Application of a Novel Au@ZIF-8 Composite in the Detection of Bisphenol A by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
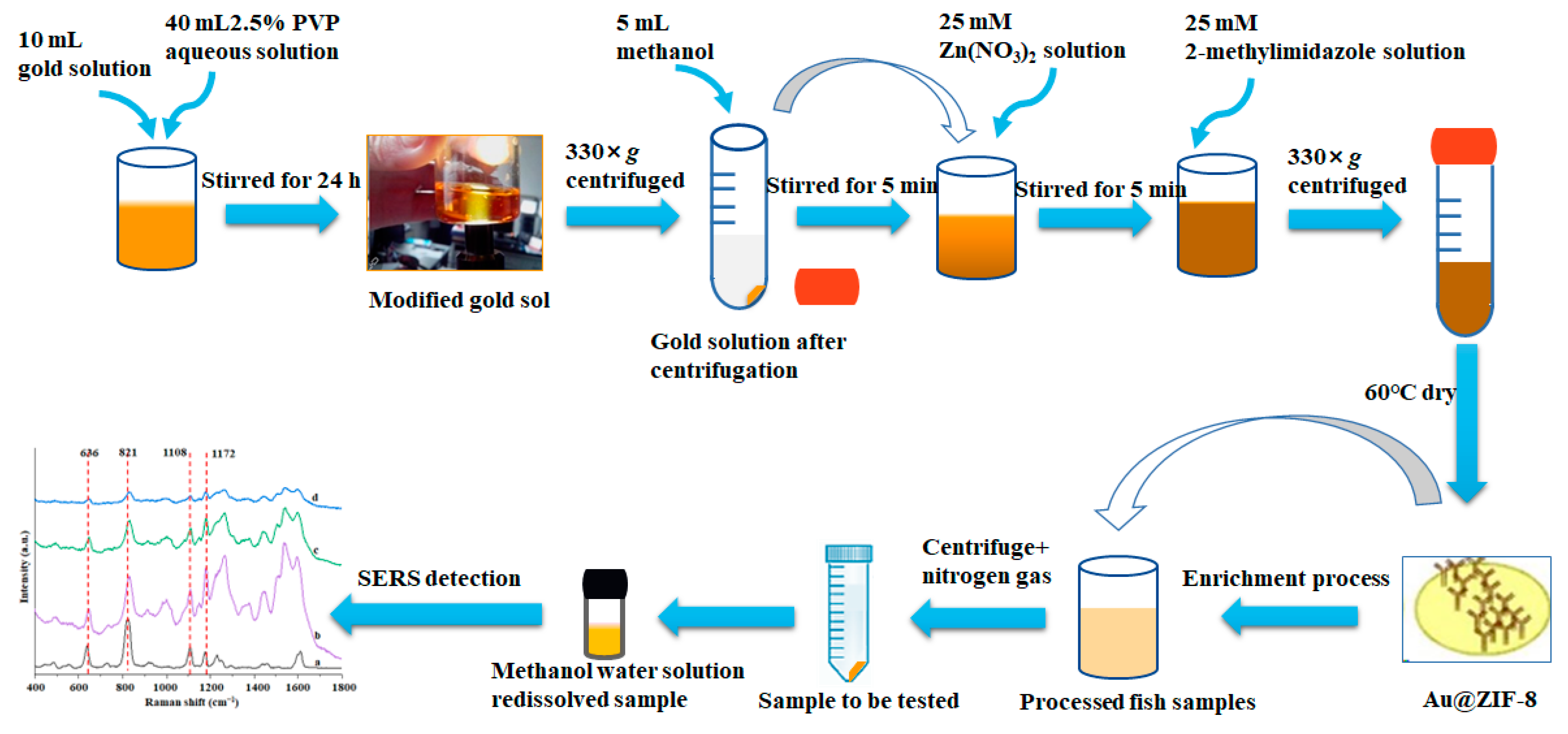
2.1. Schematic Overview of the Experimental Program
2.2. Chemicals and Reagents
2.3. Instruments
2.4. Synthesis of Au@ZIF-8
2.5. Study on the Optimum Synthesis Conditions of Au@ZIF-8
2.6. Application of Au@ZIF-8 in SERS Detection
2.6.1. SERS Detection
2.6.2. Spiking Recovery Tests
2.6.3. Detection of BPA in Fish Samples
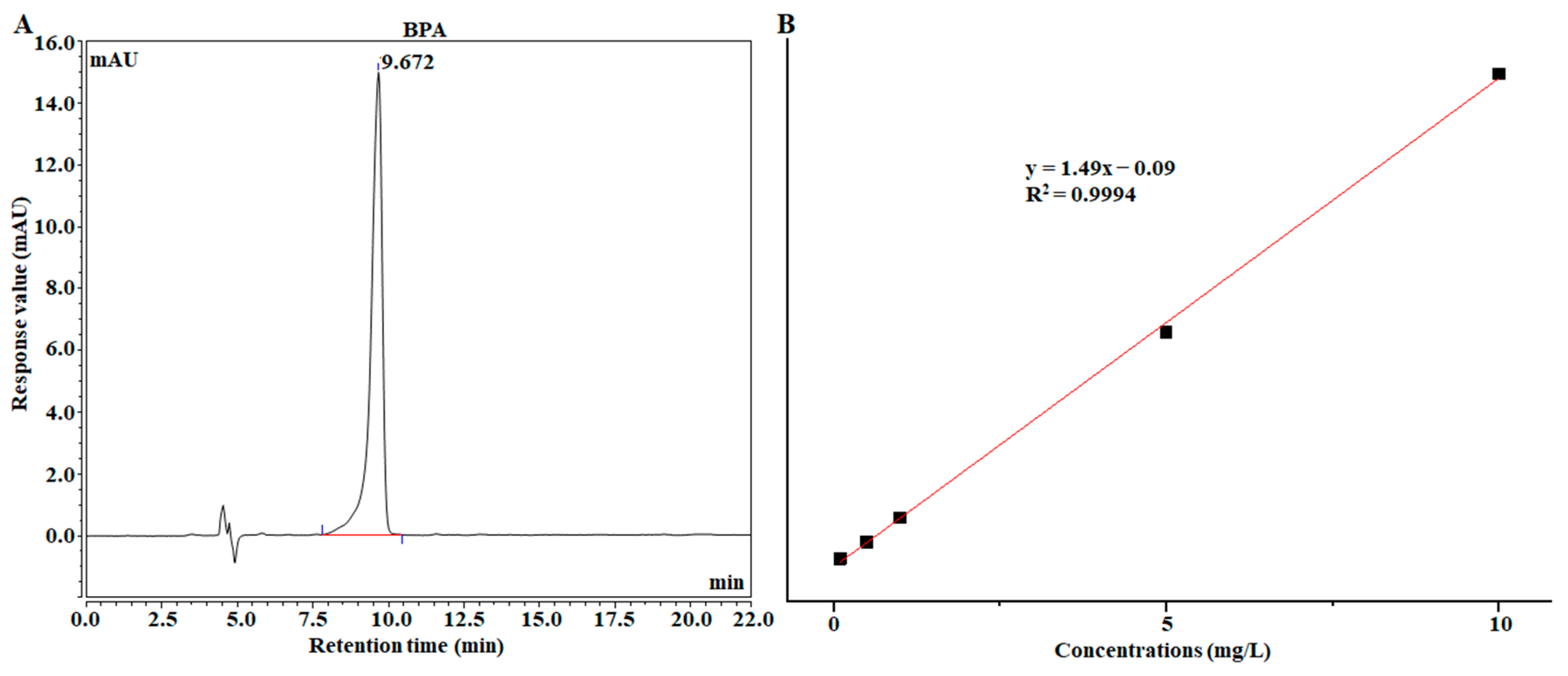
2.7. Comparison of Au@ZIF-8 SERS Detection with HPLC
2.8. Data Processing
3. Results and Discussion
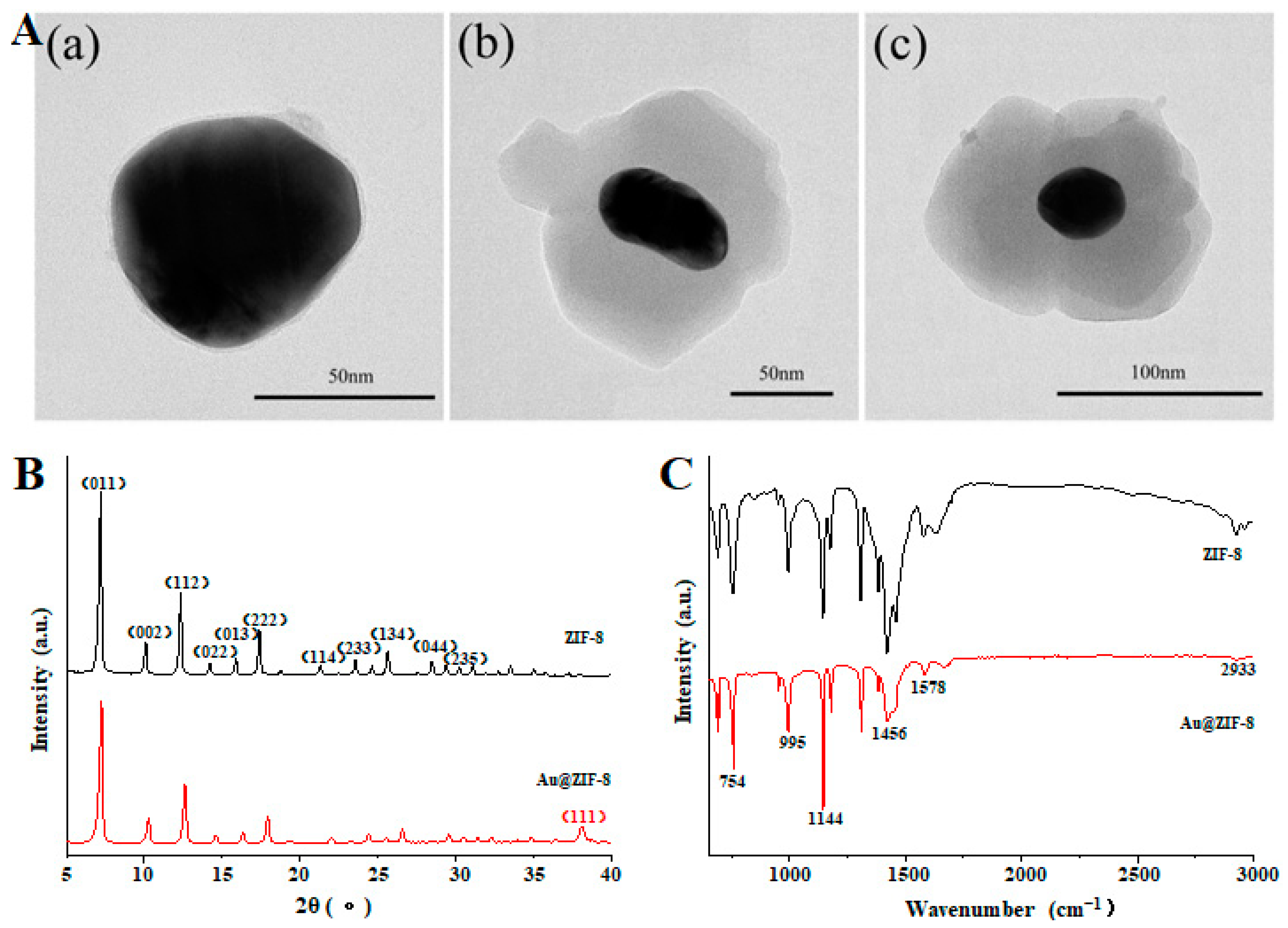
3.1. Characterization of Au@ZIF-8
3.2. The Optimum Synthesis Conditions Properties of Au@ZIF-8
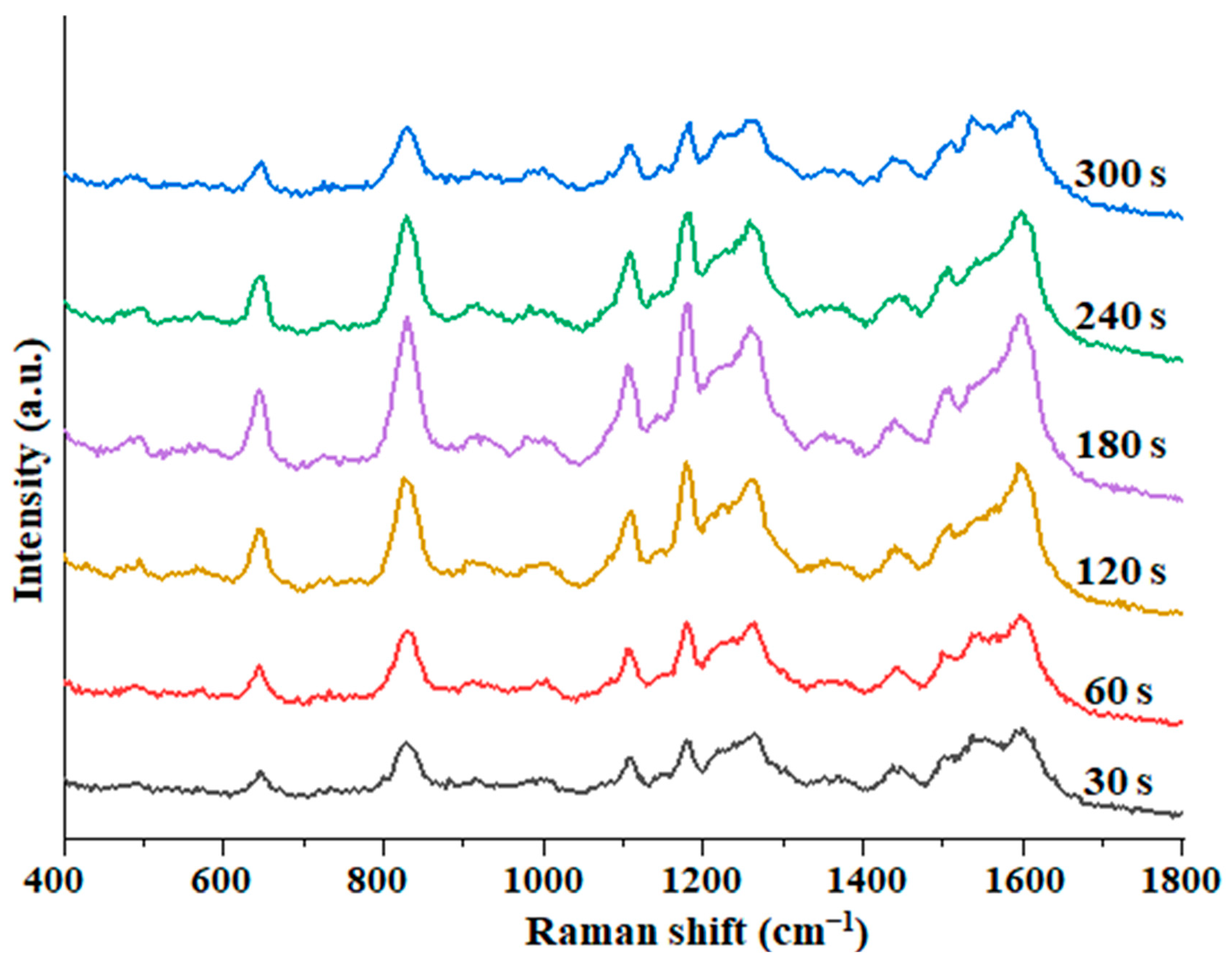
3.3. The Optimum SERS Detection Conditions of Au@ZIF-8
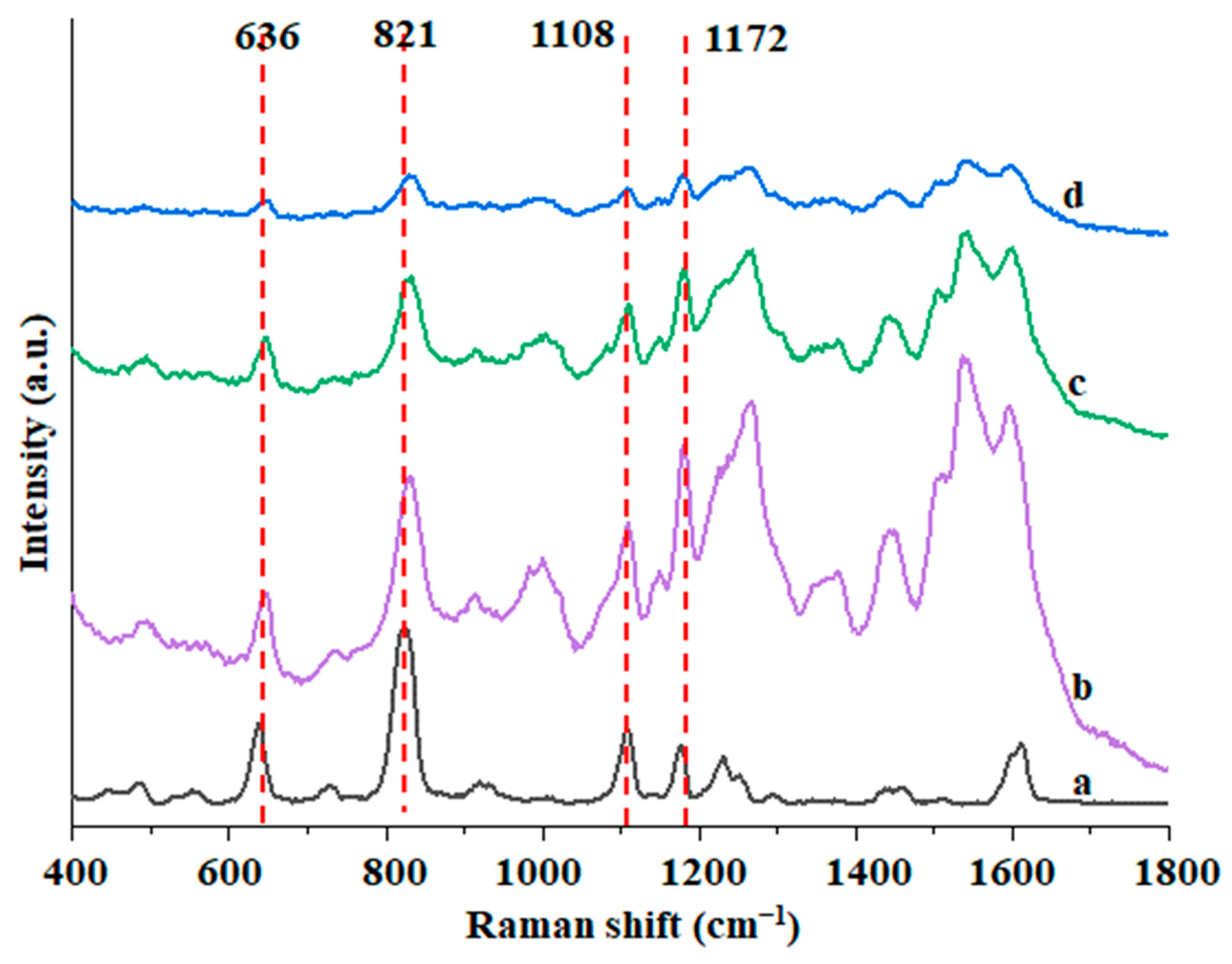
3.4. SERS Detection of Au@ZIF-8 for BPA
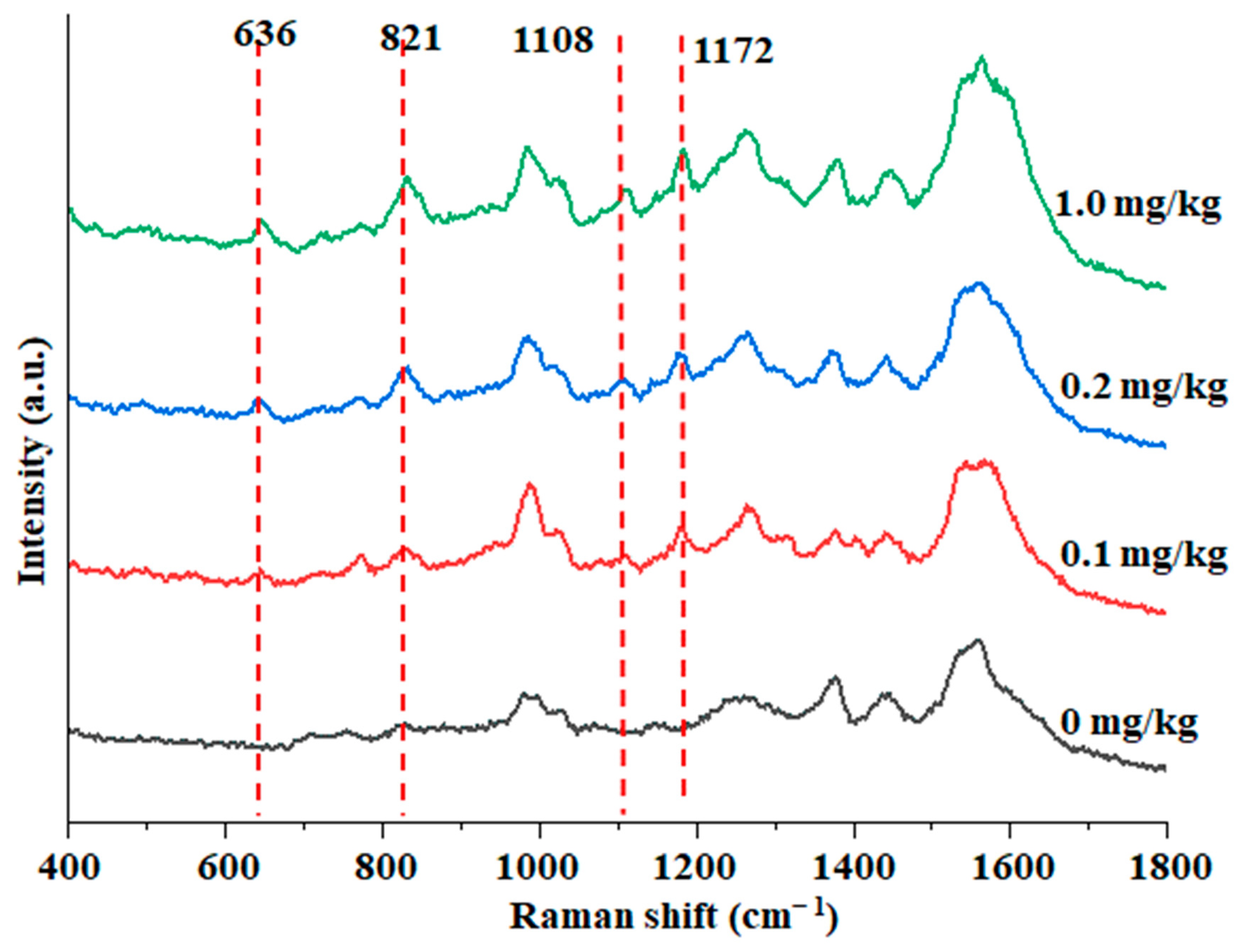
3.5. Detection of Actual Samples
3.6. Comparison of Au@ZIF-8 SERS Detection with Other SERS Methods
4. Conclusions
- There may be many pollutants in food, and the combined method of MOFs and SERS can be established for more contaminants in the future.
- The adsorption mechanism of ZIF-8 and the enhancement mechanism of Au@ZIF-8 have only been preliminarily discussed, and the related mechanism can be further explored in the future.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, S.; Guan, Q.; Liu, Q.; Qin, Z.; Rasheed, B.; Liang, X.; Yang, X. Synthesis, modifications and applications of MILs Metal-organic frameworks for environmental remediation: The cutting-edge review. Sci. Total. Environ. 2022, 810, 152279. [Google Scholar] [CrossRef] [PubMed]
- Kamali, K.; Joseph, B.; Narayana, C. Stability of zeolitic imidazolate frameworks (ZIF-7) under high pressures and its implications on storage applications of ZIFs. J. Solid State Chem. 2022, 309, 122973. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, H.-C. A metal-organic framework with entatic metal centers exhibiting high gas adsorption affinity. J. Am. Chem. Soc. 2006, 128, 11734–11735. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Wang, F.; Cui, X.; Du, X.; Lu, X. UiO series of metal-organic frameworks composites as advanced sorbents for the removal of heavy metal ions: Synthesis, applications and adsorption mechanism. Ecotoxicol. Environ. Saf. 2021, 208, 111577. [Google Scholar] [CrossRef]
- Jiang, Y.; Dong, J.; Li, R.; Sun, F.; Wu, H. Channel regulation through solvents for Cd-MOFs based on p-methoxyphenyl imidazole dicarboxylate: Synthesis, crystal structure, fluorescence, and explosive identification. J. Chin. Chem. Soc. 2022, 69, 301–309. [Google Scholar] [CrossRef]
- Ueda, S.; Hirai, Y.; Sun, H.; Matsushima, Y.; Masuhara, A.; Shiroishi, H.; Yoshida, T. Hydrothermal Synthesis and Electrochemical Evaluation of Zn-Tmla MOFs Employing Trimellitic Acid. In Electrochemical Society Meeting Abstracts 4dms18; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2018; p. 265. [Google Scholar]
- Elsabawy, K.M.; Fallatah, A.M. Microwave assisted synthesis and molecular structure visualization of ultrahigh surface area Ni-6,6′-dibromo-indigo coordinated polymeric MOFs stabilized via hydrogen bonding. Inorg. Chem. Commun. 2018, 92, 78–83. [Google Scholar] [CrossRef]
- Saidi, M.; Benomara, A.; Mokhtari, M.; Boukli-Hacene, L. Sonochemical synthesis of Zr-fumaric based metal-organic framework (MOF) and its performance evaluation in methyl violet 2B decolorization by photocatalysis. React. Kinet. Mech. Catal. Lett. 2020, 131, 1009–1021. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary Metal-Organic Framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Braga, D.; Curzi, M.; Johansson, A.; Polito, M.; Rubini, K.; Grepioni, F. Simple and quantitative mechanochemical preparation of a porous crystalline material based on a 1D coordination network for uptake of small molecules. Angew. Chem. Int. Ed. 2006, 45, 142–146. [Google Scholar] [CrossRef]
- Huang, Z.; Xiong, C.; Ying, L.; Wang, W.; Wang, S.; Ding, J.; Lu, J. A post-functional Ti-based MOFs composite for selective removal of Pb (II) from water. J. Hazard. Mater. 2022, 432, 128700. [Google Scholar] [CrossRef]
- Deng, H.; Chang, Z.; Qiu, F.; Qiao, Y.; Yang, H.; He, P.; Zhou, H. A Safe Organic Oxygen Battery Built with Li-Based Liquid Anode and MOFs Separator. Adv. Energy Mater. 2020, 10, 1903953. [Google Scholar] [CrossRef]
- Colorado-Peralta, R.; Rivera-Villanueva, J.M.; Mora-Hernández, J.M.; Morales-Morales, D.; Alfonso-Herrera, L. An overview of the role of supramolecular interactions in gas storage using MOFs. Polyhedron 2022, 224, 115995. [Google Scholar] [CrossRef]
- Hui, Y.F. Study on synthesis of a novel MOF-Cd material and adsorption performance of methylene blue. Mod. Salt Chem. Ind. 2019, 45, 45–46. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Wang, H.; Chen, Y.; Zhang, M. MOF-74 as an Efficient Catalyst for the Low-Temperature Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2016, 8, 26817–26826. [Google Scholar] [CrossRef] [PubMed]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Li, S.; Zhang, P.; Yao, Q. Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv. 2020, 10, 37600–37620. [Google Scholar] [CrossRef]
- Kukkar, P.; Kim, K.-H.; Kukkar, D.; Singh, P. Recent advances in the synthesis techniques for zeolitic imidazolate frameworks and their sensing applications. Coord. Chem. Rev. 2021, 446, 214109. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Barimah, A.O.; Chen, Q.; Li, H.; Shi, J.; El-Seedi, H.R.; Zou, X. Label-free surface enhanced Raman scattering spectroscopy for discrimination and detection of dominant apple spoilage fungus. Int. J. Food Microbiol. 2021, 338, 108990. [Google Scholar] [CrossRef]
- Duan, N.; Qi, S.; Guo, Y.C.; Xu, W.; Wu, S.; Wang, Z. Fe3O4@Au@Ag nanoparticles as surface-enhanced Raman spectroscopy substrates for sensitive detection of clenbuterol hydrochloride in pork with the use of aptamer binding. LWT 2020, 134, 110017. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Zheng, J.; He, L. Surface-enhanced Raman spectroscopy (SERS) combined techniques for high-performance detection and characterization. TrAC-Trends Anal. Chem. 2017, 90, 1–13. [Google Scholar] [CrossRef]
- Yang, F.; Wang, C.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W.; Xie, Y. Establishment of the thin-layer chromatography-surface-enhanced Raman spectroscopy and chemometrics method for simultaneous identification of eleven illegal drugs in anti-rheumatic health food. Food Biosci. 2022, 49, 101842. [Google Scholar] [CrossRef]
- Kim, H.; Trinh, B.T.; Kim, K.H.; Moon, J.; Kang, H.; Jo, K.; Akter, R.; Jeong, J.; Lim, E.-K.; Jung, J.; et al. Au@ZIF-8 SERS paper for food spoilage detection. Biosens. Bioelectron. 2021, 179, 113063. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Chen, L.; Wang, C.; Zhao, C.; Wang, H.; Ma, N.; Li, J.; Qiao, Y.; Chang, L.; Zhao, B. Highly sensitive SERS behavior and wavelength-dependence charge transfer effect on the PS/Ag/ZIF-8 substrate. Spectrochim. Acta A 2021, 247, 119126. [Google Scholar] [CrossRef]
- Yang, J.; Pan, M.; Yang, X.; Liu, K.; Song, Y.; Wang, S. Effective adsorption and in-situ SERS detection of multi-target pesticides on fruits and vegetables using bead-string like Ag NWs@ZIF-8 core-shell nanochains. Food Chem. 2022, 395, 133623. [Google Scholar] [CrossRef]
- Schiano, M.E.; Abduvakhidov, A.; Varra, M.; Albrizio, S. Aptamer-Based Biosensors for the Analytical Determination of Bisphenol A in Foodstuffs. Appl. Sci. 2020, 12, 3752. [Google Scholar] [CrossRef]
- Yang, L.; Nie, L.-Q.; Wang, J.; Li, C.-Y.; Liu, J.-M.; Wang, S. ZIF-8 sacrificial-templated hollow COF architectures enabled highly efficient enrichment, determination and regulation of food hazards from infant formulas. Food Chem. 2022, 405, 135018. [Google Scholar] [CrossRef]
- Aristiawan, Y.; Aryana, N.; Putri, D.; Styarini, D. Analytical Method Development for Bisphenol a in Tuna by Using High Performance Liquid Chromatography-UV. Procedia Chem. 2015, 16, 202–208. [Google Scholar] [CrossRef]
- Wang, B.-X.; Duan, G.; Xu, W.; Xu, C.; Jiang, J.; Yang, Z.; Wu, Y.; Pi, F. Flexible surface-enhanced Raman scatting substrates: Recent advances in their principles, design strategies, diversified material selections and applications. Crit. Rev. Food Sci. Nutr. 2022; 1–45, online ahead of print. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Li, X.; Zhang, Y.; Chen, Q.; Ye, Z.; Alqarni, Z.; Bell, S.E.J.; Xu, Y. Towards practical and sustainable SERS: A review of recent developments in the construction of multifunctional enhancing substrates. J. Mater. Chem. C 2021, 9, 11517–11552. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Hou, R.-N.; Zhu, Y.-Z.; Wang, X.-T.; Zhang, H.; Zhang, Y.-J.; Zhang, L.; Tian, Z.-Q.; Li, J.-F. Au@ZIF-8 core–shell nanoparticles as a SERS substrate for volatile organic compound gas detection. Anal. Chem. 2021, 93, 7188–7195. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Efficient Adsorptive Removal of Humic Acid from Water Using Zeolitic Imidazole Framework-8 (ZIF-8). Water Air Soil Pollut. 2015, 226, 1–17. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Qiu, J.; Liu, C.; Yu, Z.; Zhang, J.; Qiu, Z. In-situ uniform growth of ZIF-8 on 3D flower-like NiCoLDH microspheres to enhance tetracycline and doxycycline removal from wastewater: Anti-interference and stability tests. Sep. Purif. Technol. 2022, 302, 122078. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Rong, S.; Yu, H.; Gao, H.; Ding, P.; Chang, D.; Pan, H. Electrochemical immunoassay for the carcinoembryonic antigen based on Au NPs modified zeolitic imidazolate framework and ordered mesoporous carbon. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef]
- Huang, K.; Gong, S.; Zhang, L.; Zhang, H.; Li, S.; Ye, G.; Huang, F. Ultrathin ZIF-8 wrapping on Au-dotted Ag-nanowires for highly selective SERS-based CO2 gas detection. Chem. Commun. 2021, 57, 2144–2147. [Google Scholar] [CrossRef]
- Xing, L.; Wang, C.; Cao, Y.; Zhang, J.; Xia, H. Macroscopical monolayer films of ordered arrays of gold nanoparticles as SERS substrates for in situ quantitative detection in aqueous solutions. Nanoscale 2021, 13, 14925–14934. [Google Scholar] [CrossRef]
- Xue, J.-Q.; Li, D.-W.; Qu, L.-L.; Long, Y.-T. Surface-imprinted core–shell Au nanoparticles for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal. Chim. Acta 2013, 777, 57–62. [Google Scholar] [CrossRef]
- Deng, Z.-H.; Li, N.; Jiang, H.-L.; Lin, J.-M.; Zhao, R.-S. Pretreatment techniques and analytical methods for phenolic endocrine disrupting chemicals in food and environmental samples. TrAC-Trends Anal. Chem. 2019, 119, 115592. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman spectroscopic methods in food safety: A review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Yu, X.J.; Gu, Y.Y.; Feng, R.; Ge, X.Y.; Chen, M.Y.; Ni, M.L.; Hu, X.L.; Zhong, Y.Y. Simultaneously determining alkylphenols and bisphenol A residues in seafood by high-performance liquid chromatography with tandem mass spectrometry. J. Huazhong Agric. Univ. 2014, 33, 52–59. [Google Scholar] [CrossRef]
- Chen, Y.L. SERS Detection Study on Penicillins Drug and Bisphenol A in Milk; Jiamusi University: Jiamusi, China, 2018. [Google Scholar]
- Yang, Y.; Li, D.; Liu, G.; Xu, J.; Yang, L. Preparation, modification, self-assembly and surface enhanced Raman scattering of gold nanorods and its biomedical application. Sci. Sin. Chim. 2015, 45, 581–596. [Google Scholar] [CrossRef]
- Yu, X.Y. Preparation and Modification of Gold Nanostars and Application in SERS Detection; Northeast Normal University: Changchun, China, 2020. [Google Scholar]
- Ji, W.; Zhang, L.; Luo, H.S.; Song, W.; Zheng, Y.; Silver, Y. Nanoparticles Preparation and its SERS study for detection of BPA. J. Light Scat. 2016, 28, 293–296. [Google Scholar] [CrossRef]
- Sharma, M.; Pudasaini, P.R.; Ruiz-Zepeda, F.; Vinogradova, E.; Ayon, A.A. Plasmonic Effects of Au/Ag Bimetallic Multispiked Nanoparticles for Photovoltaic Applications. ACS Appl. Mater. Interfaces 2014, 6, 15472–15479. [Google Scholar] [CrossRef]
- Li, A.; Qiao, X.; Liu, K.; Bai, W.; Wang, T. Hollow metal organic framework improves the sensitivity and anti-interference of the detection of exhaled volatile organic compounds. Adv. Funct. Mater. 2022, 32, 2202805. [Google Scholar] [CrossRef]
- Huang, C.; Li, A.; Chen, X.; Wang, T. Understanding the role of metal-organic frameworks in surface-enhanced Raman scattering application. Small 2020, 16, 2004802. [Google Scholar] [CrossRef]

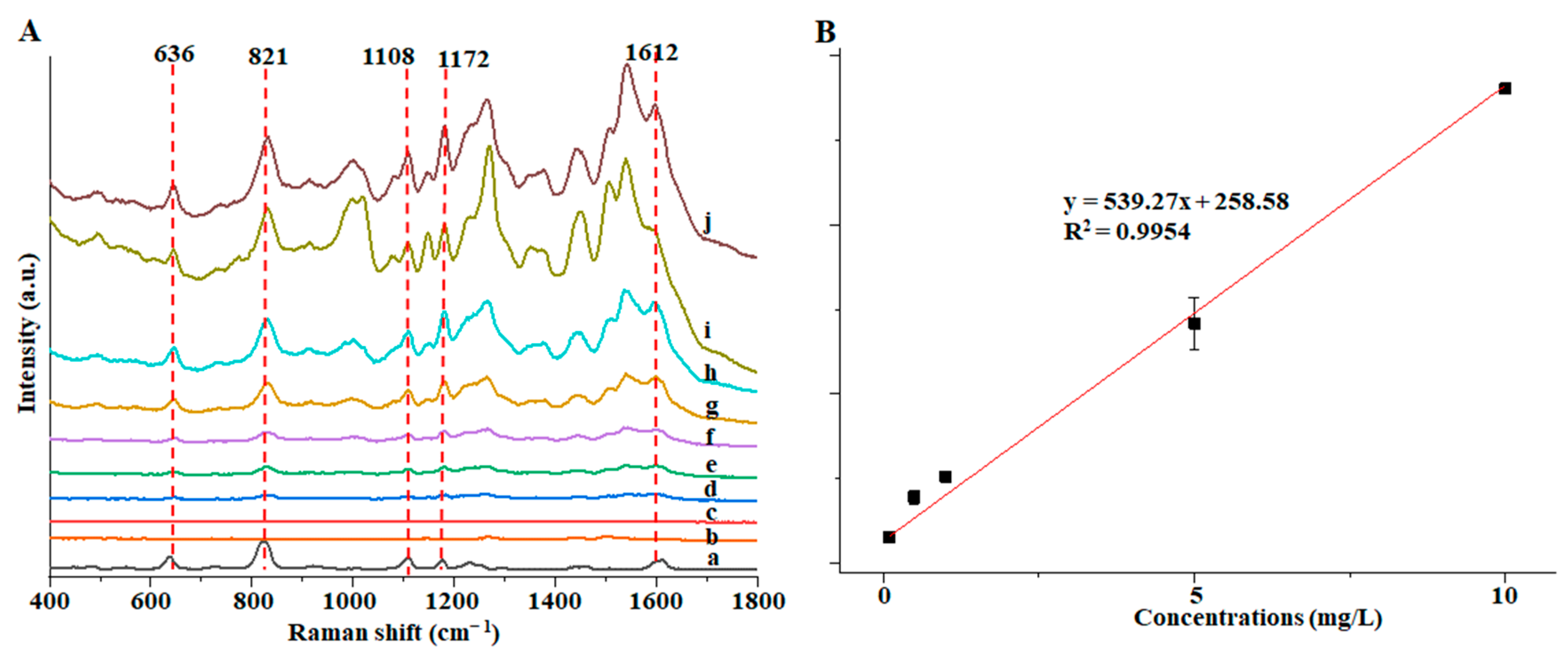
| Detection Limit (mg/L) | Linear Range (mg/L) | R2 | |
|---|---|---|---|
| ZIF-8 | 1.0 | 1–50 | 0.9846 |
| Au@ZIF-8 | 0.1 | 0.1–10 | 0.9954 |
| Material | Spike Concentration (mg/kg) | Average Recovery Rate (%) | RSD a Value (%) |
|---|---|---|---|
| ZIF-8 | 0.5 | 80.35 | 2.43 |
| 1.0 | 85.57 | 9.42 | |
| 5.0 | 84.47 | 11.81 | |
| Au@ZIF-8 | 0.1 | 102.35 | 7.58 |
| 0.2 | 99.68 | 6.14 | |
| 1.0 | 100.82 | 3.28 |
| Addition Level (mg/kg) | SERS Recovery Rate (%) | HPLC Recovery Rate (%) | Deviation from HPLC Results (%) |
|---|---|---|---|
| 0.100 | 102.4 | 99.0 | 3.43 |
| 0.200 | 99.7 | 101.4 | −1.68 |
| 1.000 | 100.8 | 100.3 | 0.50 |
| Reinforced Substrate | Actual Sample | Detection Limit | Reference |
|---|---|---|---|
| Au@ZIF-8 | Fish | 0.10 mg/L | This study |
| AuNPs | PC packaging | 7.50 mg/kg | [42] |
| AgNPs | / | 1.00 mg/L | [43] |
| Au-Ag film | / | 0.50 mg/L | [44] |
| MIPs@AgNPs | Polycarbonate toys | 0.01 mg/L | [45] |
| Functionalized Au-Ag nanoparticles | Soda water | 0.05 mg/L | [46] |
| MIP-ir-AuNPs | Drinking water | 0.12 mg/L | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Dong, X.; Cai, N.; Yang, F.; Yao, W.; Huang, L. Application of a Novel Au@ZIF-8 Composite in the Detection of Bisphenol A by Surface-Enhanced Raman Spectroscopy. Foods 2023, 12, 813. https://doi.org/10.3390/foods12040813
Xie Y, Dong X, Cai N, Yang F, Yao W, Huang L. Application of a Novel Au@ZIF-8 Composite in the Detection of Bisphenol A by Surface-Enhanced Raman Spectroscopy. Foods. 2023; 12(4):813. https://doi.org/10.3390/foods12040813
Chicago/Turabian StyleXie, Yunfei, Xianghui Dong, Nifei Cai, Fangwei Yang, Weirong Yao, and Lijun Huang. 2023. "Application of a Novel Au@ZIF-8 Composite in the Detection of Bisphenol A by Surface-Enhanced Raman Spectroscopy" Foods 12, no. 4: 813. https://doi.org/10.3390/foods12040813
APA StyleXie, Y., Dong, X., Cai, N., Yang, F., Yao, W., & Huang, L. (2023). Application of a Novel Au@ZIF-8 Composite in the Detection of Bisphenol A by Surface-Enhanced Raman Spectroscopy. Foods, 12(4), 813. https://doi.org/10.3390/foods12040813





