Modeling the Growth of Six Listeria monocytogenes Strains in Smoked Salmon Pâté
Abstract
1. Introduction
2. Materials and Methods
2.1. Smoked Salmon Pâté
2.2. Bacterial Strains and Culture Conditions
2.3. Challenge Tests
2.4. Microbiological Analyses
2.5. Physicochemical Analyses
2.6. Primary Model Fitting
2.7. Secondary Model Fitting
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Šilovs, M. Fish processing by-products exploitation and innovative fish-based food production. Res. Rural Dev. 2018, 2, 210–215. [Google Scholar] [CrossRef]
- Dutra, M.P.; Palhares, P.C.; Silva, J.R.O.; Ezequiel, I.P.; Ramos, A.L.S.; Perez, J.R.O.; Ramos, E.M. Technological and quality characteristics of cooked ham-type pâté elaborated with sheep meat. Small Rumin. Res. 2013, 115, 56–61. [Google Scholar] [CrossRef]
- Cruxen, C.E.D.S.; Thiel, P.R.; Souza, D.M.; da Costa, R.J.; Filoda, P.F.; Chaves, F.C.; Fiorentini, Â.M. Developing functional fish pâtés from Oligosarcus robustus and Loricariichythys anus with pre- and pro-biotic potentials. Food Biosci. 2021, 44, 101449. [Google Scholar] [CrossRef]
- Aquerreta, Y.; Astiasarán, I.; Mohino, A.; Bello, J. Composition of pâtés elaborated with mackerel flesh (Scomber scombrus) and tuna liver (Thunnus thynnus): Comparison with commercial fish pâtés. Food Chem. 2002, 77, 147–153. [Google Scholar] [CrossRef]
- Echarte, M.; Conchillo, A.; Ansorena, D.; Astiasarán, I. Evaluation of the nutritional aspects and cholesterol oxidation products of pork liver and fish patés. Food Chem. 2004, 86, 47–53. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e06406. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready-to-eat foods in the EU, 2010–2011 Part A: Listeria monocytogenes prevalence estimates. EFSA J. 2013, 11, 3241. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Escámez, P.S.F.; Girones, R.; Herman, L.; Koutsoumanis, K.; et al. Scientific Opinion on the Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.; Gomez, I.; Zumalacarregui, J. Prevalence and contamination levels of Listeria monocytogenes in smoked fish and pâté sold in Spain. J. Food Prot. 2001, 64, 2075–2077. [Google Scholar] [CrossRef]
- Althaus, D.; Jermini, M.; Giannini, P.; Martinetti, G.; Reinholz, D.; Nüesch-Inderbinen, M.; Lehner, A.; Stephan, R. Local outbreak of Listeria monocytogenes serotype 4b sequence type 6 due to contaminated meat pâté. Foodborne Pathog. Dis. 2017, 14, 219–222. [Google Scholar] [CrossRef]
- Cabal, A.; Allerberger, F.; Huhulescu, S.; Kornschober, C.; Springer, B.; Schlagenhaufen, C.; Wassermann-Neuhold, M.; Fötschl, H.; Pless, P.; Krause, R.; et al. Listeriosis outbreak likely due to contaminated liver pâté consumed in a tavern, Austria, December 2018. Eurosurveillance 2019, 24, 1900274. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Authority of Ireland. Recall of Union Hall smoked salmon pate due to the presence of Listeria monocytogenes. Food alert 2020.35. Available online: https://www.fsai.ie/news_centre/food_alerts/union_hall_smoked_salmon_pate.html (accessed on 16 February 2023).
- Government of South Australia. Listeria Detected in Smoked Salmon Paté. Available online: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/about+us/news+and+media/all+media+releases/listeria+detected+in+smoked+salmon+pate (accessed on 16 February 2023).
- Stavropoulou, E.; Bezirtzoglou, E. Predictive modeling of microbial behavior in food. Foods 2019, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, D.; Bolívar, A.; Pérez-Rodríguez, F.; Baka, M.; Skåra, T.; Van Impe, J.F. Isolating the effect of fat content on Listeria monocytogenes growth dynamics in fish-based emulsion and gelled emulsion systems. Food Control 2019, 108, 106874. [Google Scholar] [CrossRef]
- Verheyen, D.; Bolívar, A.; Pérez-Rodríguez, F.; Baka, M.; Skåra, T.; Van Impe, J.F. Effect of food microstructure on growth dynamics of Listeria monocytogenes in fish-based model systems. Int. J. Food Microbiol. 2018, 283, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Aryani, D.C.; den Besten, H.M.W.; Hazeleger, W.C.; Zwietering, M.H. Quantifying strain variability in modeling growth of Listeria monocytogenes. Int. J. Food Microbiol. 2015, 208, 19–29. [Google Scholar] [CrossRef]
- Begot, C.; Lebert, I.; Lebert, A. Variability of the response of 66 Listeria monocytogenes and Listeria innocua strains to different growth conditions. Food Microbiol. 1997, 14, 403–412. [Google Scholar] [CrossRef]
- Bovill, R.; Bew, J.; Cook, N.; D’Agostino, M.; Wilkinson, N.; Baranyi, J. Predictions of growth for Listeria monocytogenes and Salmonella during fluctuating temperature. Int. J. Food Microbiol. 2000, 59, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; McKellar, R.C.; Ross, W.H. Modelling the effects of various parameters on the growth of Listeria monocytogenes on liver pâté. Food Microbiol. 1995, 12, 447–453. [Google Scholar] [CrossRef]
- Hudson, J.A.; Mott, S.J. Growth of Listeria monocytogenes, Aeromonas hydrophila and Yersinia enterocolitica in pâté and a comparison with predictive models. Int. J. Food Microbiol. 1993, 20, 1–11. [Google Scholar] [CrossRef]
- Guillier, L.; Lardeux, A.-L.; Michelon, D.; Ng, P. Development of a set of Listeria monocytogenes Strains for Conducting Challenge Tests; EURL Lm: Maisons-Alfort, France, 2013; pp. 1–23. Available online: https://sitesv2.anses.fr/en/system/files/private/LIS-Cr-201317R.pdf (accessed on 20 February 2023).
- EURL Lm. EURL Lm Technical Guidance Document on Challenge Tests and Durability Studies for Assessing Shelf-Life of Ready-to-Eat Foods Related to Listeria monocytogenes; EURL Lm: Maisons-Alfort, France, 2021; pp. 1–60. Available online: https://food.ec.europa.eu/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf (accessed on 20 February 2023).
- Bolívar, A.; Tarlak, F.; Costa, J.C.C.P.; Cejudo-Gómez, M.; Bover-Cid, S.; Zurera, G.; Pérez-Rodríguez, F. A new expanded modelling approach for investigating the bioprotective capacity of Latilactobacillus sakei CTC494 against Listeria monocytogenes in ready-to-eat fish products. Food Res. Int. 2021, 147, 110545. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef]
- Tarlak, F.; Ozdemir, M.; Melikoglu, M. Predictive modelling for the growth kinetics of Pseudomonas spp. on button mushroom (Agaricus bisporus) under isothermal and non-isothermal conditions. Food Res. Int. 2020, 130, 108912. [Google Scholar] [CrossRef]
- Farber, J.M.; Daley, E. Presence and growth of Listeria monocytogenes in naturally-contaminated meats. Int. J. Food Microbiol. 1994, 22, 33–42. [Google Scholar] [CrossRef]
- Mancera-Rodriguez, L.; Muñoz-Ramirez, A.P.; Lopez-Vargas, J.H.; Simal-Gandara, J. Development, characterization and stability of a white cachama pâté-type product (Piaractus brachypomus). Food Chem. 2022, 375, 131660. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Mihnea, M.; Båth, K.; Cunha, S.C.; Fereira, R.; Fernandes, J.O.; Gonçalves, A.; Nunes, M.L.; Oliveira, H. New formulation for producing salmon pâté with reduced sodium content. Food Chem. Toxicol. 2020, 143, 111546. [Google Scholar] [CrossRef] [PubMed]
- De Jesús, A.J.; Whiting, R.C. Thermal inactivation, growth, and survival studies of Listeria monocytogenes strains belonging to three distinct genotypic lineages. J. Food Prot. 2003, 66, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Lianou, A.; Stopforth, J.D.; Yoon, Y.; Wiedmann, M.; Sofos, J.N. Growth and stress resistance variation in culture broth among Listeria monocytogenes strains of various serotypes and origins. J. Food Prot. 2006, 69, 2640–2647. [Google Scholar] [CrossRef]
- Pal, A.; Labuza, T.P.; Diez-Gonzalez, F. Evaluating the growth of Listeria monocytogenes in refrigerated ready-to-eat frankfurters: Influence of strain, temperature, packaging, lactate and diacetate, and background microflora. J. Food Prot. 2008, 71, 1806–1816. [Google Scholar] [CrossRef]
- Spanu, C.; Scarano, C.; Ibba, M.; Pala, C.; Spanu, V.; De Santis, E.P.L. Microbiological challenge testing for Listeria monocytogenes in ready-to-eat food: A practical approach. Ital. J. Food Saf. 2014, 3, 231–237. [Google Scholar] [CrossRef]
- Hunt, K.; Blanc, M.; Álvarez-Ordóñez, A.; Jordan, K. Challenge studies to determine the ability of foods to support the growth of Listeria monocytogenes. Pathogens 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Hayrapetyan, H.; Hazeleger, W.C.; Beumer, R.R. Inhibition of Listeria monocytogenes by pomegranate (Punica granatum) peel extract in meat paté at different temperatures. Food Control 2012, 23, 66–72. [Google Scholar] [CrossRef]
- Bolívar, A.; Costa, J.C.C.P.; Posada-Izquierdo, G.D.; Valero, A.; Zurera, G.; Pérez-Rodríguez, F. Modelling the growth of Listeria monocytogenes in Mediterranean fish species from aquaculture production. Int. J. Food Microbiol. 2018, 270, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.C.P.; Bover-Cid, S.; Bolívar, A.; Zurera, G.; Pérez-Rodríguez, F. Modelling the interaction of the sakacin-producing Lactobacillus sakei CTC494 and Listeria monocytogenes in filleted gilthead sea bream (Sparus aurata) under modified atmosphere packaging at isothermal and non-isothermal conditions. Int. J. Food Microbiol. 2019, 297, 72–84. [Google Scholar] [CrossRef]
- Delignette-Muller, M.L.; Cornu, M.; Pouillot, R.; Denis, J.B. Use of Bayesian modelling in risk assessment: Application to growth of Listeria monocytogenes and food flora in cold-smoked salmon. Int. J. Food Microbiol. 2006, 106, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Mejlholm, O.; Dalgaard, P. Modeling and predicting the growth boundary of Listeria monocytogenes in lightly preserved seafood. J. Food Prot. 2007, 70, 70–84. [Google Scholar] [CrossRef]
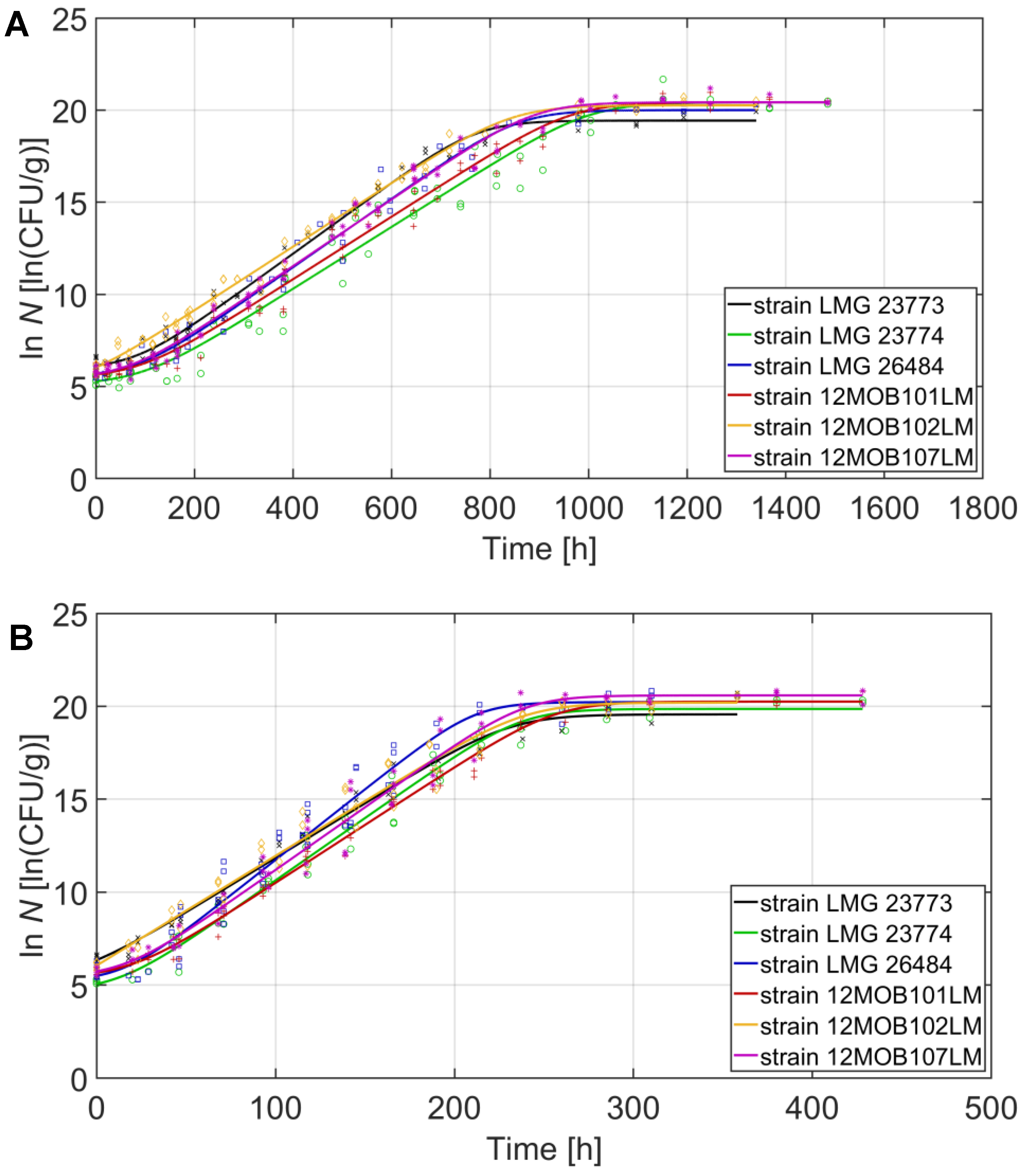
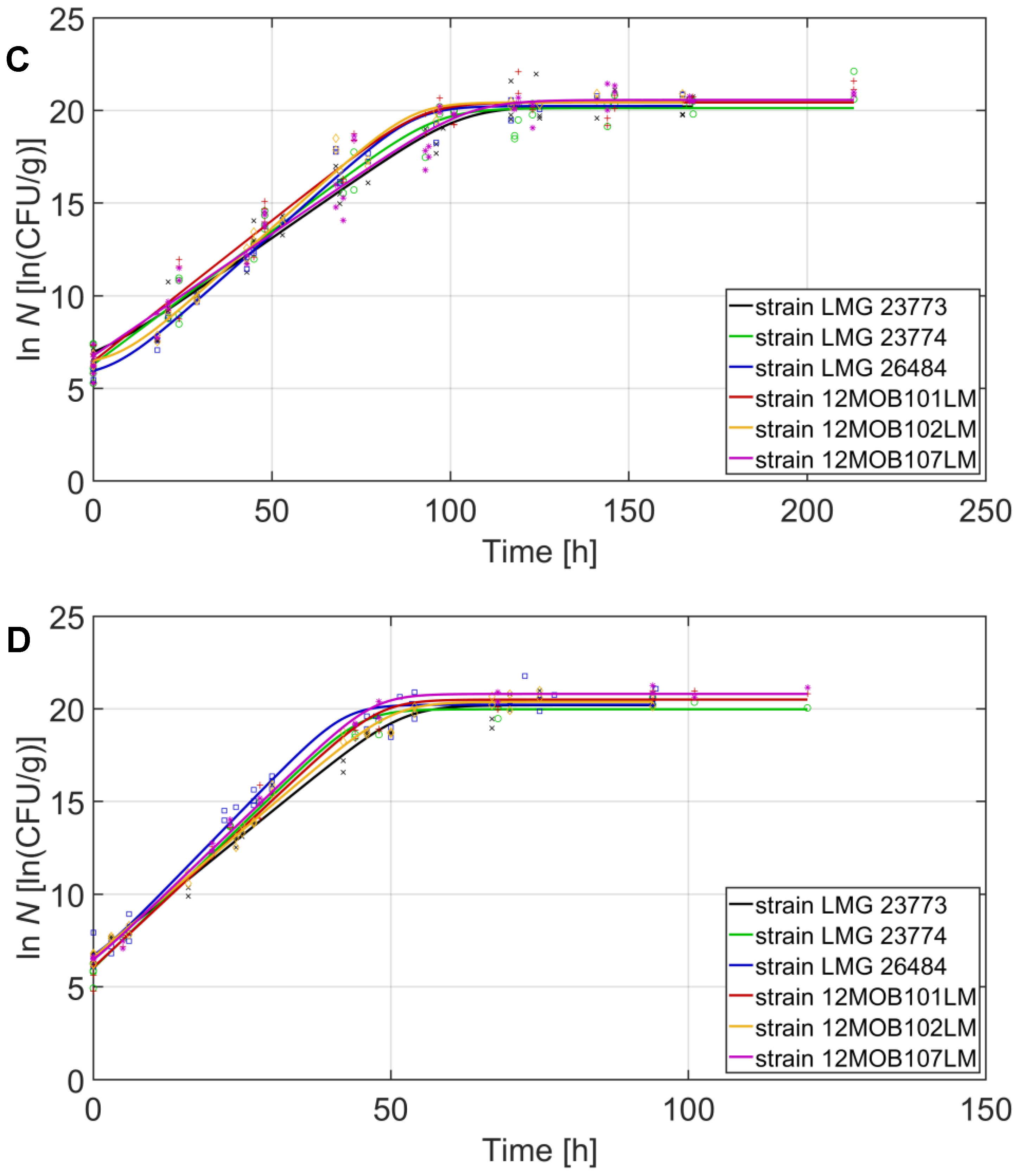

| Strain | 2 °C | 8 °C | 14 °C | 20 °C | ||||
|---|---|---|---|---|---|---|---|---|
| λ (h) | µmax (1/h) | λ (h) | µmax (1/h) | λ (h) | µmax (1/h) | λ (h) | µmax (1/h) | |
| LMG 23773 | 86.52 ± 14.68 A | 0.0194 ± 0.0005 A | 6.60 ± 7.17 A | 0.0589 ± 0.0022 C | 4.15 ± 4.34 A | 0.1339 ± 0.0083 B | NE | 0.2607 ± 0.0121 F |
| LMG 23774 | 101.91 ± 29.39 A | 0.0168 ± 0.0006 C | 16.99 ± 7.03 A | 0.0672 ± 0.0023 B | NE | 0.1437 ± 0.0099 B | NE | 0.3098 ± 0.0178 B |
| LMG 26484 | 90.58 ± 27.32 A | 0.0186 ± 0.0008 AB | 18.05 ± 7.36 A | 0.0761 ± 0.0035 A | 7.01 ± 3.07 A | 0.1714 ± 0.0083 A | 1.31 ± 2.39 A | 0.3300 ± 0.0239 A |
| 12MOB101LM | 96.07 ± 23.07 A | 0.0170 ± 0.0005 C | 22.62 ± 5.43 A | 0.0625 ± 0.0016 BC | NE | 0.1519 ± 0.0111 AB | NE | 0.3002 ± 0.0196 D |
| 12MOB102LM | 21.41 ± 15.47 B | 0.0173 ± 0.0004 ABC | NE 1 | 0.0591 ± 0.0028 C | 8.34 ± 2.83 A | 0.1715 ± 0.0078 A | NE | 0.2733 ± 0.0104 E |
| 12MOB107LM | 77.16 ± 17.12 AB | 0.0181 ± 0.0004 ABC | 19.20 ± 9.34 A | 0.0675 ± 0.0031 B | NE | 0.1316 ± 0.0081 B | 0.66 ± 1.30 A | 0.3080 ± 0.0123 C |
| Strain | b | Tmin (°C) | R2 |
|---|---|---|---|
| LMG 23773 | 0.021 ± 0.001 | −4.25 ± 0.73 | 0.995 |
| LMG 23774 | 0.023 ± 0.001 | −3.19 ± 1.00 | 0.992 |
| LMG 26484 | 0.024 ± 0.001 | −3.47 ± 0.41 | 0.999 |
| 12MOB101LM | 0.023 ± 0.001 | −3.20 ± 0.69 | 0.996 |
| 12MOB102LM | 0.022 ± 0.001 | −3.63 ± 1.03 | 0.992 |
| 12MOB107LM | 0.023 ± 0.002 | −3.43 ± 1.54 | 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolívar, A.; Garrote Achou, C.; Tarlak, F.; Cantalejo, M.J.; Costa, J.C.C.P.; Pérez-Rodríguez, F. Modeling the Growth of Six Listeria monocytogenes Strains in Smoked Salmon Pâté. Foods 2023, 12, 1123. https://doi.org/10.3390/foods12061123
Bolívar A, Garrote Achou C, Tarlak F, Cantalejo MJ, Costa JCCP, Pérez-Rodríguez F. Modeling the Growth of Six Listeria monocytogenes Strains in Smoked Salmon Pâté. Foods. 2023; 12(6):1123. https://doi.org/10.3390/foods12061123
Chicago/Turabian StyleBolívar, Araceli, Chajira Garrote Achou, Fatih Tarlak, María Jesús Cantalejo, Jean Carlos Correia Peres Costa, and Fernando Pérez-Rodríguez. 2023. "Modeling the Growth of Six Listeria monocytogenes Strains in Smoked Salmon Pâté" Foods 12, no. 6: 1123. https://doi.org/10.3390/foods12061123
APA StyleBolívar, A., Garrote Achou, C., Tarlak, F., Cantalejo, M. J., Costa, J. C. C. P., & Pérez-Rodríguez, F. (2023). Modeling the Growth of Six Listeria monocytogenes Strains in Smoked Salmon Pâté. Foods, 12(6), 1123. https://doi.org/10.3390/foods12061123








