Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. The Peach Samples Collection
2.2. The Peach Samples Preparation for Volatile Compounds Analysis
2.2.1. Extraction of Volatile Compounds
2.2.2. Volatile Compounds Detection
2.2.3. Volatile Compounds Identification
2.3. OAV Calculation of the Aroma Compounds
2.4. Sensory Evaluation
2.5. Statistic Analysis
3. Results and Discussion
3.1. Characterization the Profile of Volatile Components by HS-SPME/GC-MS
3.2. Critical Aroma Compounds Exploration from the Perspective of OAV
3.3. Critical Aroma Compounds Exploration from the Perspective of Chemometrics Methods
3.3.1. Volatile Components Modeling with PCA and OPLS-DA Approaches
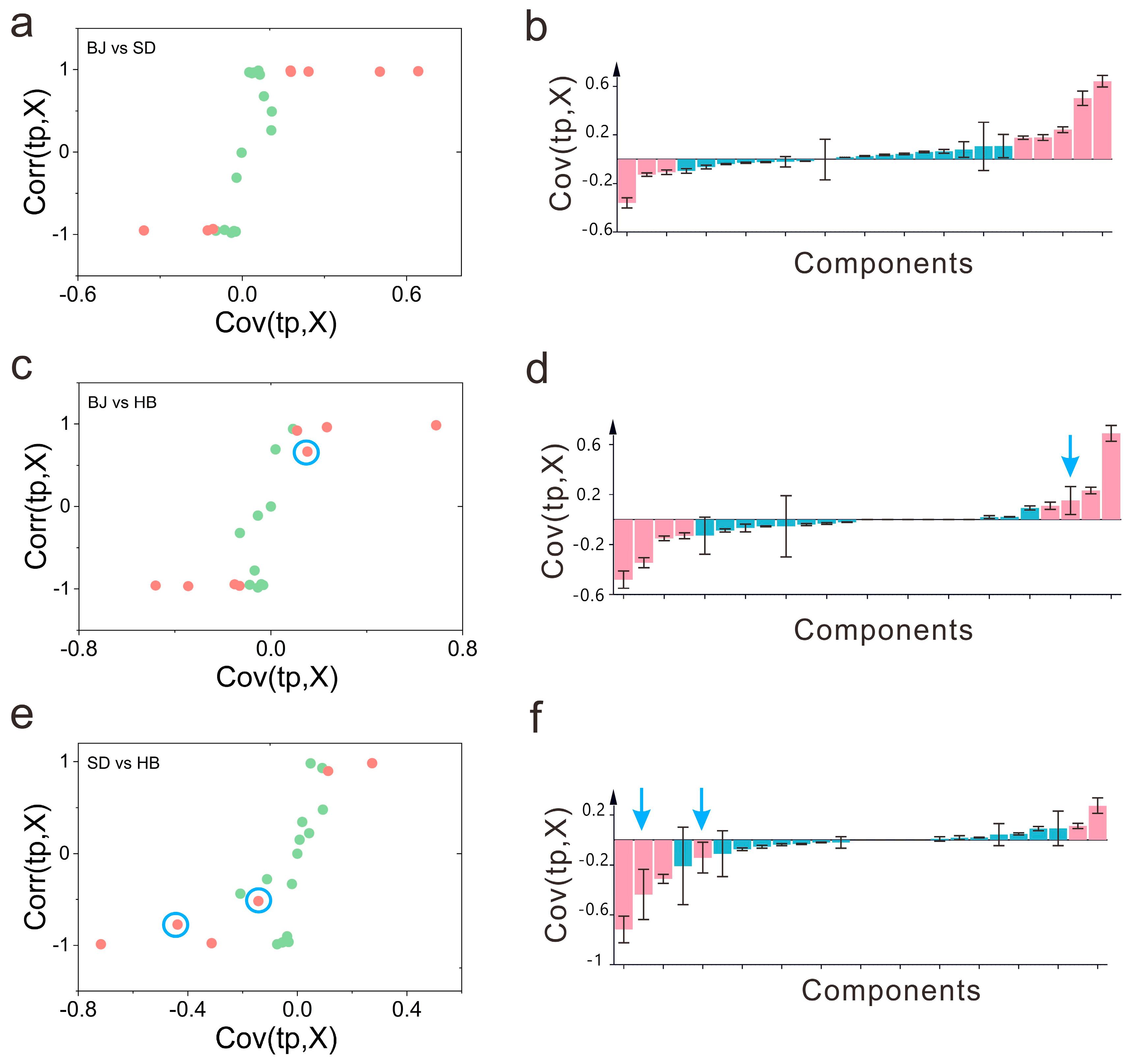
3.3.2. Critical Aroma Components Exploration by S-Plot, Jack-Knifing Confidence Interval, VIP, t-Test, and FC
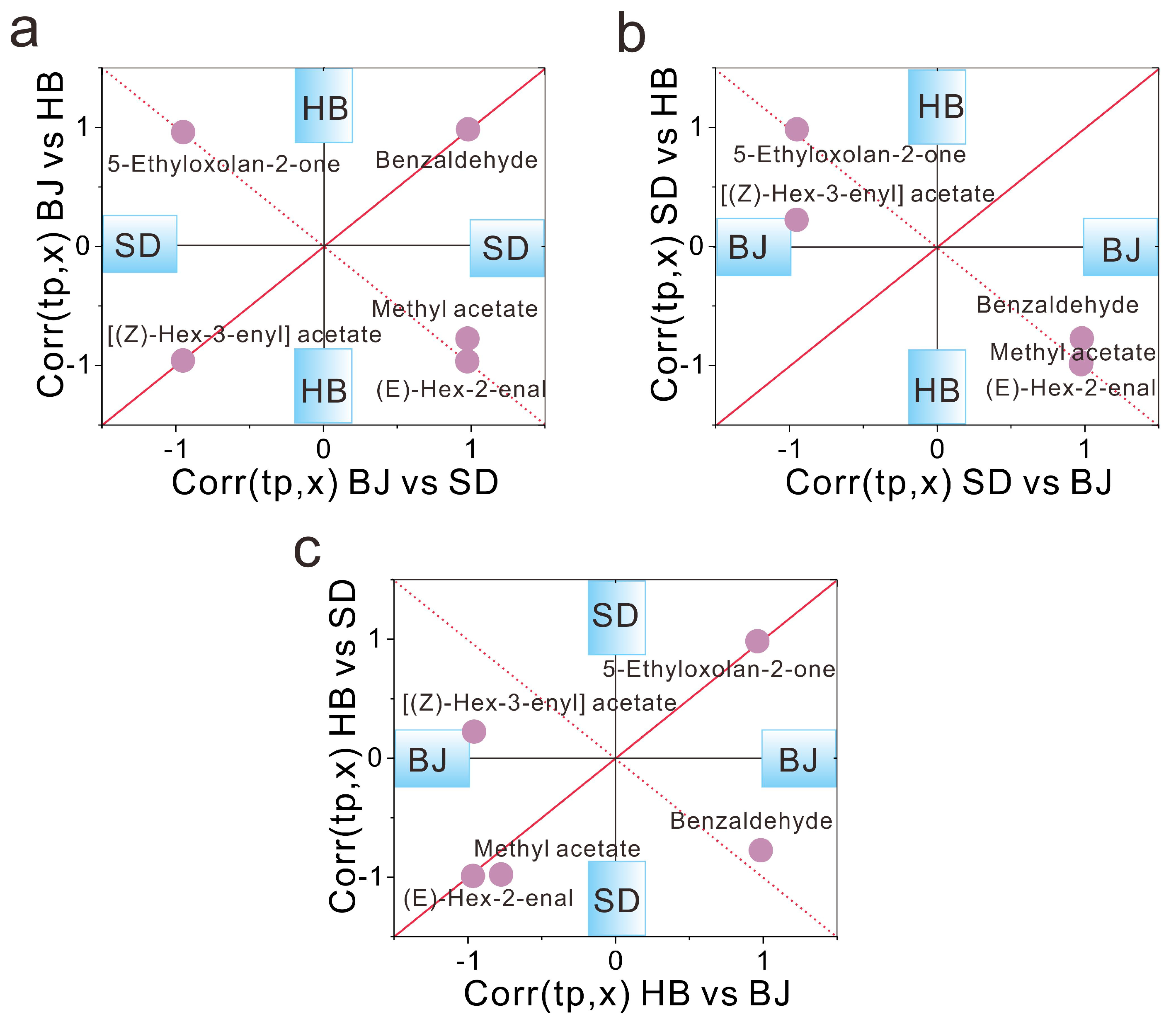
3.3.3. Critical Compounds Characterization upon the Strategy of SUS-Plot
3.4. Critical Aroma Compounds for Qualitative Discrimination Model and Potential Chemical Basis of Odors via Sensory Evaluation
3.4.1. Critical Aroma Compound: Qualitative Discrimination Model Based on PLS-DA
3.4.2. Critical Aroma Compound: Potential Chemical Basis of Odors by Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Xu, Y.; Wang, H.; Miao, Y.; Li, C.; Zhao, R.; Shi, X.; Wang, B. Physicochemical characteristics, antioxidant activities, and aroma compound analysis of seven peach cultivars (Prunus persica L. Batsch) in shihezi, xinjiang. Foods 2022, 11, 2944. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.; Tavares, E.; Bolini, H.M.A. Descriptive sensory profile and consumer study impact of different nutritive and non-nutritive sweeteners on the descriptive, temporal profile, and consumer acceptance in a peach juice matrix. Foods 2022, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, W.; Sun, B.; Li, H.; Zheng, F.; Li, J.; Meng, N. Characterization of key aroma-active compounds in two types of peach spirits produced by distillation and pervaporation by means of the sensomics approach. Foods 2022, 11, 2598. [Google Scholar] [CrossRef] [PubMed]
- Leccese, A.; Viti, R.; Bartolini, S. The effect of solvent extraction on antioxidant properties of apricot fruit. Open Life Sci. 2011, 6, 199–204. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, P.; Tian, H.; Wang, P. The presence of kiwifruit columella affects the aroma profiles of fresh and thermally treated kiwifruit juice. LWT Food Sci. Technol. 2022, 165, 113756. [Google Scholar] [CrossRef]
- Dabbou, S.; Lussiana, C.; Maatallah, S.; Gasco, L.; Hajlaoui, H.; Flamini, G. Changes in biochemical compounds in flesh and peel from Prunus persica fruits grown in Tunisia during two maturation stages. Plant Physiol. Biochem. 2016, 100, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Zhang, B.; Zhang, Y.; Cai, Z.; Song, H.; Ma, R.; Yu, M. Analysis of volatile compounds and their potential regulators in four high-quality peach (Prunus persica L.) cultivars with unique aromas. LWT Food Sci. Technol. 2022, 160, 113195. [Google Scholar] [CrossRef]
- Marsili, R. Flavor, Fragrance, and Odor Analysis; CRC Press: Boca Raton, FL, USA, 2001; Volume 115. [Google Scholar]
- Chen, C.; Liu, Z.; Yu, H.; Lou, X.; Huang, J.; Yuan, H.; Wang, B.; Xu, Z.; Tian, H. Characterization of six lactones in cheddar cheese and their sensory interactions studied by odor activity values and feller’s additive model. J. Agric. Food Chem. 2021, 70, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, H.; Chen, J.; Xie, J.; Shen, S.; Deng, Y.; Zhu, J.; Yuan, H.; Jiang, Y. Characterization of the key aroma compounds in black teas with different aroma types by using gas chromatography electronic nose, gas chromatography-ion mobility spectrometry, and odor activity value analysis. LWT Food Sci. Technol. 2022, 163, 113492. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Castellari, M.; López-Tamames, E.; Galassi, S.; Buxaderas, S. Characterisation of volatile compounds of fruit juices and nectars by HS/SPME and GC/MS. Food Chem. 2004, 87, 627–637. [Google Scholar] [CrossRef]
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Chowtivannakul, S. HS-SPME/GC-MS analysis of volatile aromatic compounds in alcohol related beverages made with mulberry fruits. Food Sci. Biotechnol. 2011, 20, 1021–1032. [Google Scholar] [CrossRef]
- Giannetti, V.; Mariani, M.B.; Mannino, P.; Marini, F. Volatile fraction analysis by HS-SPME/GC-MS and chemometric modeling for traceability of apples cultivated in the Northeast Italy. Food Control 2017, 78, 215–221. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, X.; Xing, J.; Li, N.; Li, J.; Su, Q.; Chen, Y.; Zhang, B.; Zhu, B. Accurate determination of 12 lactones and 11 volatile phenols in nongrape wines through headspace-solid-phase microextraction (HS-SPME) combined with high-resolution gas chromatography-orbitrap mass spectrometry (GC-Orbitrap-MS). J. Agric. Food Chem. 2022, 70, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A basic tool of chemometrics. J. Chemom. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Lee, L.C.; Jemain, A.A. On overview of PCA application strategy in processing high dimensionality forensic data. Microchem. J. 2021, 169, 106608. [Google Scholar] [CrossRef]
- Han, L.; Cheng, Y.; Zhang, T.; Zhou, Q.; Zhang, W.; Li, Y.; Li, G. Targeted metabolomics with a chemometric study of oxygenated heterocyclicaglycones as a tool for preliminary authenticity assessment of orange and grapefruit juices. Front. Nutr. 2022, 9, 897982. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.C.; Alves, J.D.O.; Linhare, L.A.; Egreja Filho, F.B.; Fontes, M.P. Vulnerability of tropical soils to heavy metals: A PLS-DA classification model for lead. Microchem. J. 2017, 133, 258–264. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Budiene, J.; Guclu, G.; Oussou, K.F.; Kelebek, H.; Selli, S. Elucidation of volatiles, anthocyanins, antioxidant and sensory properties of cv. Caner Pomegranate (Punica granatum L.) juices produced from three juice extraction methods. Foods 2021, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhu, J.; Xiao, Z. Characterization of the key aroma compounds in peach by gas chromatography-olfactometry, quantitative measurements and sensory analysis. Eur. Food Res. Technol. 2019, 245, 129–141. [Google Scholar] [CrossRef]
- Pino, J.A.; Trujillo, R. Characterization of odour-active compounds of sour guava (Psidium acidum [DC.] Landrum) fruit by gas chromatography-olfactometry and odour activity value. Flavour Fragr. J. 2021, 36, 207–212. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, Z.; Ye, Z.; Su, M. Multiplex analyses of the changes of aromatic compounds during the development of peach fruit using GC-MS and iTRAQ proteomic techniques. Sci. Hortic. 2018, 236, 96–105. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of aroma trait of the white-fleshed peach ‘Hu Jing Mi Lu’ and the yellow-fleshed peach ‘Jin Yuan’ based on odor activity value and odor characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Xu, Y.; Liang, J.; Chang, P.; Yan, F.; Li, M.; Liang, Y.; Zou, Z. Genome-wide association mapping for tomato volatiles positively contributing to tomato flavor. Front. Plant Sci. 2015, 6, 1042–1053. [Google Scholar] [CrossRef]
- Yahia, E.M.; Carrillo-Lopez, A. Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Peng, B.; Yu, M.; Zhang, B.; Xu, J.; Ma, R. Differences in PpAAT1 activity in high-and low-aroma peach varieties affect γ-decalactone production. Plant Physiol. 2020, 182, 2065–2080. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Xie, J.; Chen, C.; Ai, L.; Tian, H. Aroma perceptual interactions of benzaldehyde, furfural, and vanillin and their effects on the descriptor intensities of Huangjiu. Food Res. Int. 2020, 129, 108808. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, S.; Zhang, R.; Liu, S.; Zhang, C.; Li, Y.; Li, J. Characterization of honey peach (Prunus persica (L.) Batsch) aroma variation and unraveling the potential aroma metabolism mechanism through proteomics analysis under abiotic stress. Food Chem. 2022, 386, 132720. [Google Scholar] [CrossRef] [PubMed]
- Andrew Clayton, T.; Lindon, J.C.; Cloarec, O.; Antti, H.; Charuel, C.; Hanton, G.; Provost, J.P.; Le Net, J.L.; Baker, D.; Walley, R.J.; et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 2006, 440, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Michell, A.W.; Mosedale, D.; Grainger, D.J.; Barker, R.A. Metabolomic analysis of urine and serum in Parkinson’s disease. Metabolomics 2008, 4, 191–201. [Google Scholar] [CrossRef]
- Lapins, M.; Eklund, M.; Spjuth, O.; Prusis, P.; Wikberg, J.E. Proteochemometric modeling of HIV protease susceptibility. BMC Bioinform. 2008, 9, 181. [Google Scholar] [CrossRef]
- Wei, J.; Xie, G.; Zhou, Z.; Shi, P.; Qiu, Y.; Zheng, X.; Chen, T.; Su, M.; Zhao, A.; Jia, W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 2011, 129, 2207–2217. [Google Scholar] [CrossRef]
- Madala, N.E.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A. Multivariate statistical models of metabolomic data reveals different metabolite distribution patterns in isonitrosoacetophenone-elicited Nicotiana tabacum and Sorghum bicolor cells. SpringerPlus 2014, 3, 254. [Google Scholar] [CrossRef]


| No. | Volatile Compounds | RT (min) | Concentration (μg/kg) | ||
|---|---|---|---|---|---|
| BJ | SD | HB | |||
| 1 | Isocyanic acid | 4.47 | 51.92 ± 6.52 | 87.67 ± 5.35 | 92.08 ± 7.68 |
| 2 | Methyl acetate | 4.93 | 123.67 ± 12.57 | 602.58 ± 86.40 | 97.50 ± 8.99 |
| 3 | 2-Methylpropane | 5.97 | 4.83 ± 0.83 | nd | nd |
| 4 | Pentan-3-one | 6.45 | 13.17 ± 1.70 | nd | nd |
| 5 | 2-Butan-2-yloxybutane | 8.39 | 129.67 ± 11.48 | 116.92 ± 23.45 | 188.00 ± 10.82 |
| 6 | 1-(Furan-2-yl)pentan-1-one | 8.73 | 3.67 ± 0.98 | 14.00 ± 1.86 | 6.08 ± 1.62 |
| 7 | (E)-Hex-2-enal | 9.72 | 606.17 ± 52.16 | 2688.33 ± 331.83 | 65.75 ± 8.85 |
| 8 | 5-Methylhept-3-yne | 9.77 | nd | 5.33 ± 1.15 | nd |
| 9 | Ethylbenzene | 9.85 | 166.08 ± 15.37 | 87.00 ± 7.83 | 88.83 ± 7.32 |
| 10 | 1,2-Xylene | 10.18 | 264.67 ± 35.01 | 339.33 ± 46.64 | 425.92 ± 16.56 |
| 11 | Methyl hexanoate | 11.24 | nd | nd | 45.00 ± 7.77 |
| 12 | Benzaldehyde | 12.42 | 1546.42 ± 203.31 | 4858.00 ± 178.88 | 3628.92 ± 266.85 |
| 13 | (2R)-2-Hydroxy-3-methylbutanenitrile | 12.66 | nd | nd | 12.08 ± 1.62 |
| 14 | [(Z)-Hex-3-enyl] acetate | 13.25 | 1567.83 ± 248.83 | 482.25 ± 95.83 | 524.58 ± 98.45 |
| 15 | Hexyl acetate | 13.40 | 1915.08 ± 161.47 | 2290.92 ± 144.76 | 1782.25 ± 294.10 |
| 16 | (Z)-Hex-2-enyl acetate | 13.45 | 1821.17 ± 185.55 | 1826.42 ± 161.33 | 1592.25 ± 308.70 |
| 17 | (E)-Hex-3-en-1-yne | 13.75 | 7.67 ± 1.56 | nd | nd |
| 18 | Phenylmethanol | 14.27 | nd | 196.42 ± 12.44 | nd |
| 19 | 5-Ethyloxolan-2-one | 14.49 | 135.50 ± 32.10 | 11.92 ± 3.29 | 384.83 ± 45.06 |
| 20 | Octan-1-ol | 14.85 | 35.67 ± 8.97 | nd | nd |
| 21 | Nonan-1-ol | 17.19 | 143.42 ± 26.49 | 44.58 ± 6.29 | 38.17 ± 10.31 |
| 22 | [(E)-Hex-2-enyl] 3-Methylbutanoate | 18.79 | nd | 28.00 ± 3.30 | nd |
| 23 | 2,3-Dimethylbut-3-en-2-ol | 21.43 | nd | 15.42 ± 2.61 | nd |
| No. | Volatile Compounds | RI a | RI b | Odor Description c | Threshold (μg/L) d | ID e | Category |
|---|---|---|---|---|---|---|---|
| 1 | Isocyanic acid | / | n.f | MS, RI | acid | ||
| 2 | Methyl acetate | sweet, fruity | 3 | MS, RI, S | ester | ||
| 3 | 2-Methylpropane | 605 | - | / | n.f | MS, RI, S | hydrocarbon |
| 4 | Pentan-3-one | 703 | 700 | ethereal acetone | 40 | MS, RI | ketone |
| 5 | Sec-Butyl ether | 801 | - | / | n.f | MS, RI, S | ethers |
| 6 | 1-(2-Furyl) pentan-1-one | 815 | - | / | n.f | MS, RI | ketone |
| 7 | (E)-Hex-2-enal | 858 | 854 | green, leaf | 30 | MS, RI, S | aldehyde |
| 8 | 5-Methylhept-3-yne | 860 | - | / | n.f | MS, RI | hydrocarbon |
| 9 | Ethylbenzene | 863 | 864 | / | 1200 | MS, RI | benzene |
| 10 | 1,2-Xylene | 877 | - | geranium | 450 | MS, RI, S | benzene |
| 11 | Methyl hexanoate | 922 | 924 | fruity | 70 | MS, RI, S | ester |
| 12 | Benzaldehyde | 970 | 970 | sweet | 750 | MS, RI | aldehyde |
| 13 | (2R)-2-Hydroxy-3-methylbutanenitrile | 980 | - | / | n.f | MS, RI | alcohol |
| 14 | [(Z)-Hex-3-enyl] acetate | 1004 | 1009 | green, leaf | 8 | MS, RI | ester |
| 15 | Hexyl acetate | 1011 | 1010 | fruity | 10 | MS, RI, S | ester |
| 16 | (Z)-Hex-2-enyl acetate | 1013 | 1005 | / | n.f | MS, RI, S | ester |
| 17 | 3-Hexen-1-yne | 1025 | - | / | n.f | MS, RI | hydrocarbon |
| 18 | Phenylmethanol | 1047 | 1042 | floral, rose | 2500 | MS, RI, S | alcohol |
| 19 | 5-Ethyloxolan-2-one | 1056 | 1056 | sweet, coconut | 50 | MS, RI | lactone |
| 20 | Octan-1-ol | 1071 | 1068 | green | 130 | MS, RI | alcohol |
| 21 | Nonan-1-ol | 1171 | 1172 | floral rose | 50 | MS, RI, S | alcohol |
| 22 | [(E)-Hex-2-enyl] 3-methylbutanoate | 1242 | 1244 | / | n.f | MS, RI | ester |
| 23 | 2,3-Dimethylbut-3-en-2-ol | 1366 | - | / | n.f | MS, RI | alcohol |
| Volatile Compounds | OAV Values | ||
|---|---|---|---|
| BJ | SD | HB | |
| Methyl acetate | 41.22 | 200.90 | 32.50 |
| (E)-Hex-2-enal | 20.21 | 89.61 | 2.19 |
| Benzaldehyde | 2.06 | 6.48 | 4.84 |
| [(Z)-Hex-3-enyl] acetate | 195.98 | 60.28 | 65.60 |
| Hexyl acetate | 191.51 | 229.10 | 178.00 |
| Nonan-1-ol | 2.87 | 0.89 | 0.76 |
| 5-Ethyloxolan-2-one | 2.71 | 0.24 | 7.70 |
| Modeling Parameters | R2p(X) | R2(X) | R2(Y) | Q2(Y) | Components | Intercept R2 |
|---|---|---|---|---|---|---|
| BJ vs. SD | 0.737 | 0.861 | 0.988 | 0.983 | 1 predictive + 1 orthogonal | 0.078 |
| BJ vs. HB | 0.680 | 0.846 | 0.980 | 0.969 | 1 predictive + 1 orthogonal | 0.058 |
| SD vs. HB | 0.641 | 0.729 | 0.994 | 0.984 | 1 predictive + 1 orthogonal | 0.126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, B.; Zhang, R.; Liu, S.; Yang, S.; Li, Y.; Li, J. Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics. Foods 2023, 12, 837. https://doi.org/10.3390/foods12040837
Li Q, Li B, Zhang R, Liu S, Yang S, Li Y, Li J. Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics. Foods. 2023; 12(4):837. https://doi.org/10.3390/foods12040837
Chicago/Turabian StyleLi, Qianqian, Bei Li, Rong Zhang, Shuyan Liu, Shupeng Yang, Yi Li, and Jianxun Li. 2023. "Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics" Foods 12, no. 4: 837. https://doi.org/10.3390/foods12040837
APA StyleLi, Q., Li, B., Zhang, R., Liu, S., Yang, S., Li, Y., & Li, J. (2023). Flavoromics Approach in Critical Aroma Compounds Exploration of Peach: Correlation to Origin Based on OAV Combined with Chemometrics. Foods, 12(4), 837. https://doi.org/10.3390/foods12040837





