [Google Scholar] [CrossRef]
- Bąkowski, M.; Kiczorowska, B.; Samolińska, W.; Klebaniuk, R.; Lipiec, A. Silver and Zinc Nanoparticles in Animal Nutrition—A Review. Ann. Anim. Sci. 2018, 18, 879–898. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Amara, S.; Ben Slama, I.; Mrad, I.; Rihane, N.; Khemissi, W.; El Mir, L.; Ben Rhouma, K.; Abdelmelek, H.; Sakly, M. Effects of zinc oxide nanoparticles and/or zinc chloride on biochemical parameters and mineral levels in rat liver and kidney. Hum. Exp. Toxicol. 2014, 33, 1150–1157. [Google Scholar] [CrossRef]
- Singh, N.; Das, M.K.; Gautam, R.; Ramteke, A.; Rajamani, P. Assessment of intermittent exposure of zinc oxide nanoparticle (ZNP)–mediated toxicity and biochemical alterations in the splenocytes of male Wistar rat. Environ. Sci. Pollut. Res. 2019, 26, 33642–33653. [Google Scholar] [CrossRef]
- Zalewski, P.D.; Truong-Tran, A.Q.; Grosser, D.; Jayaram, L.; Murgia, C.; Ruffin, R.E. Zinc metabolism in airway epithelium and airway inflammation: Basic mechanisms and clinical targets. A review. Pharmacol. Ther. 2005, 105, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Pie, J.-E.; Kim, Y.-R.; Lee, H.R.; Son, S.W.; Kim, M.-K. Effects of zinc oxide nanoparticles on gene expression profile in human keratinocytes. Mol. Cell. Toxicol. 2012, 8, 113–118. [Google Scholar] [CrossRef]
- Muqbil, I.; Banu, N. Enhancement of pro-oxidant effect of 7,12-dimethylbenz(a)anthracene (DMBA) in rats by pre-exposure to restraint stress. Cancer Lett. 2006, 240, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Singh, N.P. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006, 231, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Boone, C.W.; Steele, V.E.; Crowell, J.A.; Lubet, R.; Doody, L.A.; Greenwald, P. Development of breast cancer chemopreventive drugs. J. Cell Biochem. 1993, 17, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R. Experimental basis for the prevention of breast cancer. Eur. J. Cancer 2000, 36, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Grand, L.C.; Brillantes, F.P. Mammary Cancer Induced by a Single Feeding of Polynuclear Hydrocarbons, and its Suppression. Nature 1961, 189, 204–207. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Sosnoski, D.M.; Mastro, A.M. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010, 12, 1–11. [Google Scholar] [CrossRef]
- Barros, A.C.S.D.; Muranaka, E.N.K.; Jo Mori, L.; Pelizon, C.H.T.; Iriya, K.; Giocondo, G.; Pinotti, J.A. Induction of experimental mammary carcinogenesis in rats with 7,12-dimethylbenz(a)anthracene. Rev. Hosp. Clínicas 2004, 59, 257–261. [Google Scholar] [CrossRef]
- Bobrowska-Korczak, B.; Domanska, K.; Skrajnowska, D.; Wrzesien, R.; Giebultowicz, J.; Bielecki, W.; Wyrebiak, R.; Piotrowska, U.; Sobczak, M.; Kałużna-Czaplińska, J. Nanosized zinc, epigenetic changes and its relationship with DMBA induced breast cancer in rats. Rev. Anal. Chem. 2020, 39, 200–208. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.-F.; Li, D.-Y.; Chen, Y.-C.; Zhao, L.-J.; Liu, X.-G.; Guo, Y.-F.; Shen, J.; Lin, X.; Deng, J.; et al. The good, the bad, and the ugly of calcium supplementation: A review of calcium intake on human health. Clin. Interv. Aging 2018, 13, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Major, P.; Lortholary, A.; Hon, J.; Abdi, E.; Mills, G.; Menssen, H.D.; Yunus, F.; Bell, R.; Body, J.; Quebe-Fehling, E.; et al. Zoledronic Acid Is Superior to Pamidronate in the Treatment of Hypercalcemia of Malignancy: A Pooled Analysis of Two Randomized, Controlled Clinical Trials. J. Clin. Oncol. 2001, 19, 558–567. [Google Scholar] [CrossRef]
- Stewart, A.F. Hypercalcemia Associated with Cancer. N. Engl. J. Med. 2005, 352, 373–379. [Google Scholar] [CrossRef]
- Taverna, S.; Giusti, I.; D’Ascenzo, S.; Pizzorno, L.; Dolo, V. Breast Cancer Derived Extracellular Vesicles in Bone Metastasis Induction and Their Clinical Implications as Biomarkers. Int. J. Mol. Sci. 2020, 21, 3573. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Park, S.H.; Eber, M.R.; Peters, C.M.; Shiozawa, Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016, 23, 825–832. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef]
- Hameister, R.; Lohmann, C.H.; Dheen, S.T.; Singh, G.; Kaur, C. The effect of TNF-α on osteoblasts in metal wear-induced periprosthetic bone loss. Bone Jt. Res. 2020, 9, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L. The Role of Calcium in Inflammation-Associated Bone Resorption. Biomolecules 2018, 8, 69. [Google Scholar] [CrossRef]
- Canaff, L.; Zhou, X.; Hendy, G.N. The Proinflammatory Cytokine, Interleukin-6, Up-regulates Calcium-sensing Receptor Gene Transcription via Stat1/3 and Sp1/3. J. Biol. Chem. 2008, 283, 13586–13600. [Google Scholar] [CrossRef]
- Zaichick, V.; Zaichick, S. The Ca, Cl, Mg, Na, and P Mass Fractions in Human Bone Affected by Ewing’s Sarcoma. Biol. Trace Elem. Res. 2014, 159, 32–38. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Osteopath. Med. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium- and strontium-co-substituted hydroxyapatite: The effects of doped-ions on the structure and chemico-physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Zhu, X.; Manson, J.A.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Zofkova, I.; Davis, M.; Blahos, J. Trace Elements Have Beneficial, as Well as Detrimental Effects on Bone Homeostasis. Physiol. Res. 2017, 66, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and Hormonal Effects of Magnesium Deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. J. Trace Elem. Med. Biol. 2015, 32, 86–106. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Pivotal role of boron supplementation on bone health: A narrative review. J. Trace Elem. Med. Biol. 2020, 62, 126577. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Biţă, A.; Bejenaru, L.E.; Bejenaru, C.; Croitoru, O.; Rău, G.; Rogoveanu, O.-C.; Florescu, D.N.; Neamţu, J.; Scorei, I.D.; et al. Calcium Fructoborate for Bone and Cardiovascular Health. Biol. Trace Elem. Res. 2016, 172, 277–281. [Google Scholar] [CrossRef]
- Pizzorno, L. Nothing Boring About Boron. Integr. Med. 2015, 14, 35–48. [Google Scholar]
- JamaliMoghadamSiahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Moghadam, E.S.; Abbasian, L.; Manshadi, S.A.D.; Salehi, M.; et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef]
- Uluisik, I.; Karakaya, H.C.; Koc, A. The importance of boron in biological systems. J. Trace Elem. Med. Biol. 2018, 45, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Meacham, S.L. Growing Evidence for Human Health Benefits of Boron. J. Evid. -Based Complement. Altern. Med. 2011, 16, 169–180. [Google Scholar] [CrossRef]
- Engström, A.; Michaëlsson, K.; Vahter, M.; Julin, B.; Wolk, A.; Åkesson, A. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 2012, 50, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Youness, E.R.; Mohammed, N.A.; Morsy, F. Cadmium impact and osteoporosis: Mechanism of action. Toxicol. Mech. Methods 2012, 22, 560–567. [Google Scholar] [CrossRef]
- Al-Ghafari, A.; Elmorsy, E.; Fikry, E.; Alrowaili, M.; Carter, W.G. The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress. PLoS ONE 2019, 14, e0225341. [Google Scholar] [CrossRef]
- Das, S.C.; Al-Naemi, H.A. Cadmium Toxicity: Oxidative Stress, Inflammation and Tissue Injury. Occup. Dis. Environ. Med. 2019, 7, 144–163. [Google Scholar] [CrossRef]
- Yang, P.; Yang, X.; Sun, L.; Han, X.; Xu, L.; Gu, W.; Zhang, M. Effects of cadmium on oxidative stress and cell apoptosis in Drosophila melanogaster larvae. Sci. Rep. 2022, 12, 4762. [Google Scholar] [CrossRef] [PubMed]
- Sughis, M.; Penders, J.; Haufroid, V.; Nemery, B.; Nawrot, T.S. Bone resorption and environmental exposure to cadmium in children: A cross—Sectional study. Environ. Health 2011, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; De Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms ofCandida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef] [PubMed]

| Groups | s | m | n |
|---|---|---|---|
| Added Zn | 0 | 4.6 mg/mL | 4.6 mg/mL |
| Total Zn | 76.9 mg/kg diet * | 230.7 mg/kg diet * | 230.7 mg/kg diet * |
| Groups | s | m | n | p Value |
|---|---|---|---|---|
| Body weight (g) | 231.0 ± 13.8 | 230.1 ± 17.2 | 230.4 ± 10.2 | n.s. |
| Mass of femur (g) | 0.904 ± 0.052 a | 0.964 ± 0.05 b | 0.951 ± 0.028 b | 0.05 |
| Groups | s | n | m | |||
|---|---|---|---|---|---|---|
| Elements (n = 8) | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD |
| As (ppb) | 43.2 | 45.9 ± 10.02 | 34.4 | 38.2 ± 15.04 | 53.55 | 53.96 ± 8.892 |
| Ba (ppb) | 2999 | 2980 ± 252 | 2826 | 2841 ± 116 | 2889 | 2935 ± 24 |
| Cr (ppb) | 122.8 | 123 ± 37 | 99 | 102 ± 28 | 105 | 108 ± 26 |
| Cu (ppb) | 569 | 535 ± 115 | 666 | 747 ± 265 | 506 | 532 ± 104 |
| Fe (ppb) | 63,791 | 66,503 ± 12,930 | 63,896 | 65,433 ± 9266 | 69,727 | 69,766 ± 16,199 |
| K (ppm) | 1226 | 1241 ± 154 | 1354 | 1339 ± 79 | 1391 | 1348 ± 168 |
| Mn (ppb) | 315.8 | 311 ± 37 | 322 | 319 ± 46 | 294 | 287 ± 6 |
| Na (ppm) | 3947 | 3874 ± 352 | 3759 | 3746 ± 70 | 3766 | 3780 ± 141 |
| Ni (ppm) | 47.2 | 37.7 ± 24.0 | 22.5 | 27.2 ± 26.2 | 59.7 | 47.0 ± 21.5 |
| Rb (ppb) | 1483 | 1491 ± 155 | 1508 | 1507 ± 101 | 1428 | 1494 ± 254 |
| Se (ppb) | 112 | 107 ± 16 | 94 | 95 ± 12 | 112 | 108 ± 15 |
| Sr (ppb) | 39,026 | 39,402 ± 2926 | 39,744 | 40,169 ± 1839 | 38,989 | 38,475 ± 2091 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrajnowska, D.; Idkowiak, J.; Szterk, A.; Ofiara, K.; Augustyniak, K.; Bobrowska-Korczak, B. Effect of Nano- and Microzinc Supplementation on the Mineral Composition of Bones of Rats with Induced Mammary Gland Cancer. Foods 2023, 12, 1348. https://doi.org/10.3390/foods12061348
Skrajnowska D, Idkowiak J, Szterk A, Ofiara K, Augustyniak K, Bobrowska-Korczak B. Effect of Nano- and Microzinc Supplementation on the Mineral Composition of Bones of Rats with Induced Mammary Gland Cancer. Foods. 2023; 12(6):1348. https://doi.org/10.3390/foods12061348
Chicago/Turabian StyleSkrajnowska, Dorota, Jakub Idkowiak, Arkadiusz Szterk, Karol Ofiara, Kinga Augustyniak, and Barbara Bobrowska-Korczak. 2023. "Effect of Nano- and Microzinc Supplementation on the Mineral Composition of Bones of Rats with Induced Mammary Gland Cancer" Foods 12, no. 6: 1348. https://doi.org/10.3390/foods12061348
APA StyleSkrajnowska, D., Idkowiak, J., Szterk, A., Ofiara, K., Augustyniak, K., & Bobrowska-Korczak, B. (2023). Effect of Nano- and Microzinc Supplementation on the Mineral Composition of Bones of Rats with Induced Mammary Gland Cancer. Foods, 12(6), 1348. https://doi.org/10.3390/foods12061348






