A Food Waste-Derived Organic Liquid Fertiliser for Sustainable Hydroponic Cultivation of Lettuce, Cucumber and Cherry Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Seedlings
2.1.2. Liquid Fertilisers
2.2. Experimental Setup
2.3. Analytical Methods
3. Results and Discussions
3.1. Nutrients
3.2. Cations
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campuzano, R.; González-Martínez, S. Characteristics of the organic fraction of municipal solid waste and methane production: A review. Waste Manag. 2016, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Renju, B. Strategies for resource recovery from the organic fraction of municipal solid waste. Case Stud. Chem. Environ. Eng. 2021, 3, 100098–100106. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; Food and Agriculture Organisation of the United Nations: Dusseldorf, Germany, 2011. [Google Scholar]
- FAO. Key Facts on Food Loss and Waste You Should Know! Food and Agriculture Organization of the United Nations: Dusseldorf, Germany, 2012. [Google Scholar]
- Kumar, N.; Goel, S. Characterization of Municipal Solid Waste (MSW) and a proposed management plan for Kharagpur, West Bengal, India. Resour. Conserv. Recycl. 2009, 53, 166–174. [Google Scholar] [CrossRef]
- Garnett, T. Where are the best opportunities for reducing greenhouse gas emissions in the food system. Food Policy 2011, 36, S23–S32. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Horan, N.J.; Salter, M. Energy optimisation from co-digested waste using two-phase process to generate hydrogen and methane. Int. J. Hydrogen Energy 2011, 36, 4792–4799. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Li, P.; Yin, L.; Liu, D.; Zhing, Y.; Li, W.; Zheng, G. A novel alternate feeding mode for semi-continuous anaerobic co-digestion of food waste with chicken manure. Bioresour. Technol. 2014, 164, 309–314. [Google Scholar] [CrossRef]
- Tong, H.; Shen, Y.; Zhang, J.; Wang, C.H.; Ge, T.S.; Tong, Y.W. A comparative life cycle assessment on four waste-to-energy scenarios for food waste generated in eateries. Appl. Energy 2019, 249, 28–36. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Touliatos, D.; Dodd, I.C.; Mcainsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 84–91. [Google Scholar] [CrossRef]
- Zhao, C.; Chavan, S.; He, X.; Zhou, M.; Cazzonelli, C.I.; Chen, Z.H.; Tissue, D.T.; Ghannoum, O. Smart glass impacts stomatal sensitivity of greenhouse Capsicum through altered light. J. Exp. Bot. 2021, 272, 3235–3248. [Google Scholar] [CrossRef]
- Lin, T.; Goldsworthy, M.; Chavan, S.; Liang, W.; Maier, C.; Ghannoum, O.; Cazzonelli, C.I.; Tissue, D.T.; Lan, Y.C.; Sethuvenkatraman, S.; et al. A novel cover material improves cooling energy and fertigation efficiency for glasshouse eggplant production. Energy 2022, 4, 123871. [Google Scholar] [CrossRef]
- Jones, J. Hydroponics: Its history and use in plant nutrition studies. Plant Nutr. 1982, 5, 1003–1030. [Google Scholar] [CrossRef]
- Nicola, S.; Hoeberechts, J.; Fontana, E. Comparison between Traditional and Soilless Culture Systems to Produce Rocket (Eruca sativa) with Low Nitrate Content; International Society for Horticultural Science: Leuven, Belgium, 2005; Volume 697, pp. 549–555. [Google Scholar]
- Flores, H.; Villalobos, J.R.; Ahumada, O.; Uchanski, M.; Meneses, C.; Sanchez, O. Use of supply chain planning tools for efficiently placing small farmers into high-value, vegetable markets. Comput. Electron. Agric. 2019, 157, 205–217. [Google Scholar] [CrossRef]
- Rabbi, B.; Chen, Z.H.; Sethuvenkatraman, S. Protected cropping in warm climates: A review of humidity control and cooling methods. Energies 2019, 12, 2737. [Google Scholar] [CrossRef]
- Smith, G. An Overview of the Australian Protected Cropping Industry [EB/OL]. Available online: https://www.graemesmithconsulting.com/images/documents (accessed on 27 May 2019).
- Bae, K.S.; Chung, S.O.; Kim, K.D.; Hur, S.O.; Kim, H.J. Implementation of remote monitoring scenario using CDMA short message service for protected crop production environment. J. Biosyst. Eng. 2011, 36, 279–284. [Google Scholar] [CrossRef]
- Baille, A. Greenhouse structure and equipment for improving crop production in mild winter climates. In International Symposium Greenhouse Management for Better Yield & Quality in MildWinter Climates. Acta Hort. 1997, 491, 37–48. [Google Scholar]
- He, X.; Qiao, Y.; Liu, Y.; Dendler, L.; Yin, C.; Martin, F. Environmental impact assessment of organic and conventional tomato production in urban greenhouses of Beijing city, China. J. Clean. Prod. 2016, 134, 251–258. [Google Scholar] [CrossRef]
- He, X.; Maier, C.; Chavan, S.G.; Zhao, C.C.; Alagoz, Y.; Cazzonelli, C.; Ghannoum, O.; Tissue, D.T.; Chen, Z.H. Light-altering cover materials and sustainable greenhouse production of vegetables: A review. Plant Growth Regul. 2021, 95, 1–7. [Google Scholar] [CrossRef]
- Domingues, D.S.; Takahshi, H.W.; Camara, C.A.P.; Nixdorf, S.L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012, 84, 53–61. [Google Scholar] [CrossRef]
- Brechner, M.; Both, A.J. Hydroponic Lettuce Handbook; Cornell Controlled Environment Agriculture—Cornell University CEA Programme: Ithaca, NY, USA, 2013. [Google Scholar]
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009, 41, 1275–1281. [Google Scholar] [CrossRef]
- Tatlioglu, T. Cucumber (Cucumis sativus L.). In Genetic Improvement of Vegetable Crop; Pearson Press: London, UK, 1993; pp. 197–224. [Google Scholar]
- Grewal, H.S.; Maheshwari, B.; Parks, S.E. Water and nutrient use efficiency of a low-cost hydroponic greenhouse for a cucumber crop: An Australian case study. Agric. Water Manag. 2011, 98, 841–846. [Google Scholar] [CrossRef]
- Parks, S.E.; Worrall, R.J.; Low, C.T.; Jarvis, J.A. Initial efforts to improve the management of substrates in greenhouse vegetable production in Australia. Acta Hort. 2009, 819, 331–336. [Google Scholar] [CrossRef]
- Halbrooks, M.C.; Wilcox, G.E. Tomato Plant Development and Elemental Accumulation1. J. Am. Soc. Hortic. Sci. 1980, 105, 826–828. [Google Scholar] [CrossRef]
- Maia, J.T.L.S.; Martinez, H.E.P.; Clemente, J.M.; Ventrella, M.C.; Milagres, C.C. Growth, nutrient concentration, nutrient accumulation and visual symptoms of nutrient deficiencies in cherry tomato plants. Semina Ciênc. Agrar. 2019, 40, 585–598. [Google Scholar] [CrossRef]
- Snyder, R.G. Publication-Cooperative Extension Service. In Greenhouse Tomato Handbook; Mississippi State University: Starkville, MS, USA, 1992. [Google Scholar]
- EngindenizI, S.; Yuksel, T. Economic analysis of organic greenhouse lettuce production in Turkey. Sci. Agric. 2006, 63, 285–290. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.-C.; Show, P.L. Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Hagare, D.; Jayasena, V.; Swick, R.; Chen, Z.; Rahman, M.; Boyle, N.; Ghodrat, M. Production of chicken feed and liquid fertiliser from food waste. Waste Manag. 2021, 131, 386–393. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Hagare, D.; Chen, Z.H.; Jayasena, V.; Shahrivar, A.A.; Panatta, O.; Liang, W.; Boyle, N. Growing lettuce and cucumber in a hydroponic system using food waste derived organic liquid fertiliser. Environ. Sustain. 2022, 5, 325–334. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca sativa L. var. Sucrine) Growth Performance in Complemented Aquaponic Solution Outperforms Hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Sublett, W.L.; Barickman, T.C.; Sams, C.E. The Effect of Environment and Nutrients on Hydroponic Lettuce Yield, Quality, and Phytonutrients. Horticulturae 2018, 4, 48. [Google Scholar] [CrossRef]
- Parker, J.B.; James, L.; Parks, S.; Tesoriero, L.; Ryland, A.; Ekman, J.; Jarvis, J. Greenhouse Cucumber Production; NSW Government: Sydney, Australia, 2019.
- Sung, J.K.; Lee, S.Y.; Lee, Y.J.; Kim, R.Y.; Lee, J.Y.; Lee, J.S.; Jang, B.C. Influence of Calcium Supply on the Growth, Calcium and Oxalate Contents, Mineral Nutrients and Ca-oxalate Crystal Formation of Cucumber. Korean J. Soil Sci. Fert. 2010, 43, 471–477. [Google Scholar]
- Colla, G.; Rouphael, Y.; Rea, E.; Cardelli, M. Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinisation. Sci. Hortic. 2012, 135, 177–185. [Google Scholar] [CrossRef]
- Dutch Fest HydroGrow. Hydroponic Solutions. 2020. Available online: https://www.hydroponicsolutions.com.au/shop/dutch-fest-hydrogrow-a-b-set (accessed on 10 January 2020).
- Bugbee, B. Nutrient Management in Recirculating Hydroponic Culture. South Pac. Soil. Cult. Conf. Acta Hort. 2004, 648, 99–112. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability—A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1984; p. 44. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: San Diego, CA, USA, 1995; pp. 11–19. [Google Scholar]

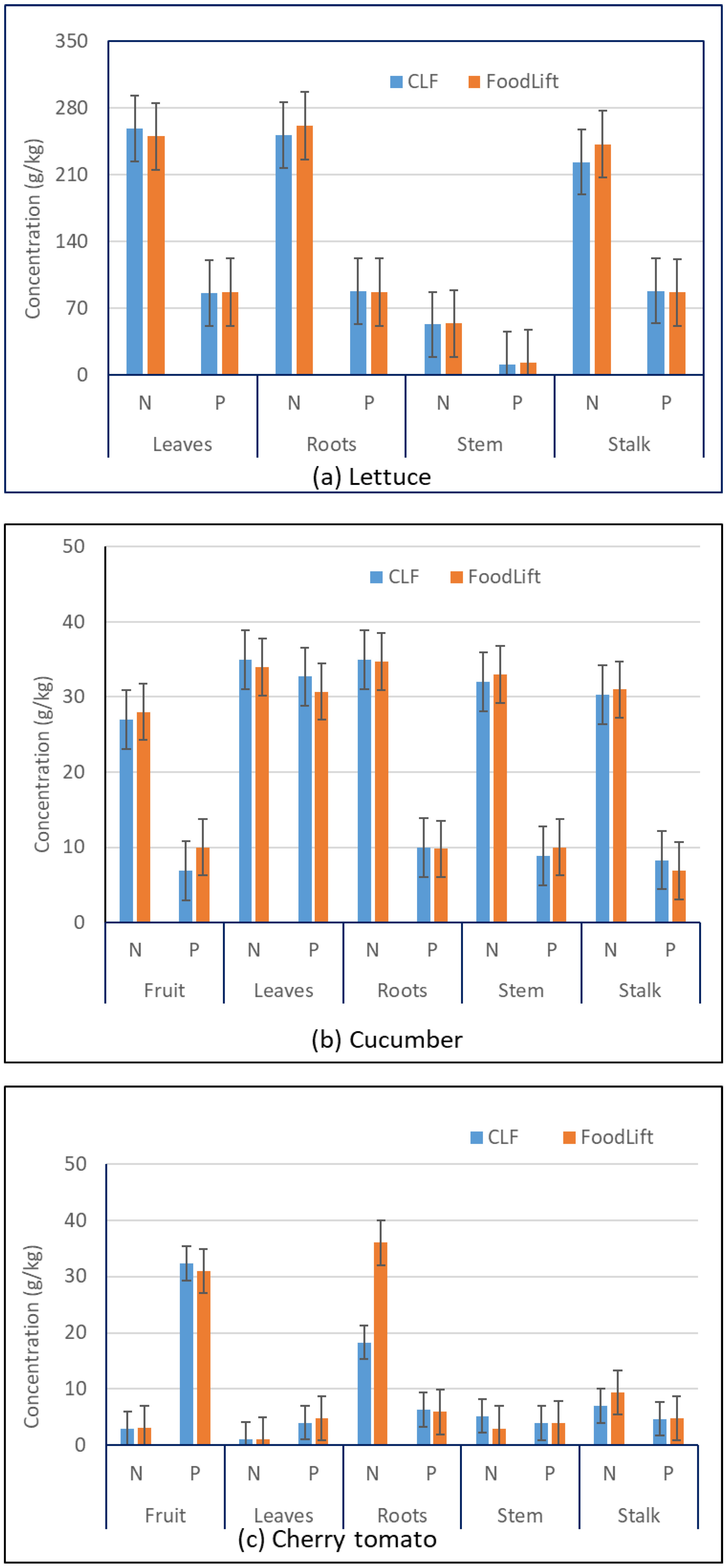
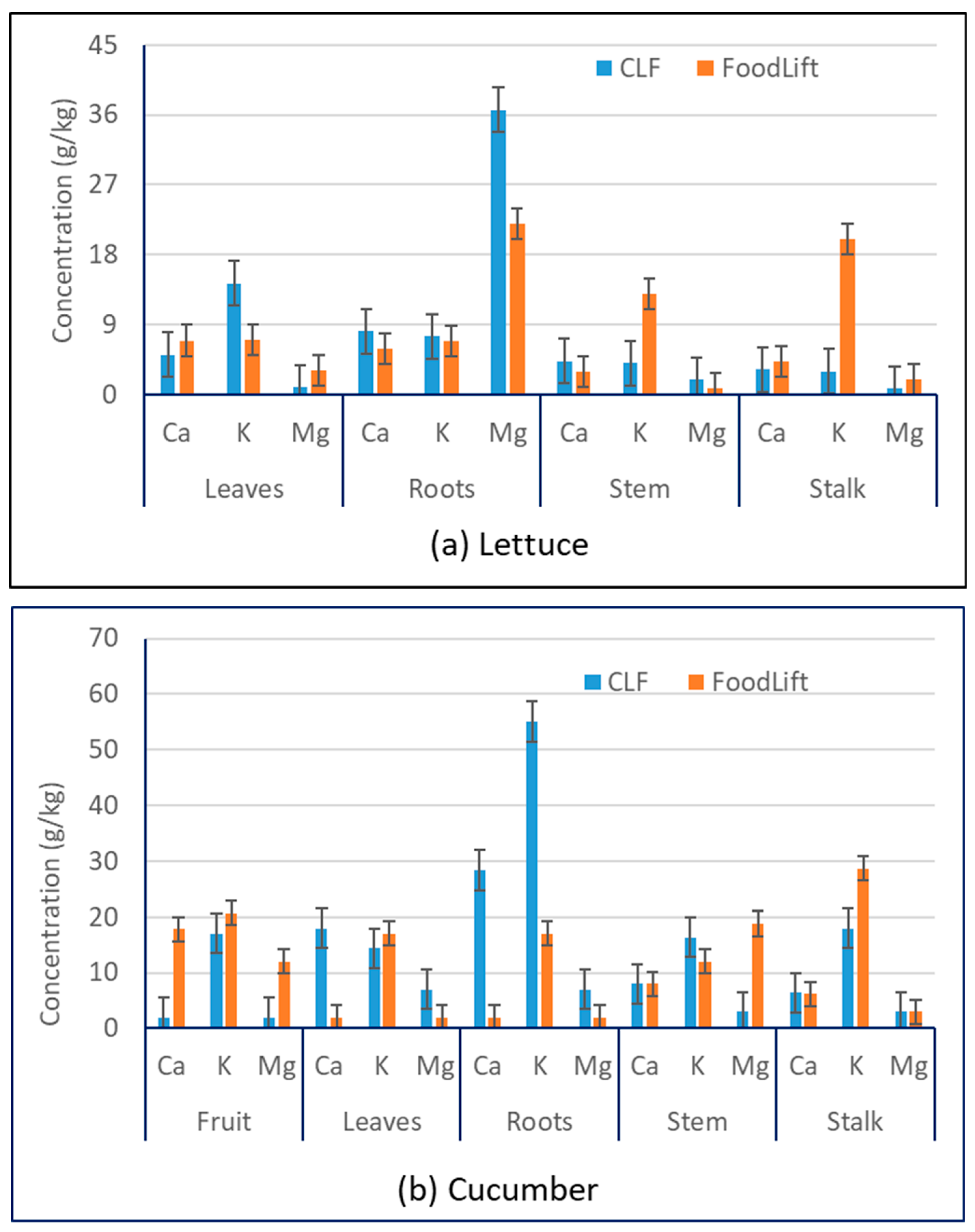
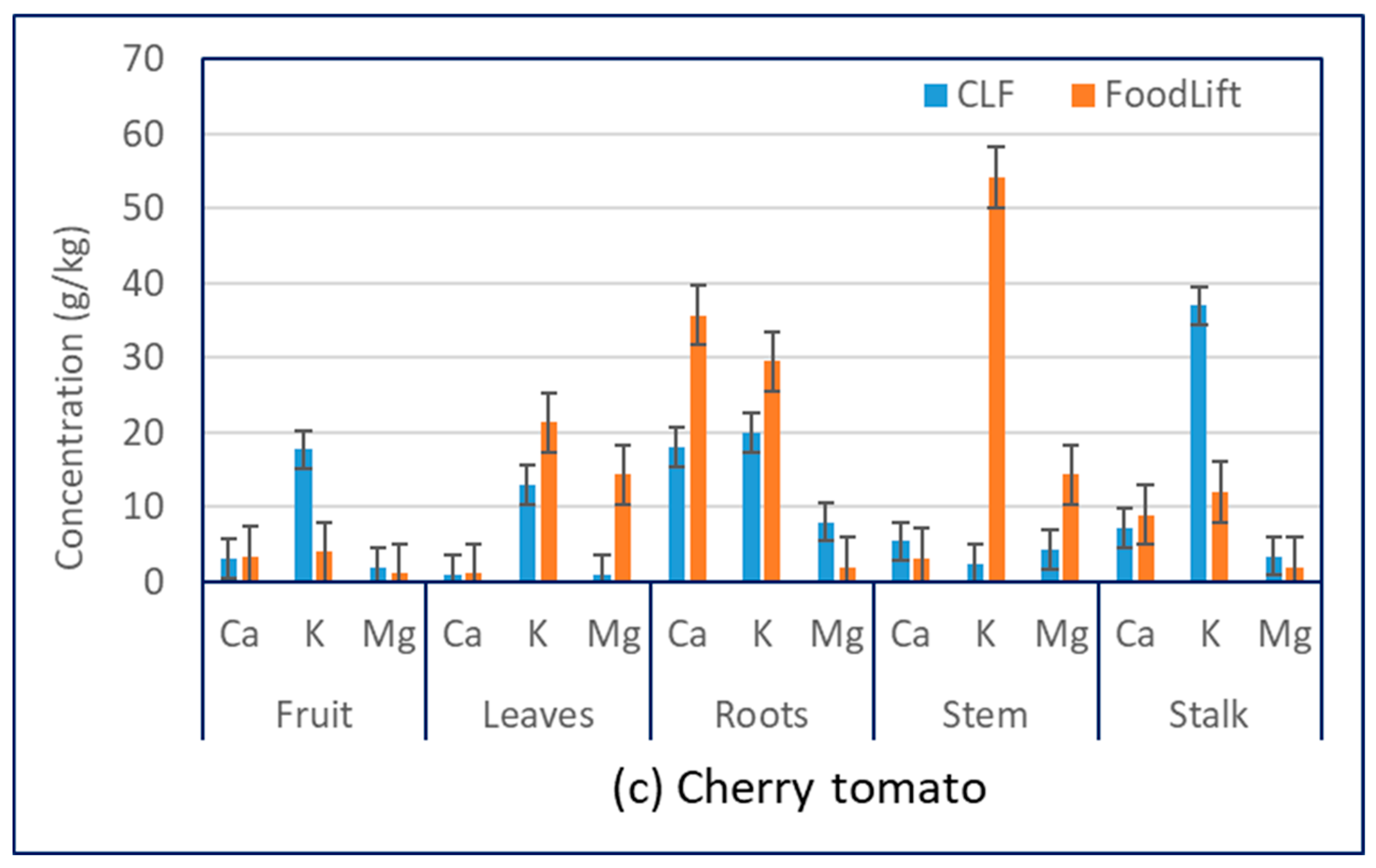
| Plant | Structural Part | N, g/kg | P, g/kg | Ca, g/kg | Mg, g/kg | K, g/kg | Reference |
|---|---|---|---|---|---|---|---|
| Lettuce | Leaf | 3 | 8.6 | Delaide et al. [36] | |||
| Leaf | 5.7–6.7 | Sublett et al. [37] | |||||
| Continental cucumber | Leaf | 25–45 | 45–40 | 25–50 | 3–15 | 3–7 | Parker et al. [38] |
| Root | 37 | 10 | 11.5 | 2.9 | 27.6 | Sung et al. [39] | |
| Fruit | 27.3 | 9.6 | 2.1 | 2 | 21.8 | Colla et al. [40] | |
| Stem | 32.3 | 9.7 | 26.9 | 4 | 61 | Sung et al. [39] | |
| Cherry tomato | Leaf | 33.4 | 3.7 | 21.3 | 5.4 | 20.7 | Maia et al. [30] |
| Root | 24.5 | 6.3 | 4.3 | 6.9 | 18.7 | Maia et al. [30] | |
| Fruit | 29 | 22 | 31.9 | Maia et al. [30] | |||
| Stem | 28.8 | 4.4 | 8.4 | 3.8 | 22.2 | Maia et al. [30] |
| Parameters | Unit | Lettuce | Cucumber/Cherry Tomato | ||||
|---|---|---|---|---|---|---|---|
| FoodLift Feed Solution | CLF Feed Solution | Growing Stage | Flowering Stage | ||||
| FoodLift Feed Solution | CLF Feed Solution | FoodLift Feed Solution | CLF Feed Solution | ||||
| pH | 5.5 ± 0.3 | 5.5 ± 0.3 | 5.5 ± 0.3 a 6.5 ± 0.3 b | 5.5 ± 0.3 a 6.5 ± 0.3 b | 5.5 ± 0.3 a 6.5 ± 0.3 b | 5.5 ± 0.3 a 6.5 ± 0.3 b | |
| Water temperature | °C | 24 | 24 | 24 | 24 | 24 | 24 |
| Dissolved oxygen (DO) | mg/L | >7 | >7 | >7 | >7 | >7 | >7 |
| N | mg/L | 96 | 96 | 96 | 96 | 160 | 160 |
| P | mg/L | 38 | 40 | 38 | 40 | 64 | 160 |
| K | mg/L | 260 | 460 | 260 | 460 | 433 | 288 |
| Ca | mg/L | 81 | 70 | 81 | 70 | 162 | 140 |
| Mg | mg/L | 9 | 32 | 9 | 32 | 18 | 64 |
| Plant 1 | Plant 2 | Plant 3 | Plant 4 | Average (SD) | |
|---|---|---|---|---|---|
| FM yield for fruits grown using FoodLift, g/plant | 0 | 0 | 23 | 102 | 31 (48) |
| FM yield for fruits grown using CLF, g/plant | 33 | 339 | 308 | 428 | 277 (170) |
| Produce | Parts | Macronutrients and Cations | ||||
|---|---|---|---|---|---|---|
| N | P | Ca | K | Mg | ||
| Lettuce | Leaves | 0.39 | 0.35 | 0.048 * | 0.0007 *** | 0.016 * |
| Roots | 0.35 | 0.14 | 0.012 * | 0.16 | 0.0008 *** | |
| Stem | 0.38 | 0.178 | 0.001 ** | 0.0005 *** | 0.003 ** | |
| Stalk | 0.3 | 0.41 | 0.044 * | 0.00006 *** | 0.0013 ** | |
| Cucumber | Fruits | 0.47 | 0.3 | 0.001 ** | 0.060 | 0.0017 ** |
| Leaves | 0.43 | 0.28 | 0.0007 *** | 0.107 | 0.000002 *** | |
| Roots | 0.5 | 0.42 | 0.003 ** | 0.0006 *** | 0.0104 * | |
| Stem | 0.48 | 0.48 | 0.5 | 0.0167 * | 0.0055 ** | |
| Stalk | 0.44 | 0.15 | 0.35 | 0.043 * | 0.226 | |
| Tomato | Fruits | 0.33 | 0.15 | 0.28 | 0.00005 *** | 0.003 ** |
| Leaves | 0.46 | 0.042 * | 0.33 | 0.053 | 0.0068 ** | |
| Roots | 0.0007 *** | 0.22 | 0.0002 *** | 0.0019 ** | 0.0047 ** | |
| Stem | 0.012 * | 0.26 | 0.005 ** | 0.0004 *** | 0.012 * | |
| Stalk | 0.038 * | 0.44 | 0.028 * | 0.000003 *** | 0.022 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, Z.; Hagare, D.; Liu, M.-H.; Panatta, O.; Hussain, T.; Memon, S.; Noorani, A.; Chen, Z.-H. A Food Waste-Derived Organic Liquid Fertiliser for Sustainable Hydroponic Cultivation of Lettuce, Cucumber and Cherry Tomato. Foods 2023, 12, 719. https://doi.org/10.3390/foods12040719
Siddiqui Z, Hagare D, Liu M-H, Panatta O, Hussain T, Memon S, Noorani A, Chen Z-H. A Food Waste-Derived Organic Liquid Fertiliser for Sustainable Hydroponic Cultivation of Lettuce, Cucumber and Cherry Tomato. Foods. 2023; 12(4):719. https://doi.org/10.3390/foods12040719
Chicago/Turabian StyleSiddiqui, Zuhaib, Dharmappa Hagare, Min-Hang Liu, Orousa Panatta, Tanveer Hussain, Sheeraz Memon, Amber Noorani, and Zhong-Hua Chen. 2023. "A Food Waste-Derived Organic Liquid Fertiliser for Sustainable Hydroponic Cultivation of Lettuce, Cucumber and Cherry Tomato" Foods 12, no. 4: 719. https://doi.org/10.3390/foods12040719
APA StyleSiddiqui, Z., Hagare, D., Liu, M.-H., Panatta, O., Hussain, T., Memon, S., Noorani, A., & Chen, Z.-H. (2023). A Food Waste-Derived Organic Liquid Fertiliser for Sustainable Hydroponic Cultivation of Lettuce, Cucumber and Cherry Tomato. Foods, 12(4), 719. https://doi.org/10.3390/foods12040719








