Modulation Effects of Sargassum pallidum Extract on Hyperglycemia and Hyperlipidemia in Type 2 Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Extract from Sargassum Pallidum
2.3. Animals and Experimental Design
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Biochemical Analysis
2.6. Histopathological Examination
2.7. Determination of Antioxidant Parameters and Key Glycometabolism Enzymes
2.8. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR) Assay
2.9. Cecal Microbiota Analysis
2.10. Untargeted Metabolome Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Composition of SPPE
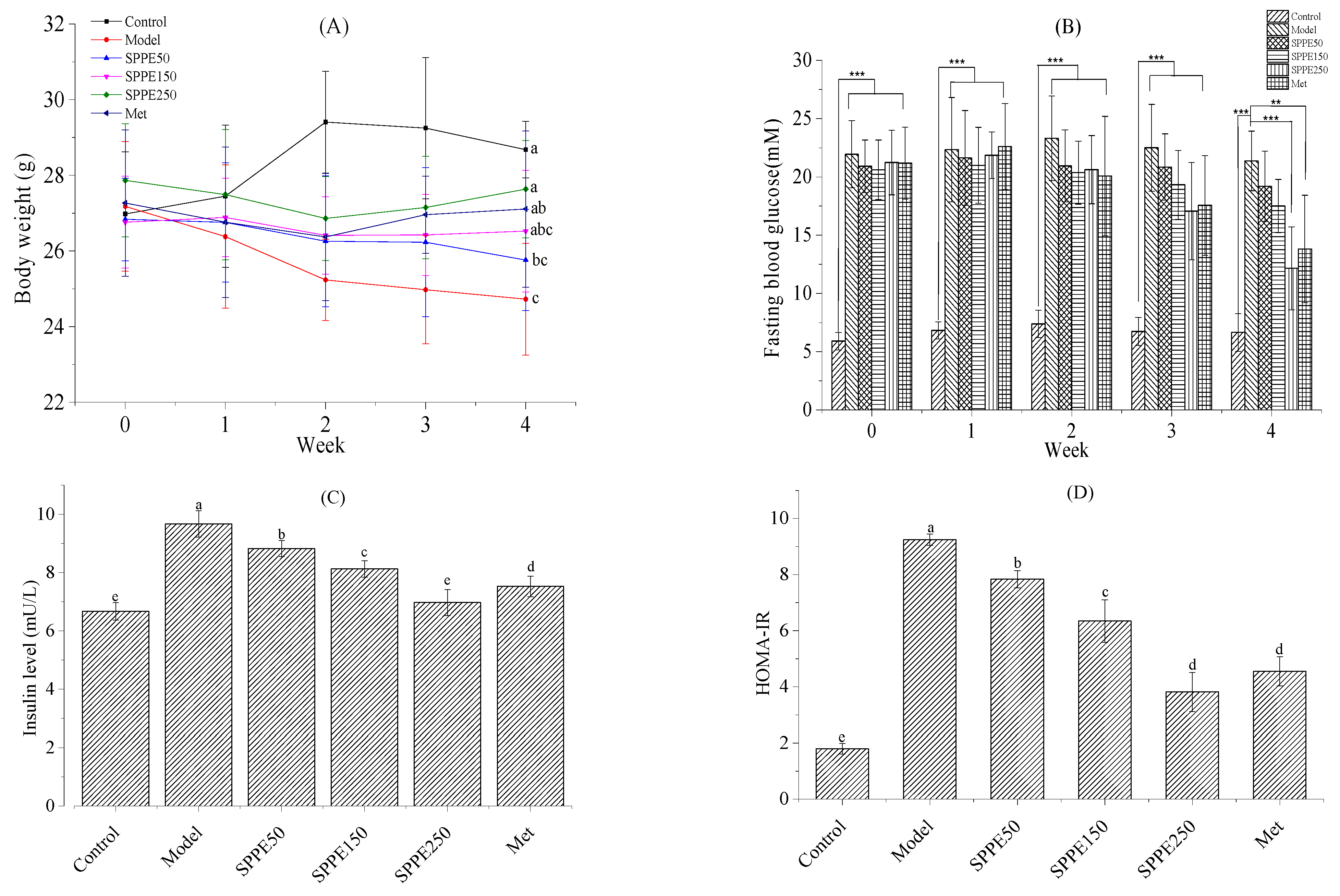
3.2. Effect of SPPE on Body Weight
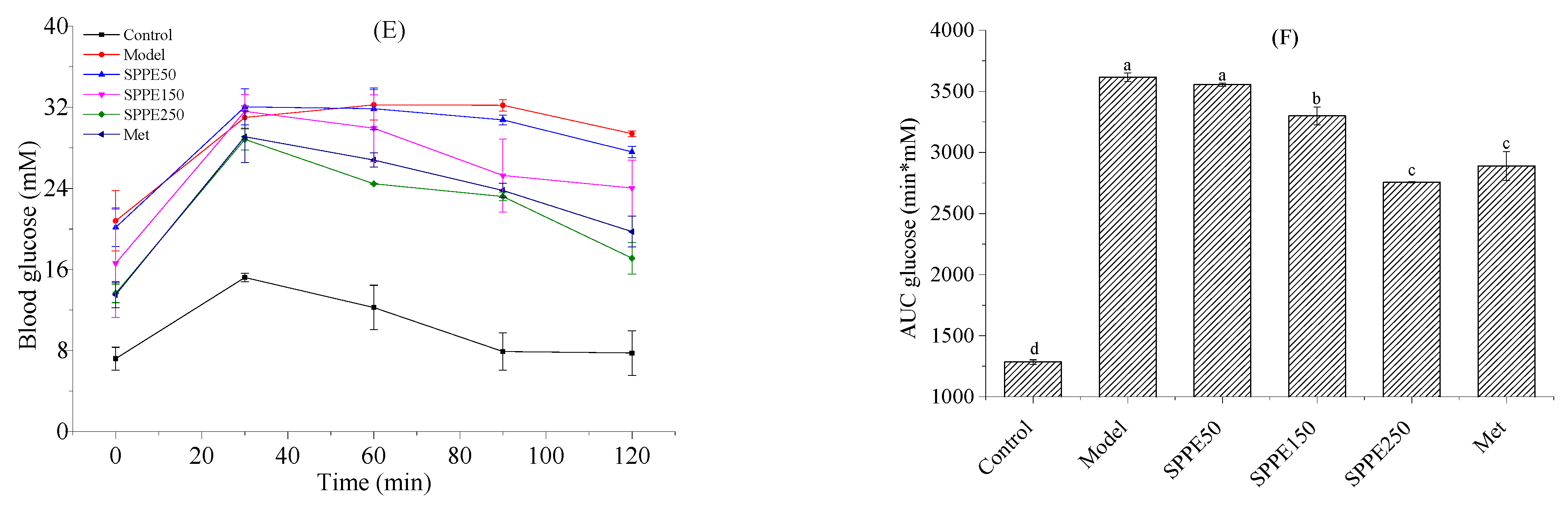
3.3. Effect of SPPE on Glucose Homeostasis
3.4. Effect of SPPE on Serum and Hepatic Lipid Profiles
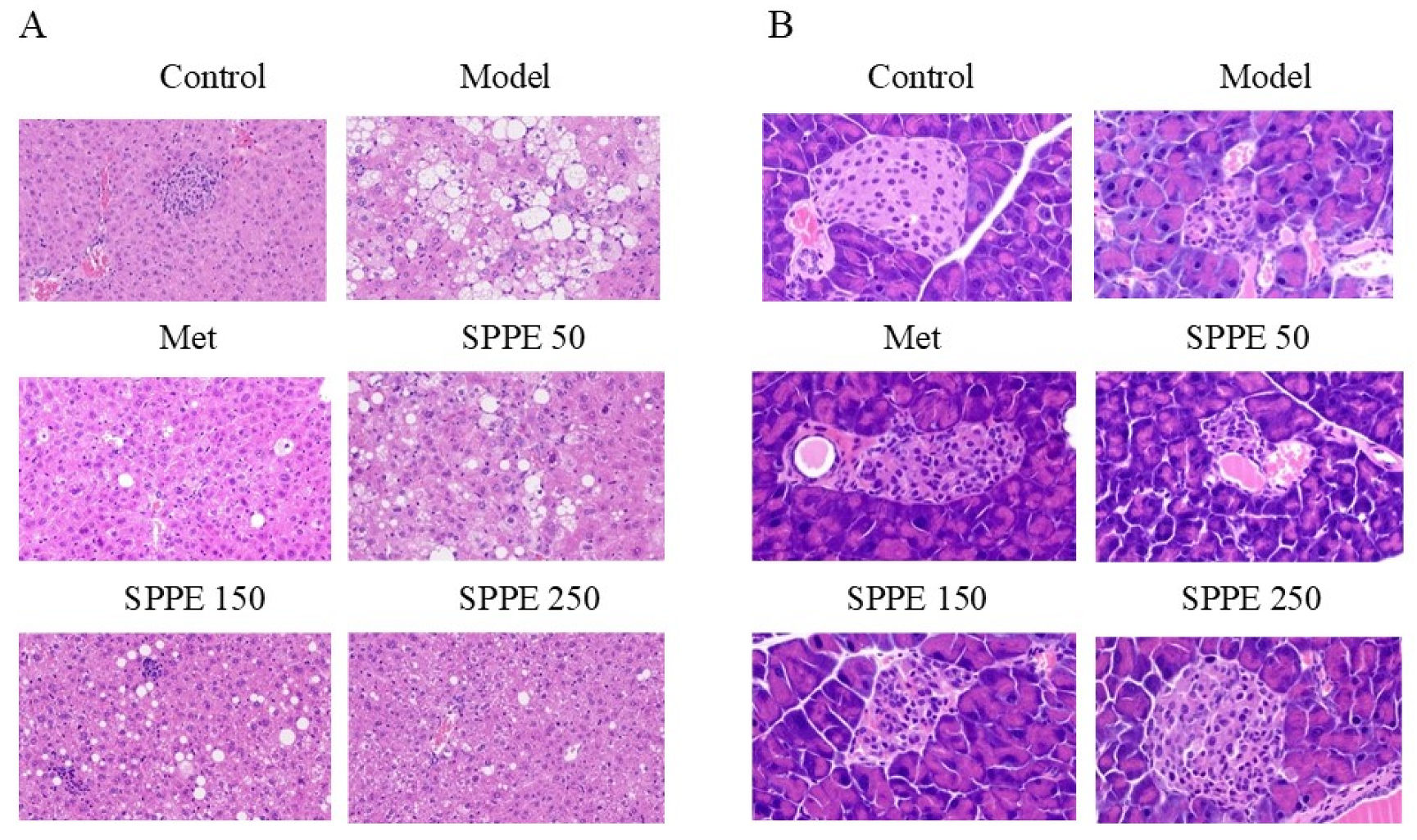
3.5. Effect of SPPE on the Histomorphology of Liver and Pancreas Tissues
3.6. Effect of SPPE on AST, ALT and Oxidative Stress
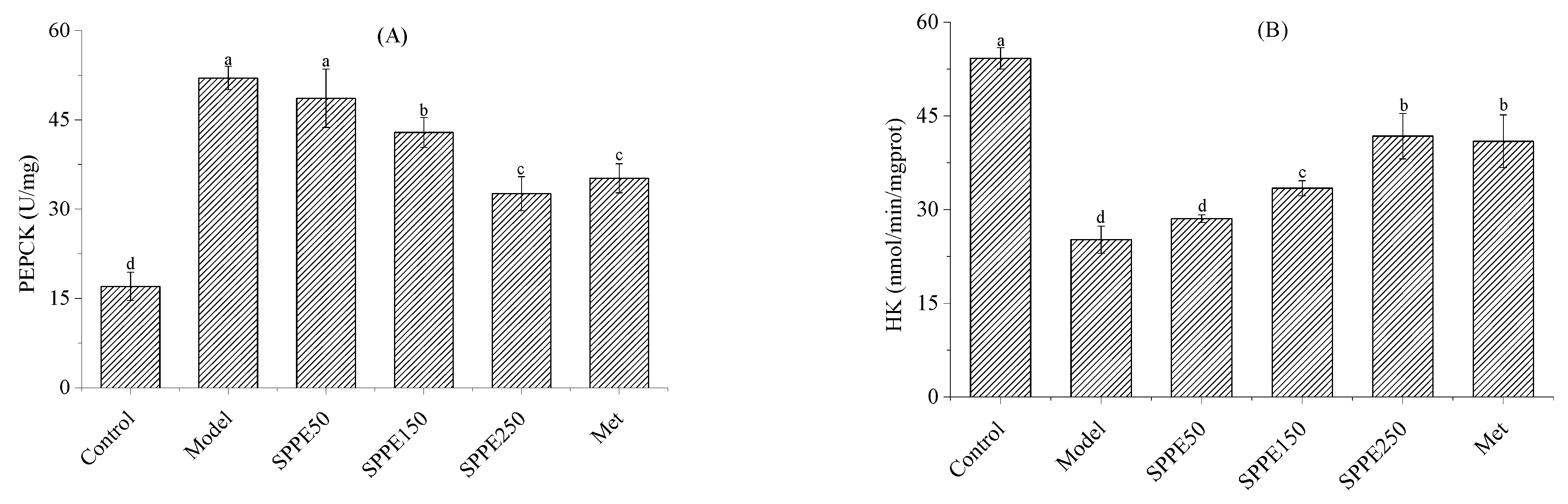
3.7. Effect of SPPE on Activities of PEPCK and HK
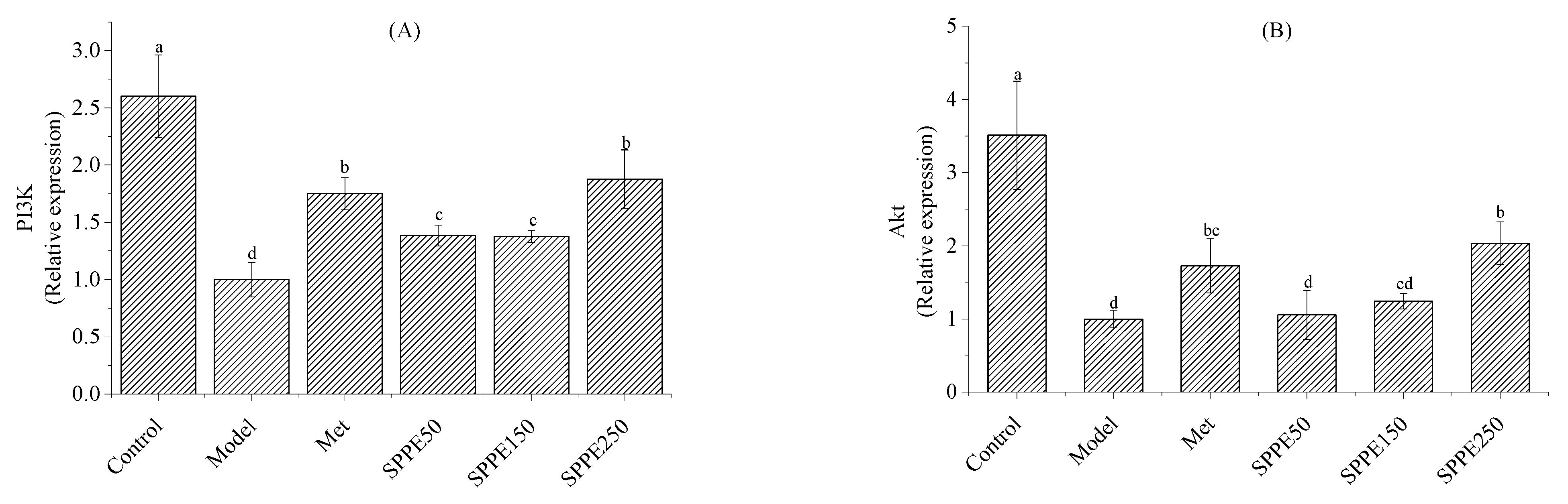
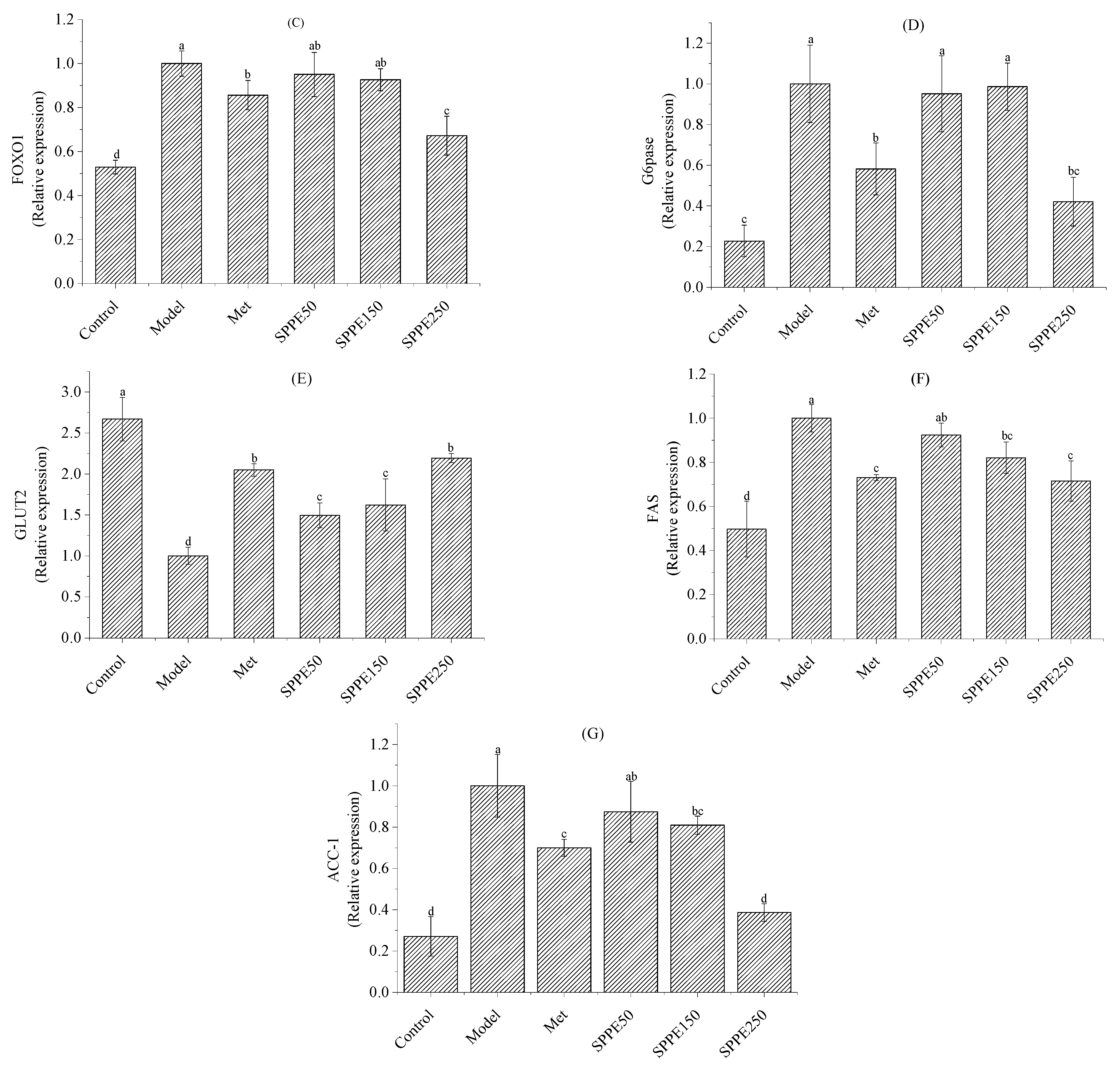
3.8. Effect of SPPE on mRNA Expression of Glucose and Lipid Metabolism Genes
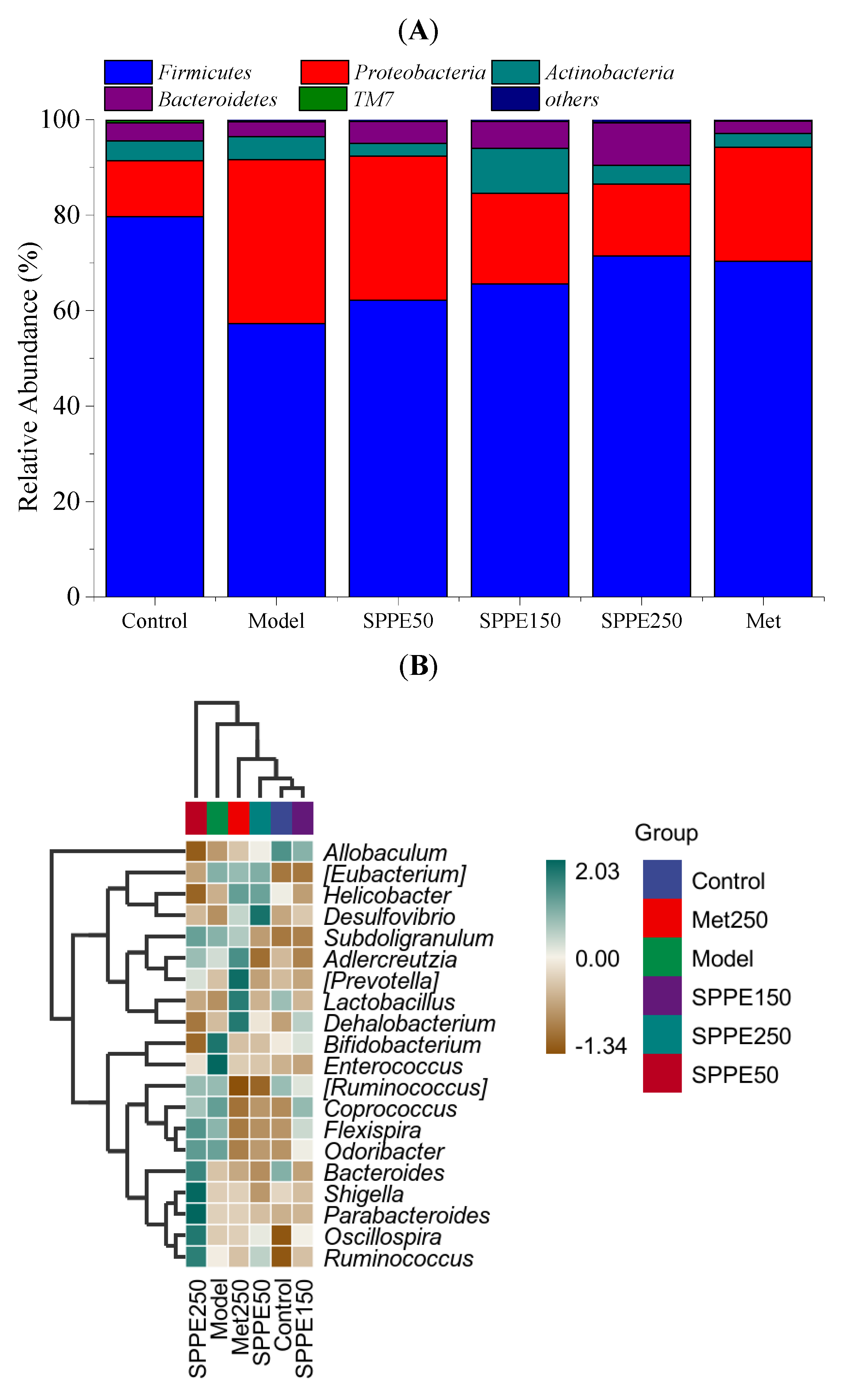
3.9. Effect of SPPE on Gut Microbiota
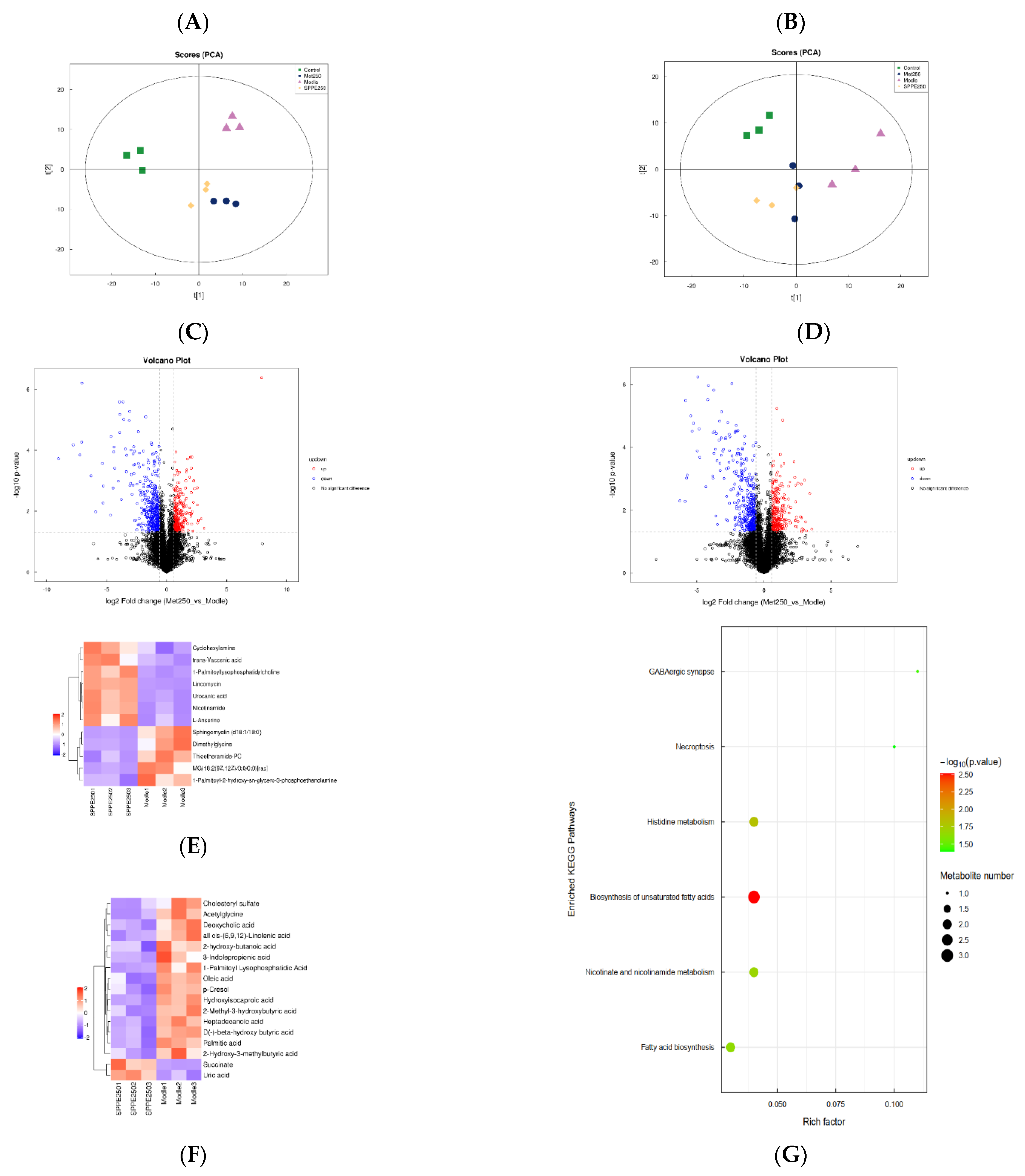
3.10. Effect of SPPE on Serum Metabolites
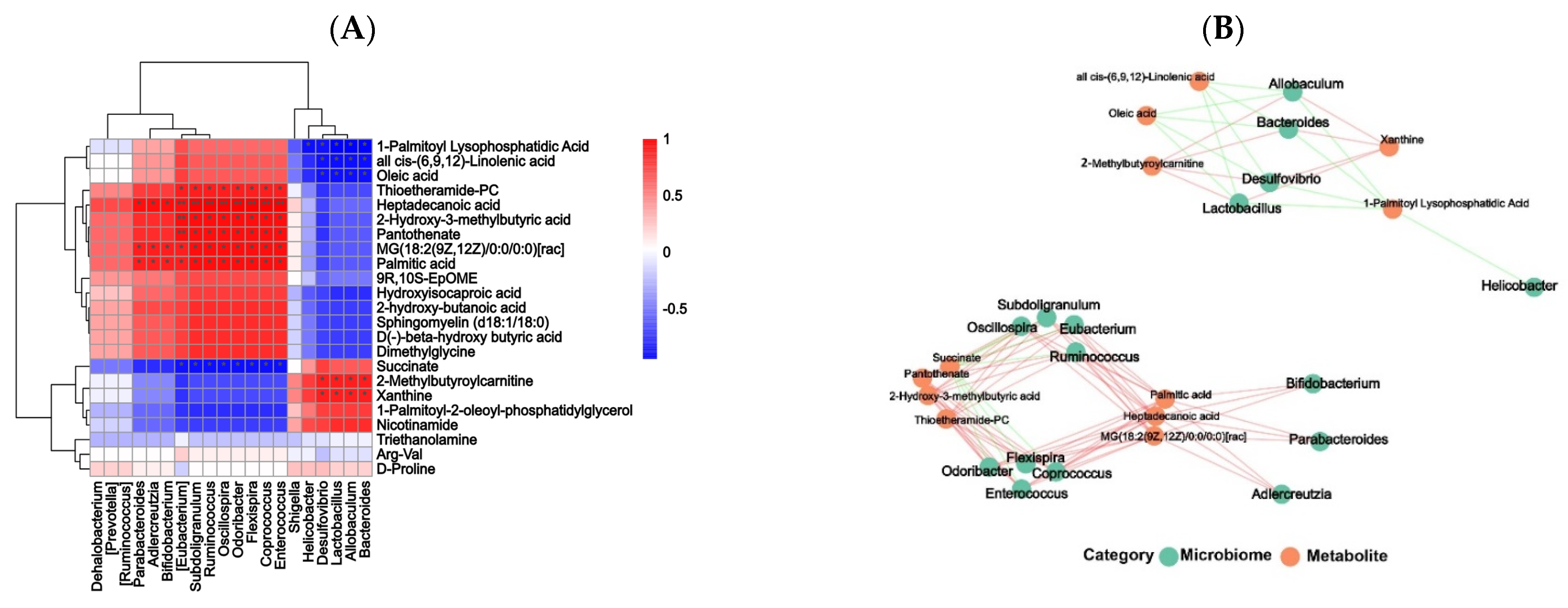
3.11. Correlation Analysis between Gut Microbiota and Differential Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Fu, X. Spheroidization on Fructus Mori polysaccharides to enhance bioavailability and bioactivity by anti-solvent precipitation method. Food Chem. 2019, 300, 125245. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-P.; Wang, W.-D.; Chen, C.; Fu, X.; Liu, R.-H. Effect of Fructus Mori. bioactive polysaccharide conjugation on improving functional and antioxidant activity of whey protein. Int. J. Biol. Macromol. 2020, 148, 761–767. [Google Scholar]
- Tran, H.G.; Vu, M.T.; Nguyen, V.; Hoang, T.M.H.; Ngo, T.H.T.; Phan, T.H.; Nguyen, X.N.; Nguyen, T.M.H. Two new neolignans from the roots of Platycodon grandiflorus and their hypolipidemic effects. Phytochem. Lett. 2023, 57, 167–171. [Google Scholar] [CrossRef]
- Dou, Z.-M.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Guo, R.; Zheng, B.; Li, T.; Liu, R.H. IRS-1/PI3K/Akt pathway and miRNAs are involved in whole grain highland barley (Hordeum vulgare L.) ameliorating hyperglycemia of db/db mice. Food Funct. 2020, 11, 9535–9546. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, R.H.; Kamel, E.M.; Mahmoud, A.M.; El-Bassuony, A.A.; Bin-Jumah, M.; Lamsabhi, A.M.; Ahmed, S.A. Rumex dentatus L. phenolics ameliorate hyperglycemia by modulating hepatic key enzymes of carbohydrate metabolism, oxidative stress and PPARγ in diabetic rats. Food Chem. Toxicol. 2020, 138, 111202. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Liu, R.H. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: Characterization and hypoglycemic activity. Ind. Crops Prod. 2017, 100, 1–11. [Google Scholar] [CrossRef]
- Zhao, C.; Wan, X.; Zhou, S.; Cao, H. Natural Polyphenols: A Potential Therapeutic Approach to Hypoglycemia. eFood 2020, 1, 107–118. [Google Scholar] [CrossRef]
- Kang, M.-C.; Wijesinghe, W.A.J.P.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef]
- Lv, Y.; Hao, J.; Liu, C.; Huang, H.; Ma, Y.; Yang, X.; Tang, L. Anti-diabetic effects of a phenolic-rich extract from Hypericum attenuatum Choisy in KK-Ay mice mediated through AMPK /PI3K/Akt/GSK3β signaling and GLUT4, PPARγ, and PPARα expression. J. Funct. Foods 2019, 61, 103506. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, Q.; Zhang, J.; Liu, S.; Li, C.; Wang, L. Glycolipid Metabolism and Metagenomic Analysis of the Therapeutic Effect of a Phenolics-Rich Extract from Noni Fruit on Type 2 Diabetic Mice. J. Agric. Food Chem. 2022, 70, 2876–2888. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, B.; Li, C.; Huang, Q.; Fu, X.; Liu, R.H. Structure and in vitro hypoglycemic activity of a homogenous polysaccharide purified from Sargassum pallidum. Food Funct. 2019, 10, 2828–2838. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, C.; You, L.; Dong, H.; Fu, X. Changes of digestive and fermentation properties of Sargassum pallidum polysaccharide after ultrasonic degradation and its impacts on gut microbiota. Int. J. Biol. Macromol. 2020, 164, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, C.; Fu, X. Screening α-glucosidase inhibitors from four edible brown seaweed extracts by ultra-filtration and molecular docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Chun, C.; Qiang, H.; Xiong, F.; Rui-Hai, L. Investigation into the mechanisms of quercetin-3-O-glucuronide inhibiting α-glucosidase activity and non-enzymatic glycation by spectroscopy and molecular docking. Food Funct. 2021, 12, 7825–7835. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.-J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.-H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Fan, D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019, 10, 2538–2551. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572–3585. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, Z.; Zhao, Y.; Kang, C.; Zhang, X.; Tian, Y.; Yu, J.; Cao, H.; Wang, M. The anti-diabetic activity of polyphenols-rich vinegar extract in mice via regulating gut microbiota and liver inflammation. Food Chem. 2022, 393, 133443. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, D.; Chen, J.; Li, Y.-H.; Zhang, Z.; Hidayat, K.; Wan, Z.; Xu, J.-Y.; Qin, L.-Q. Lactoferrin improves hepatic insulin resistance and pancreatic dysfunction in high-fat diet and streptozotocin-induced diabetic mice. Nutr. Res. 2022, 103, 47–58. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Sweet potato (Ipomoea batatas L.) leaf polyphenols ameliorate hyperglycemia in type 2 diabetes mellitus mice. Food Funct. 2021, 12, 4117–4131. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zuo, J.; Cheng, Y.; Zhang, Y.; Zhang, Z.; Wu, M.; Yang, Y.; Tong, H. Ethanol extract of Sargarsum fusiforme alleviates HFD/STZ-induced hyperglycemia in association with modulation of gut microbiota and intestinal metabolites in type 2 diabetic mice. Food Res. Int. 2021, 147, 110550. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yamasaki, M.; Katsube, T.; Shiwaku, K. Effects of quercetin derivatives from mulberry leaves: Improved gene expression related hepatic lipid and glucose metabolism in short-term high-fat fed mice. Nutr. Res. Pract. 2014, 9, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Xu, N.; Zhang, X.-C.; Zhu, Y.-Y.; Liu, S.-W.; Chang, Y.-N. Chrysin Improves Glucose and Lipid Metabolism Disorders by Regulating the AMPK/PI3K/AKT Signaling Pathway in Insulin-Resistant HepG2 Cells and HFD/STZ-Induced C57BL/6J Mice. J. Agric. Food Chem. 2021, 69, 5618–5627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, L.; Dong, D.; Xu, L.; Yin, L.; Qi, Y.; Han, X.; Lin, Y.; Liu, K.; Peng, J. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013, 141, 2108–2116. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, F.G.; Zhang, W.S.; Pan, A.; Yang, Y.L.; Liu, J.F.; Li, P.; Liu, B.L.; Qi, L.W. Ginsenoside Rg1 Inhibits Glucagon-Induced Hepatic Gluconeogenesis through Akt-FoxO1 Interaction. Theranostics 2017, 7, 4001–4012. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. A New Possible Mechanism by Which Punicalagin Protects against Liver Injury Induced by Type 2 Diabetes Mellitus: Upregulation of Autophagy via the Akt/FoxO3a Signaling Pathway. J. Agric. Food Chem. 2019, 67, 13948–13959. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.-H.; Shon, M.-Y.; Kong, R.; Seo, Y.-S.; Zhou, T.; Kim, D.-Y.; Kim, Y.-S.; Kwon, D.-Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement. Altern. Med. 2017, 17, 341. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, M.; Zhang, R.; You, L.; Li, T.; Liu, R.H. Whole Grain Brown Rice Extrudate Ameliorates the Symptoms of Diabetes by Activating the IRS1/PI3K/AKT Insulin Pathway in db/db Mice. J. Agric. Food Chem. 2019, 67, 11657–11664. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Kucuk, O.; Tuzcu, M.; Sahin, N.; Ojalvo, S.P.; Komorowski, J. Effects of Magnesium Biotinate Supplementation on Serum Insulin, Glucose, and Lipid Parameters Along with Gene Expressions of Intermediary Metabolism in Rats. Res. Sq. 2020, 34, 9–19. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, A.; Sikdar, S.; Paul, A.; Ghosh, S.; Khuda-Bukhsh, A.R. [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol. Lett. 2012, 210, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kashyap, D.; Sak, K.; Tuli, H.S.; Sharma, A.K. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm. Pat. Anal. 2018, 7, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Irii, H.; Tahara, Y.; Ishii, H.; Hirao, A.; Udagawa, H.; Hiramoto, M.; Yasuda, K.; Takanishi, A.; Shibata, S.; et al. Synthesis of a New [6]-Gingerol Analogue and Its Protective Effect with Respect to the Development of Metabolic Syndrome in Mice Fed a High-Fat Diet. J. Med. Chem. 2011, 54, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Horiuchi, Y.; Hironao, K.-Y.; Kitakaze, T.; Yamashita, Y.; Ashida, H. Prevention effect of quercetin and its glycosides on obesity and hyperglycemia through activating AMPKα in high-fat diet-fed ICR mice. J. Clin. Biochem. Nutr. 2020, 67, 75–83. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Jeong, M.-Y.; Jang, H.-M.; Kim, D.-H. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci. Lett. 2019, 698, 51–57. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Jiang, Y.; Pan, R.; Zhou, Y.; Wu, S.; Wang, R.; Zhuang, K.; Zhang, W.; Li, T.; Man, C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-R.; Lin, C.-S.; Chang, C.-J.; Lin, T.-L.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Lu, C.-C.; Young, J.D.; Lai, H.-C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Germani, Y.; Dauga, C.; Duval, P.; Huerre, M.; Levy, M.; Pialoux, G.; Sansonetti, P.; Grimont, P.A.D. Strategy for the detection of Helicobacter species by amplification of 16S rRNA genes and identification of H. felis in a human gastric biopsy. Res. Microbiol. 1997, 148, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Ren, D.; Li, T.; Niu, P.; Zhang, X.; Yang, X.; Xiao, J. Fu Brick Tea Manages HFD/STZ-Induced Type 2 Diabetes by Regulating the Gut Microbiota and Activating the IRS1/PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2022, 70, 8274–8287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Q.; Guo, S.; Lu, Y.-Y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.-X.; Wang, H.-Q.; Zhang, W.-H.; Duan, J.-A. Lycium barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother. 2020, 121, 109559. [Google Scholar] [CrossRef]
- Yu, B.; Li, A.H.; Muzny, D.; Veeraraghavan, N.; Vries, P.S.D.; Bis, J.C.; Musani, S.K.; Alexander, D.; Morrison, A.C.; Franco, O.H.; et al. Association of Rare Loss-Of-Function Alleles in HAL, Serum Histidine. Circ. Cardiovasc. Genet. 2015, 8, 351–355. [Google Scholar] [CrossRef]
- Yun, H.; Sun, L.; Wu, Q.; Zong, G.; Qi, Q.; Li, H.; Zheng, H.; Zeng, R.; Liang, L.; Lin, X. Associations among circulating sphingolipids, β-cell function, and risk of developing type 2 diabetes: A population-based cohort study in China. PLoS Med. 2020, 17, e1003451. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Li, J.; Liu, W.; Warda, M.; Cui, B.; El-Aty, A.M.A. Oligosaccharides derived from Lycium barbarum ameliorate glycolipid metabolism and modulate the gut microbiota community and the faecal metabolites in a type 2 diabetes mouse model: Metabolomic bioinformatic analysis. Food Funct. 2022, 13, 5416–5429. [Google Scholar] [CrossRef]
- Svingen, G.F.T.; Schartum-Hansen, H.; Pedersen, E.R.; Ueland, P.M.; Tell, G.S.; Mellgren, G.; Njølstad, P.R.; Seifert, R.; Strand, E.; Karlsson, T.; et al. Prospective Associations of Systemic and Urinary Choline Metabolites with Incident Type 2 Diabetes. Clin. Chem. 2016, 62, 755–765. [Google Scholar] [CrossRef]
- Yang, Z.; Dan, W.; Li, Y.; Zhou, X.; Liu, T.; Shi, C.; Li, R.; Zhang, Y.; Zhang, J.; Yan, J.; et al. Untargeted metabolomics analysis of the anti-diabetic effect of Red ginseng extract in Type 2 diabetes Mellitus rats based on UHPLC-MS/MS. Biomed. Pharmacother. 2022, 146, 112495. [Google Scholar] [CrossRef]

| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| PI3K | sense | ACACCACGGTTTGGACTATGG |
| antisense | GGCTACAGT AGTGGGCTTGG | |
| Akt | sense | GCCGGTGACAGACGATACT |
| antisense | TGGCATTCACGTTTGTGGAGC | |
| FOXO1 | sense | GAGTTAGTGAGCAGGCTACAT |
| antisense | TTTGGACTGCTCCTCAGTTCC | |
| G6pase | sense | GGAGTCTTGTCAGGCATTGCT |
| antisense | CGGAGGCTGGCATTGTAGAT | |
| GLUT2 | sense | GATCGCTCCAACCACACTCA |
| antisense | CTGAGGCCAGCAA TC TGACTA | |
| ACC-1 | sense | CGCCAACAATGGTATTGCAGC |
| antisense | TCG GATTGCACGTTCATTTCG | |
| FAS | sense | GCGGGTTCGTGAAACTGATAA |
| antisense | GCAAAATGGGCCTCCTTGATA | |
| β-Actin | sense | GATCGATGCCGGTGCTAAGA |
| antisense | TCCTATGGGAGAACGGCAGA |
| Group | Serum TG (mmol/L) | Serum TC (mmol/L) | Serum HDL-c (mmol/L) | Serum LDL-c (mmol/L) | Hepatic TG (mmol/L) | Hepatic TC (mmol/L) |
|---|---|---|---|---|---|---|
| Control | 0.98 ± 0.05 d | 2.85 ± 0.22 d | 2.33 ± 0.10 a | 0.50 ± 0.03 cd | 0.26 ± 0.01 e | 0.13 ± 0.01 e |
| Model | 3.36 ± 0.06 a | 6.37 ± 0.37 a | 0.82 ± 0.02 f | 0.78 ± 0.05 a | 0.50 ± 0.01 a | 0.40 ± 0.01 a |
| SPPE50 | 2.71 ± 0.12 b | 5.68 ± 0.30 b | 1.15 ± 0.03 e | 0.70 ± 0.13 ab | 0.44 ± 0.03 b | 0.29 ± 0.03 b |
| SPPE150 | 1.42 ± 0.15 c | 5.10 ± 0.01 b | 1.42 ± 0.02 d | 0.63 ± 0.08 bc | 0.38 ± 0.02 c | 0.22 ± 0.02 c |
| SPPE250 | 0.95 ± 0.06 d | 4.06 ± 0.07 c | 1.98 ± 0.07 b | 0.47 ± 0.08 d | 0.26 ± 0.03 e | 0.16 ± 0.01 d |
| Met | 1.34 ± 0.11 c | 4.36 ± 0.19 c | 1.76 ± 0.14 c | 0.52 ± 0.02 cd | 0.32 ± 0.01 d | 0.18 ± 0.03 d |
| Group | Index | |
|---|---|---|
| Chao | Shannon | |
| Control | 767.08 ± 35.34 c | 5.20 ± 0.25 b |
| Model | 916.16 ± 65.12 b | 6.19 ± 0.23 a |
| SPPE50 | 952.16 ± 38.82 b | 5.90 ± 0.15 a |
| SPPE150 | 966.26 ± 48.21 b | 5.97 ± 0.21 a |
| SPPE250 | 1004.20 ± 55.62 a | 6.72 ± 0.31 a |
| Met | 652.75 ± 45.32 d | 4.60 ± 0.18 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Chen, C.; Fu, X. Modulation Effects of Sargassum pallidum Extract on Hyperglycemia and Hyperlipidemia in Type 2 Diabetic Mice. Foods 2023, 12, 4409. https://doi.org/10.3390/foods12244409
Xie X, Chen C, Fu X. Modulation Effects of Sargassum pallidum Extract on Hyperglycemia and Hyperlipidemia in Type 2 Diabetic Mice. Foods. 2023; 12(24):4409. https://doi.org/10.3390/foods12244409
Chicago/Turabian StyleXie, Xing, Chun Chen, and Xiong Fu. 2023. "Modulation Effects of Sargassum pallidum Extract on Hyperglycemia and Hyperlipidemia in Type 2 Diabetic Mice" Foods 12, no. 24: 4409. https://doi.org/10.3390/foods12244409
APA StyleXie, X., Chen, C., & Fu, X. (2023). Modulation Effects of Sargassum pallidum Extract on Hyperglycemia and Hyperlipidemia in Type 2 Diabetic Mice. Foods, 12(24), 4409. https://doi.org/10.3390/foods12244409








