Effects of Hevea brasiliensis Intercropping on the Volatiles of Pandanus amaryllifolius Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
Growing Environment
Experiment Design
2.2.2. Determination of Plant Samples
Sample Collection and Pretreatment
Qualitative Analysis
Quantitative Analysis
2.2.3. Determination of Soil Samples
Sample Collection
Sample Analysis
2.2.4. Determination of Ecosystem Environment
2.2.5. Statistical Analysis
3. Results and Discussion
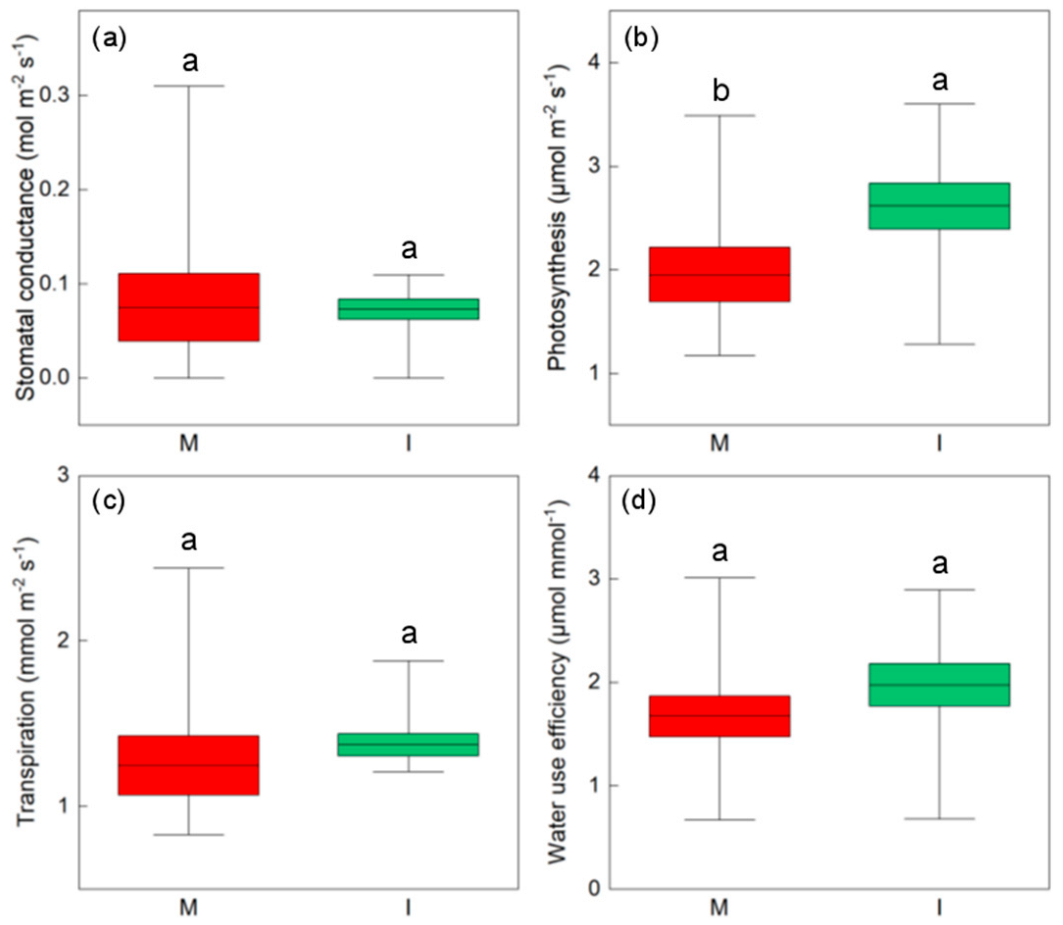
3.1. Effects of Intercropping Pattern on Soil Physico-Chemical Properties and Ecosystem Micro-Environment
3.2. Effects of Intercropping Pattern on Pandanus amaryllifolius Volatile Substances
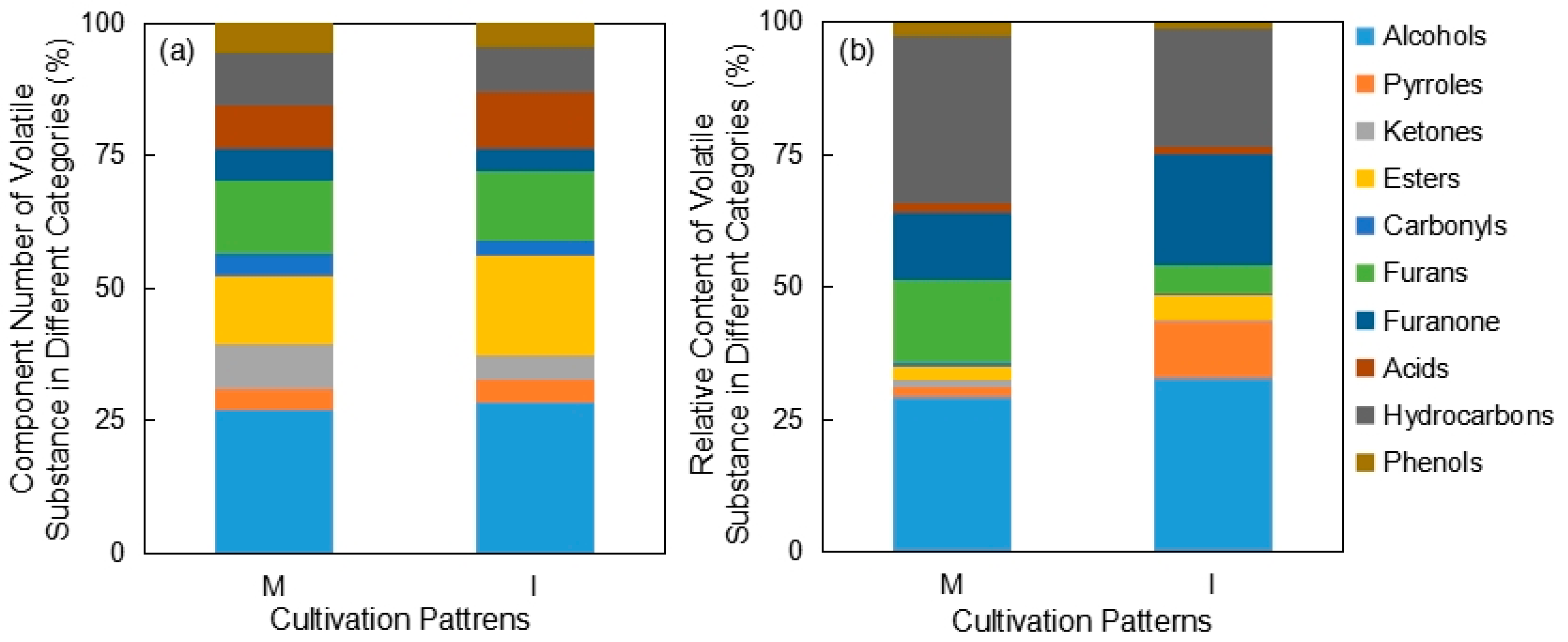
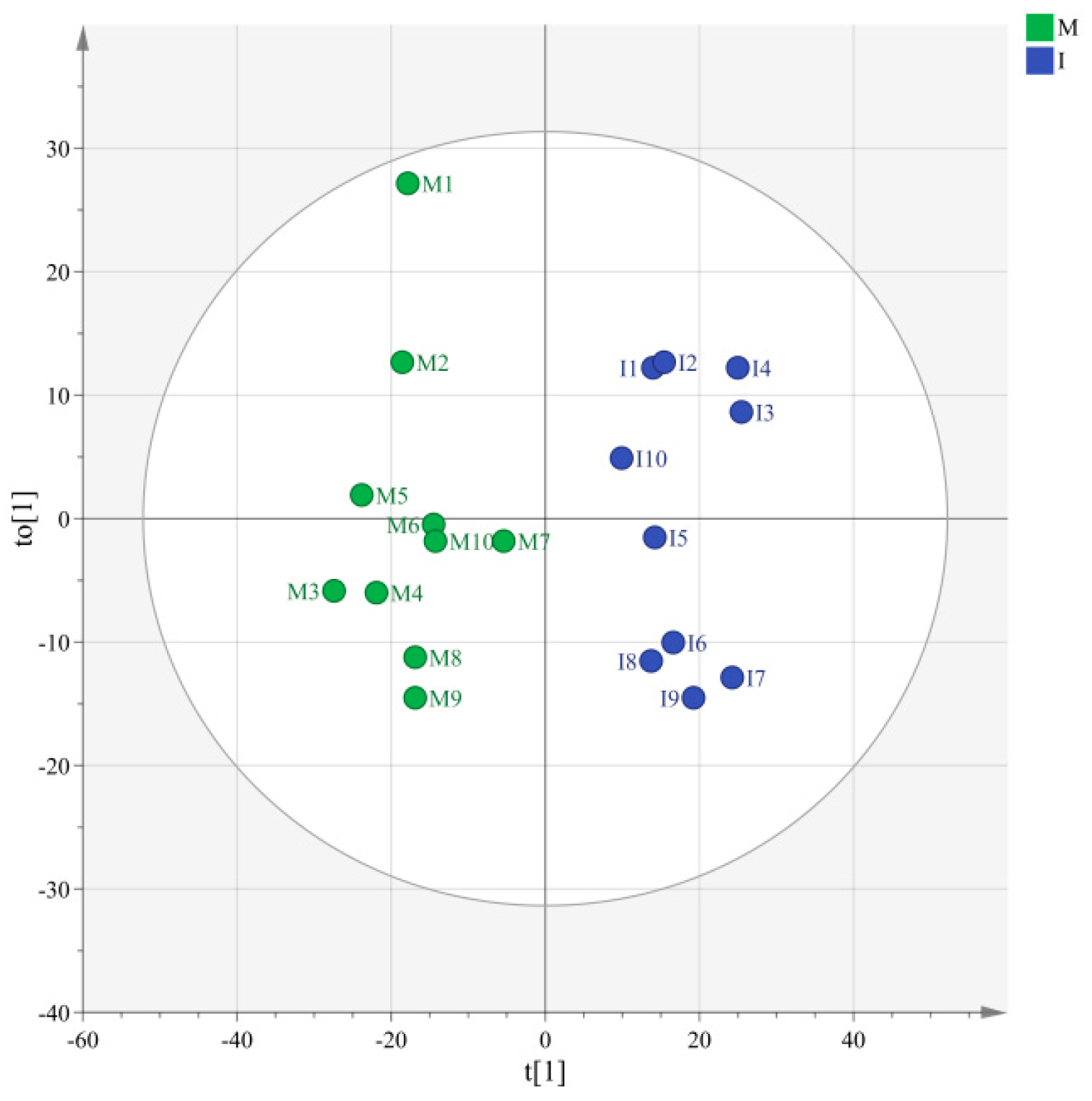
3.2.1. Comparison of Pandanus amaryllifolius Volatile Substances under Different Cultivation Patterns
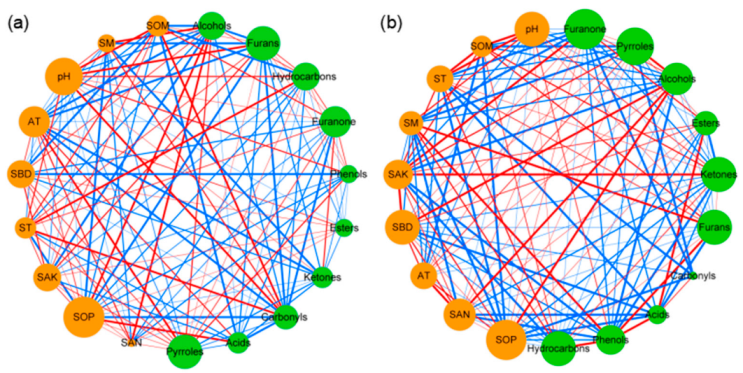
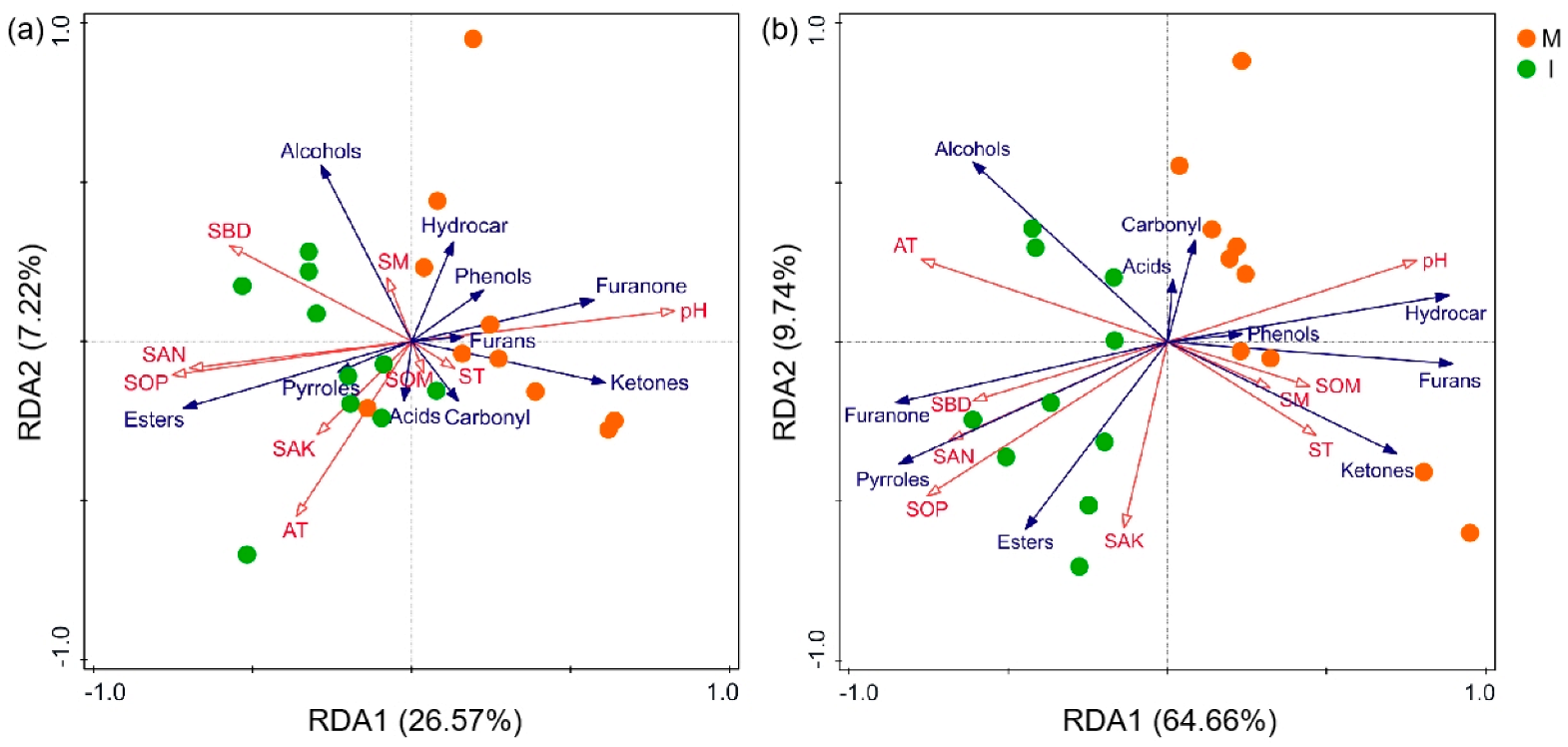
3.2.2. Co-Occurrence Network Analysis between Soil Properties and Volatile Substances of Pandanus amaryllifolius
3.2.3. Correlation among Soil Physico-Chemical Properties, Ecosystem Micro-Environment and Volatile Substances of Pandanus amaryllifolius
3.3. Key Regulated Factors of Intercropping Pattern on Volatile Substances
3.3.1. Key Regulated Factors of Intercropping Pattern on the Component Numbers of Pandanus amaryllifolius Volatile Substances in Different Categories
3.3.2. Key Regulated Factors of Intercropping Pattern on the Relative Contents of Pandanus amaryllifolius Volatile Substances in Different Categories
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Category | Component | Relative Content (%) | |
|---|---|---|---|---|
| Pandanus amaryllifolius Monoculture | Pandanus amaryllifolius and Hevea brasiliensis Intercropping | |||
| 1 | Alcohols | 1-Propanol | 0.35 | 0.31 |
| 2 | 3,7,11-Trimethyl-1-dodecanol | 0.31 | 0.66 | |
| 3 | 2-Tetradecanol | 0.06 | - | |
| 4 | Trans-hex-3-en-1-ol | - | 0.07 | |
| 5 | 3-Methyl-1,5-pentanediol | - | 0.02 | |
| 6 | 2-Hexadecanol | 0.05 | 0.01 | |
| 7 | 1,3-Dimethoxy-2-propanol | - | 0.03 | |
| 8 | 2-Methylhexadecan-1-ol | 0.05 | 0.04 | |
| 9 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 2.24 | 6.43 | |
| 10 | Phytol | 14.22 | 15.89 | |
| 11 | 1-Heptatriacotanol | 3.08 | 0.78 | |
| 12 | 1-Octen-3-ol | 0.02 | - | |
| 13 | (3β,5Z,7E)-9,10-Secocholesta-5,7,10(19)-Triene-3,24,25-triol | 0.04 | - | |
| 14 | Stigmasterol | 1.68 | - | |
| 15 | β-Sitosterol | 7.20 | 8.52 | |
| 16 | Pyrroles | 2-Acetyl-1-pyrroline | 1.85 | 10.68 |
| 17 | Ketones | 2,4-Pentanedione,3-(phenylmethyl) | 0.06 | 0.01 |
| 18 | Acetol | 0.36 | 0.32 | |
| 19 | Acetoin | 0.05 | - | |
| 20 | N-(4-Oxocyclohexyl) acetamide | 0.04 | - | |
| 21 | Oxacyclododecan-2-one | - | - | |
| 22 | Cyclohexan-1,2-dion | - | 0.02 | |
| 23 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one | 0.81 | - | |
| 24 | Neocurdione | 0.04 | - | |
| 25 | Esters | 2-Hydroxy-gama-butyrolactone | 0.08 | - |
| 26 | Hexadecanoic acid, 1-(Hydroxymethyl)-1,2-ethanediyl ester | 0.24 | 1.11 | |
| 27 | 1,2-Propanediol,1-acetate | 0.26 | 0.06 | |
| 28 | 2,3-Epoxypropyl acetate | 0.02 | - | |
| 29 | α-Methylene-γ-butyrolactone | 0.02 | - | |
| 30 | Ethyl hexadecanoate | 0.39 | 0.97 | |
| 31 | Lactic acid | 0.02 | - | |
| 32 | Propanoic acid, 2-hydroxy | 0.02 | 0.03 | |
| 33 | 2,3-Dihydroxypropyl (9Z,12Z,15Z)-9,12,15-octadecatrienoate | 0.16 | 0.37 | |
| 34 | Ethyl linoleate | 0.26 | 0.94 | |
| 35 | 2,3-Dihydroxypropyl (9Z,12Z,15Z)-9,12,15-octadecatrienoate | 0.35 | 0.34 | |
| 36 | Hexadecyl 2-ethylhexanoate | 0.10 | - | |
| 37 | Dimethyl phthalate | 0.46 | 0.64 | |
| 38 | 1,2-Benzenedicarboxylic acid, butyl octyl ester | 0.10 | 0.32 | |
| 39 | Carbonyls | 1-Methyl-1H-pyrazole-4-carbaldehyde | - | 0.02 |
| 40 | Methylglyoxal | 0.08 | 0.05 | |
| 41 | Succindialdehyde | 0.21 | - | |
| 42 | 5-Octadecenal | 0.36 | 0.28 | |
| 43 | 4-Octadecenal | - | 0.03 | |
| 44 | 8-Octadecenal | 0.02 | - | |
| 45 | Furans | 5-Hydroxymethylfurfural | 10.81 | 3.07 |
| 46 | 2-formylfuran | 1.37 | 0.89 | |
| 47 | Furfurylalcohol | 1.52 | 0.98 | |
| 48 | 2,3-Dihydrobenzofuran | 1.96 | 0.18 | |
| 49 | Furanone | 5-Methylfurfural | 0.15 | - |
| 50 | 3-Methyl-2(5H)-furanone | 12.63 | 21.06 | |
| 51 | Acids | Lactic acid | 0.45 | 0.35 |
| 52 | 3-(Acetylthio)-2-methylpropanoic acid | 0.03 | - | |
| 53 | L(+)-Arginine | 0.08 | 0.07 | |
| 54 | 3-Hydroxydodecanoic acid | 0.10 | 0.13 | |
| 55 | 17-Octadecynoic acid | 0.02 | - | |
| 56 | 9-Hexadecenoic acid | 0.01 | 0.04 | |
| 57 | Ricinoleic acid | 0.03 | 0.05 | |
| 58 | 11-Octadecenoic acid | 0.32 | 0.17 | |
| 59 | Trans-13-Octadecenoic acid | - | 0.35 | |
| 60 | Oleic Acid | 0.56 | 0.07 | |
| 61 | Hydrocarbons | 1-(Benzyloxy)-2,4-difluorobenzene | 0.07 | - |
| 62 | 6-Methyloctadecane | 0.01 | - | |
| 63 | Squalene | 26.29 | 8.27 | |
| 64 | Neophytadiene | 5.24 | 14.17 | |
| 65 | Phenols | Probucol | 0.02 | - |
| 66 | 2,4-Di-tert-butylphenol | 1.30 | 1.21 | |
| 67 | Phenol, 2,5-bis(1,1-dimethylethyl) | 0.07 | - | |
| 68 | Vitamin E | 1.34 | - | |
References
- Routray, W.; Rayaguru, K. Chemical constituents and post-harvest prospects of pandanus amaryllifolius leaves: A review. Food Rev. Int. 2010, 26, 230–245. [Google Scholar] [CrossRef]
- Ningrum, A.; Minh, N.N.; Schreiner, M. Carotenoids and norisoprenoids as carotenoid degradation products in pandan leaves (Pandanus amaryllifolius Roxb.). Int. J. Food Prop. 2015, 18, 1905–1914. [Google Scholar] [CrossRef]
- Wakte, K.V.; Thengane, R.J.; Jawali, N.; Nadaf, A.B. Optimization of HS-SPME conditions for quantification of 2-acetyl-1-pyrroline and study of other volatiles in Pandanus amaryllifolius Roxb. Food Chem. 2010, 121, 595–600. [Google Scholar] [CrossRef]
- Wakte, K.V.; Nadaf, A.B.; Thengane, R.J.; Jawali, N. Pandanus amaryllifolius Roxb. cultivated as a spice in coastal regions of India. Genet. Resour. Crop Evol. 2009, 56, 735–740. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern. Med. 2013, 13, 341. [Google Scholar] [CrossRef]
- Patil, S.B.; Hublikar, L.V.; Raghavendra, N.; Shanbhog, C.; Kamble, A. Synthesis and exploration of anticancer activity of silver nanoparticles using Pandanus amaryllifolius Roxb. leaf extract: Promising approach against lung cancer and breast cancer cell lines. Biologia 2021, 76, 3533–3545. [Google Scholar] [CrossRef]
- Prabha, S.P.; Karthik, C.; Chandrika, S.H. Phytol-A biosurfactant from the aquatic weed hydrilla verticillata. Biocatal. Agric. Biotechnol. 2019, 17, 736–742. [Google Scholar] [CrossRef]
- Placido, D.; Dong, N.; Amer, B.; Dong, C.; Ponciano, G.; Kahlon, T.; Whalen, M.; Baidoo, E.E.K.; Mcmahan, C. Downregulation of squalene synthase broadly Impacts Isoprenoid biosynthesis in guayule. Metabolites 2022, 12, 303. [Google Scholar] [CrossRef]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; Mcnerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef]
- Chiabchalard, A.; Nooron, N. Antihyperglycemic effects of Pandanus amaryllifolius Roxb. leaf extract. Pharmacogn. Mag. 2015, 11, 117. [Google Scholar] [CrossRef]
- Ooi, L.S.M.; Sun, S.S.M.; Ooi, V.E.C. Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae). Int. J. Biochem. Cell Biol. 2004, 36, 1440–1446. [Google Scholar] [CrossRef]
- Wakte, K.V.; Zanan, R.L.; Thengane, R.J.; Jawali, N.; Nadaf, A.B. Identification of elite population of Pandanus amaryllifolius Roxb. for higher 2-acetyl-1-pyrroline and other volatile contents by HS-SPME/GC-FID from peninsular India. Food Anal. Method 2012, 5, 1276–1288. [Google Scholar] [CrossRef]
- Moreira, S.L.S.; Pires, C.V.; Marcatti, G.E.; Santos, R.H.S.; Imbuzeiro, H.M.A.; Fernandes, R.B.A. Intercropping of coffee with the palm tree, macauba, can mitigate climate change effects. Agric. For. Meteorol. 2018, 256, 379–390. [Google Scholar] [CrossRef]
- Clermont-Dauphin, C.; Dissataporn, C.; Suvannang, N.; Pongwichian, P.; Maeght, J.; Hammecker, C.; Jourdan, C. Intercrops improve the drought resistance of young rubber trees. Agron. Sustain. Dev. 2018, 38, 56. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agr. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Cuartero, J.; Antonio Pascual, J.; Vivo, J.; özbolat, O.; Sánchez-Navarro, V.; Egea-Cortines, M.; Zornoza, R.; Martinez Mena, M.; Garcia, E.; Ros, M. A first-year melon/cowpea intercropping system improves soil nutrients and changes the soil microbial community. Agr. Ecosyst. Environ. 2022, 328, 107856. [Google Scholar] [CrossRef]
- Hong, Y.; Heerink, N.; Jin, S.; Berentsen, P.; Zhang, L.; van der Werf, W. Intercropping and agroforestry in China-Current state and trends. Agr. Ecosyst. Environ. 2017, 244, 52–61. [Google Scholar] [CrossRef]
- Gou, F.; Yin, W.; Hong, Y.; van der Werf, W.; Chai, Q.; Heerink, N.; van Ittersum, M.K. On yield gaps and yield gains in intercropping: Opportunities for increasing grain production in northwest China. Agric. Syst. 2017, 151, 96–105. [Google Scholar] [CrossRef]
- Du, J.; Han, T.; Gai, J.; Yong, T.; Sun, X.; Wang, X.; Yang, F.; Liu, J.; Shu, K.; Liu, W.; et al. Maize-soybean strip intercropping: Achieved a balance between high productivity and sustainability. J. Integr. Agric. 2018, 17, 747–754. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Wu, J.; Jiang, X.; Zhu, X. Can intercropping with the cash crop help improve the soil physico-chemical properties of rubber plantations? Geoderma 2019, 335, 149–160. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, S.; Zhang, X.; Zhao, K.; Chen, H.Y.H. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Xiao, J.; Yin, X.; Ren, J.; Zhang, M.; Tang, L.; Zheng, Y. Complementation drives higher growth rate and yield of wheat and saves nitrogen fertilizer in wheat and faba bean intercropping. Field Crop. Res. 2018, 221, 119–129. [Google Scholar] [CrossRef]
- Duan, Y.; Shen, J.; Zhang, X.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W.; Zhu, X. Effects of soybean–tea intercropping on soil-available nutrients and tea quality. Acta Physiol. Plant. 2019, 41, 140. [Google Scholar] [CrossRef]
- Maffei, M.; Mucciarelli, M. Essential oil yield in peppermint/soybean strip intercropping. Field Crop. Res. 2003, 84, 229–240. [Google Scholar] [CrossRef]
- Jost, T.; Heymann, T.; Glomb, M.A. Efficient analysis of 2-acetyl-1-pyrroline in foods using a novel derivatization trategy and LC-MS/MS. J. Agric. Food Chem. 2019, 67, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Seng Kean Loh, Y.B.C.M.; Hamid, N.S.A. Process optimisation of encapsulated pandan (Pandanus amaryllifolius) powder using spray-drying method. J. Sci. Food Agric. 2005, 85, 1999–2004. [Google Scholar] [CrossRef]
- Yiming Zhong, A.Z.X.Q.; Ying, Z.J.W.J. Effects of Intercropping Pandanus amaryllifolius on soil properties and microbial community composition in Areca Catechu plantations. Forests 2022, 13, 1814. [Google Scholar] [CrossRef]
- Bai, Y.; Li, B.; Xu, C.; Raza, M.; Wang, Q.; Wang, Q.; Fu, Y.; Hu, J.; Imoulan, A.; Hussain, M.; et al. Intercropping walnut and tea: Effects on soil nutrients, enzyme activity, and microbial communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef]
- Zou, X.; Shi, P.; Zhang, C.; Si, T.; Wang, Y.; Zhang, X.; Yu, X.; Wang, H.; Wang, M. Rotational strip intercropping of maize and peanuts has multiple benefits for agricultural production in the northern agropastoral ecotone region of China. Eur. J. Agron. 2021, 129, 126304. [Google Scholar] [CrossRef]
- Jiang, Y.; Khan, M.U.; Lin, X.; Lin, Z.; Lin, S.; Lin, W. Evaluation of maize/peanut intercropping effects on microbial assembly, root exudates and peanut nitrogen uptake. Plant Physiol. Biochem. 2022, 171, 75–83. [Google Scholar] [CrossRef]
- Paramita Bhattacharjee, A.K.; Singhal, R.S. Supercritical carbon dioxide extraction of 2-acetyl-1-pyrroline from Pandanus amaryllifolius Roxb. Food Chem. 2005, 91, 255–259. [Google Scholar] [CrossRef]
- Mo, Z.; Tang, Y.; Ashraf, U.; Pan, S.; Duan, M.; Tian, H.; Wang, S.; Tang, X. Regulations in 2-acetyl-1-pyrroline contents in fragrant rice are associated with water-nitrogen dynamics and plant nutrient contents. J. Cereal Sci. 2019, 88, 96–102. [Google Scholar] [CrossRef]
- Funsueb, S.C.K.S.M.; Krongchai, C.; Mahatheeranont, S.; Kittiwachana, S. Prediction of 2-acetyl-1-pyrroline content in grains of Thai jasmine rice based on planting condition, plant growth and yield component data using chemometrics. Chemometr. Intell. Lab. 2016, 156, 203–210. [Google Scholar] [CrossRef]
- Chen, K.; Hu, S.; Jiang, Y. Efects of nitrogen fertilizer on the Bt protein content in transgenic rice and nitrogen metabolism mechanism. J. Plant Growth Regul. 2021, 41, 2375–2385. [Google Scholar] [CrossRef]
- Cocozza, C.; Brilli, F.; Pignattelli, S.; Pollastri, S.; Brunetti, C.; Gonnelli, C.; Tognetti, R.; Centritto, M.; Loreto, F. The excess of phosphorus in soil reduces physiological performances over time but enhances prompt recovery of salt-stressed Arundo donax plants. Plant Physiol. Biochem. 2020, 151, 556–565. [Google Scholar] [CrossRef]
- Chauhan, N.K.; Semwal, M.P.; Singh, D.; Singh, B.; Rauthan, S. Influence of various plant spacing on growth, herbage yield, essential oil yield and aroma content of palmarosa (Cymbopogon martinii Roxb.) at different harvest under agro-climatic condition of doon valley. J. Essent. Oil Bear. Plants. 2017, 20, 1587–1593. [Google Scholar] [CrossRef]
- Liu, C.; Cai, Q.; Liao, P.; Jiang, X.; Tang, X.; Yang, Q.; Zhou, L. Effects of Fallopia multiflora–Andrographis paniculata intercropping model on yield, quality, soil nutrition and rhizosphere microorganisms of F multiflora. Plant Soil 2021, 467, 465–481. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Wang, J.; Dawuda, M.M.; Liao, W.; Meng, X.; Yuan, H.; Xie, J.; Tang, Z.; Lyu, J.; et al. Heme is involved in the exogenous ALA-promoted growth and antioxidant defense system of cucumber seedlings under salt stress. BMC Plant Biol. 2022, 22, 329. [Google Scholar] [CrossRef]
- Telci, I.; Demirtas, I.; Bayram, E.; Arabaci, O.; Kacar, O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.). Ind. Crop. Prod. 2010, 32, 588–592. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, Y.; Yan, B.; Zhan, Z.; Chi, X.; Xu, Y.; Guo, X.; Cui, X.; Wang, T.; Wang, S.; et al. Diverse Intercropping patterns enhance the productivity and volatile oil yield of atractylodes lancea (Thunb.) DC. Front. Plant Sci. 2021, 12, 663730. [Google Scholar] [CrossRef]
| Soil Physico-Chemical Properties | Cultivation Patterns | |
|---|---|---|
| Pandanus amaryllifolius Monoculture | Pandanus amaryllifolius and Hevea brasiliensis Intercropping | |
| AT (°C) | 33.50 ± 0.32 b | 34.52 ± 0.31 a |
| ST (°C) | 20.24 ± 0.10 a | 20.10 ± 0.09 a |
| pH | 7.15 ± 0.08 a | 6.15 ± 0.03 b |
| SM (%) | 18.88 ± 0.55 a | 17.62 ± 0.79 a |
| SBD (g cm−3) | 1.78 ± 0.06 b | 2.02 ± 0.08 a |
| SOM (g kg−1) | 17.44 ± 2.08 a | 15.25 ± 1.17 a |
| SAK (mg kg−1) | 38.13 ± 1.60 a | 41.85 ± 1.45 a |
| SAN (mg kg−1) | 51.95 ± 2.65 b | 82.96 ± 2.02 a |
| SOP (mg kg−1) | 5.44 ± 0.24 b | 15.93 ± 0.67 a |
| Network Metrics | Component Numbers of Volatile Substances in Different Categories | Relative Contents of Volatile Substances in Different Categories |
|---|---|---|
| Number of Nodes | 19 | 19 |
| Number of Edges | 130 | 135 |
| Number of Positive Correlations | 60 | 61 |
| Number of Negative Correlations | 70 | 74 |
| Percentage of the Positive Link (P%) | 46.15 | 45.19 |
| P% from Soil Properties to Volatile Substances Categories | 52.59 | 53.90 |
| Average Connectivity (avgK) | 2.46 | 5.20 |
| Average Clustering Coefficient (avgCC) | 0.58 | 0.71 |
| Average Path Length (APL) | 1.86 | 2.11 |
| Graph Density | 0.21 | 0.37 |
| Sequence | Component Numbers of Volatile Substances in Different Categories | Relative Contents of Volatile Substance in Different Categories | ||||
|---|---|---|---|---|---|---|
| Explains | F Value | p Value | Explains | F Value | p Value | |
| pH | 13.50 | 2.80 | 0.04 | 40.90 | 12.40 | 0.01 |
| AT | 4.40 | 0.90 | 0.45 | 18.30 | 7.60 | 0.01 |
| SOP | 3.40 | 0.60 | 0.60 | 5.90 | 2.70 | 0.04 |
| SOM | 1.80 | 0.30 | 0.88 | 3.30 | 1.60 | 0.18 |
| SBD | 4.20 | 0.90 | 0.52 | 2.10 | 1.00 | 0.39 |
| SAK | 4.90 | 1.00 | 0.42 | 2.00 | 0.90 | 0.42 |
| SAN | 2.60 | 0.50 | 0.73 | 3.00 | 1.50 | 0.19 |
| ST | 1.20 | 0.20 | 0.95 | 2.10 | 1.00 | 0.38 |
| SM | 3.90 | 0.80 | 0.50 | 1.90 | 0.90 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Lu, Z.; Yu, H.; Zhang, Y.; Qin, X.; Ji, X.; He, S.; Zong, Y.; Zhong, Y.; Li, L. Effects of Hevea brasiliensis Intercropping on the Volatiles of Pandanus amaryllifolius Leaves. Foods 2023, 12, 888. https://doi.org/10.3390/foods12040888
Zhang A, Lu Z, Yu H, Zhang Y, Qin X, Ji X, He S, Zong Y, Zhong Y, Li L. Effects of Hevea brasiliensis Intercropping on the Volatiles of Pandanus amaryllifolius Leaves. Foods. 2023; 12(4):888. https://doi.org/10.3390/foods12040888
Chicago/Turabian StyleZhang, Ang, Zhiqing Lu, Huan Yu, Yaoyu Zhang, Xiaowei Qin, Xunzhi Ji, Shuzhen He, Ying Zong, Yiming Zhong, and Lihua Li. 2023. "Effects of Hevea brasiliensis Intercropping on the Volatiles of Pandanus amaryllifolius Leaves" Foods 12, no. 4: 888. https://doi.org/10.3390/foods12040888
APA StyleZhang, A., Lu, Z., Yu, H., Zhang, Y., Qin, X., Ji, X., He, S., Zong, Y., Zhong, Y., & Li, L. (2023). Effects of Hevea brasiliensis Intercropping on the Volatiles of Pandanus amaryllifolius Leaves. Foods, 12(4), 888. https://doi.org/10.3390/foods12040888






