The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Apples
2.2. Chemicals
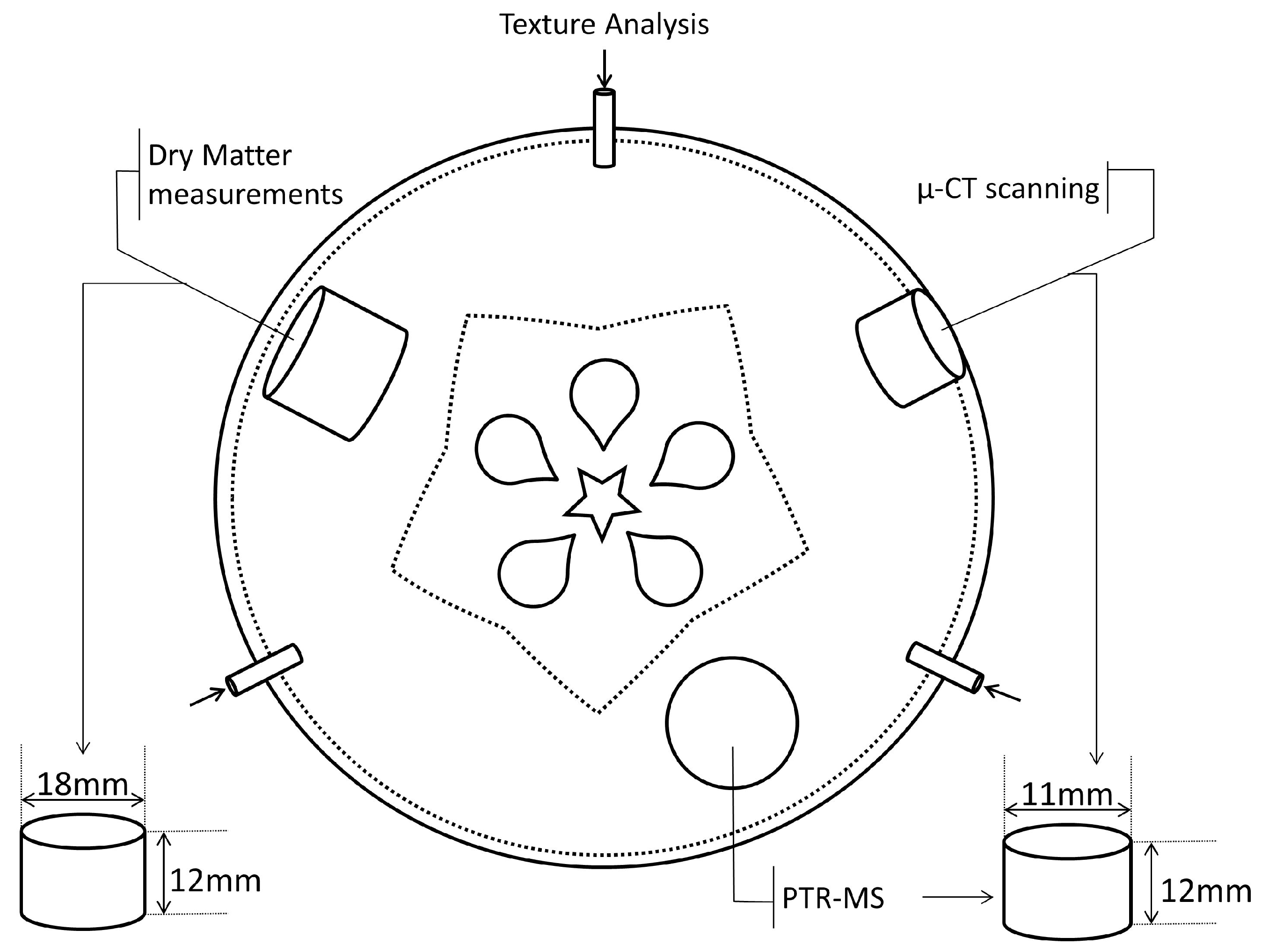
2.3. Texture Analyzer
2.4. Soluble Solids Content and Dry Matter Concentration
2.5. Headspace Volatile Organic Compound Measurement
2.6. X-ray Micro-CT Scanning and Image Analysis
2.7. Data Analysis
2.7.1. Headspace Analysis
2.7.2. Statistical Analysis
3. Results
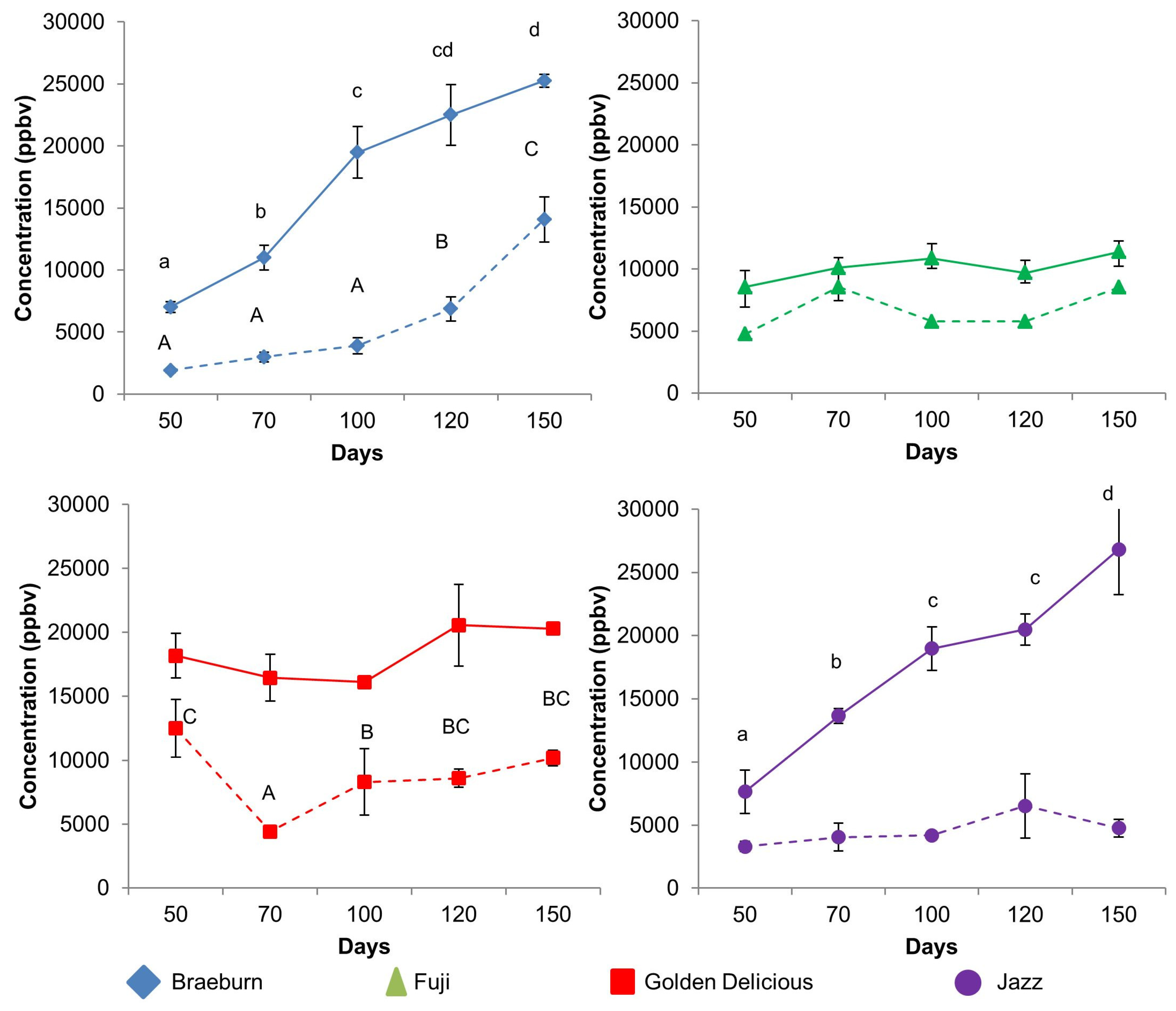
3.1. Headspace Analysis of VOCs from Fresh Cut Cylinders
3.1.1. Comparing Untreated and SF-Treated Apples
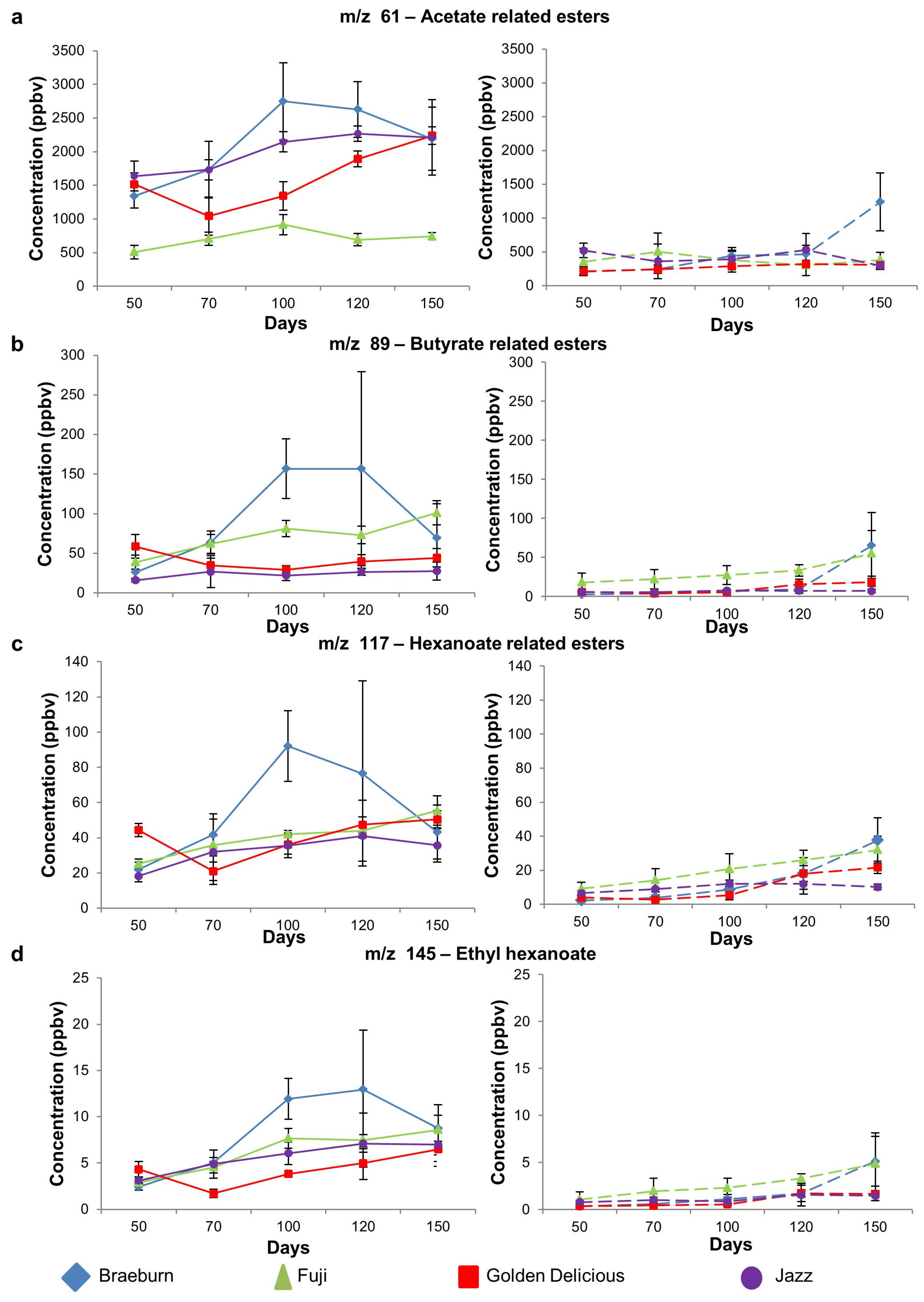
3.1.2. Ester Compounds
3.1.3. Aldehyde Compounds
3.1.4. Alcohol Compounds
3.2. Texture, Physico-Chemical and µ-CT Results
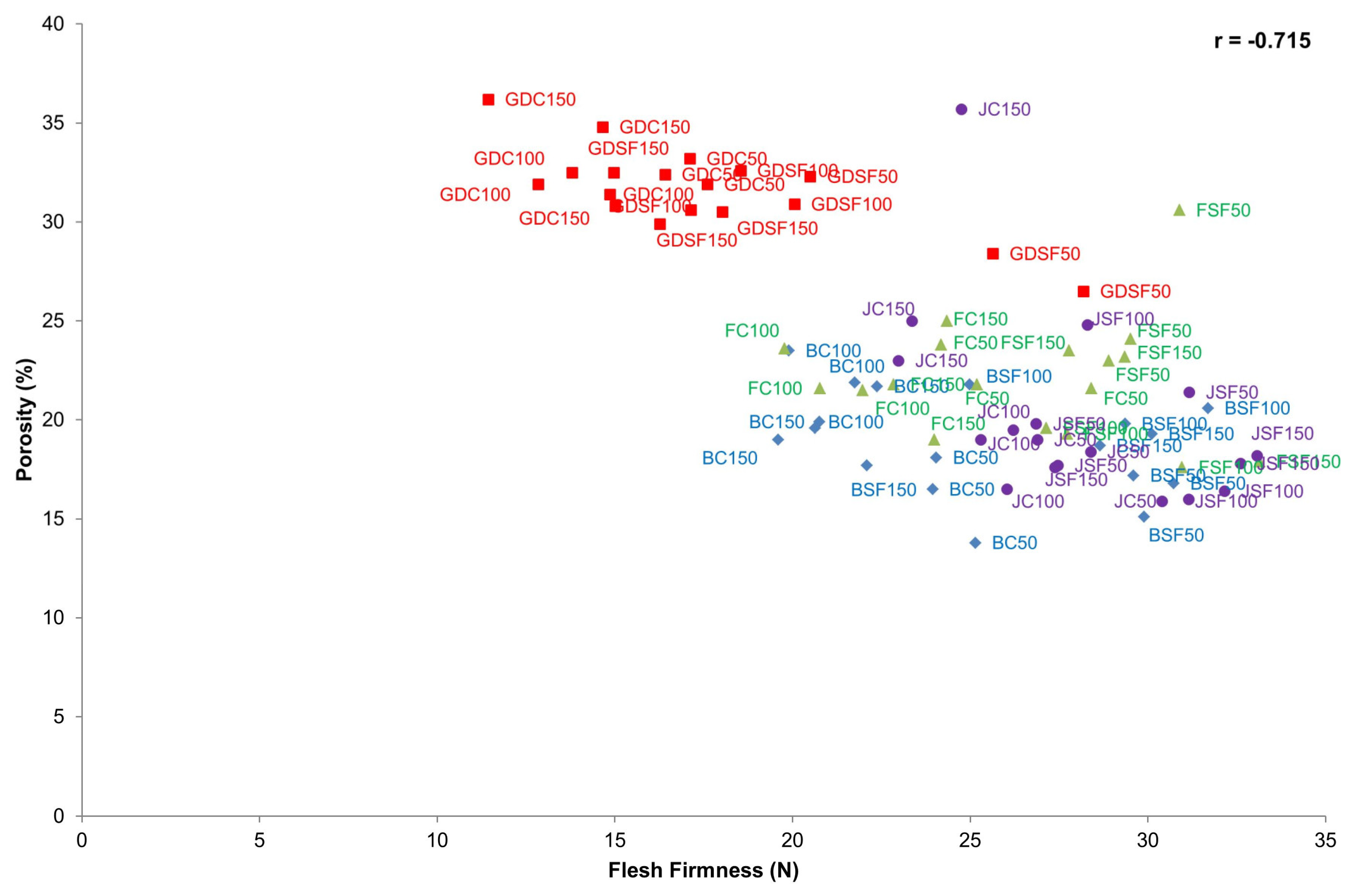
3.2.1. Relation between Porosity and Flesh Firmness
3.2.2. The Relationship between Connectivity and Anisotropy to Porosity
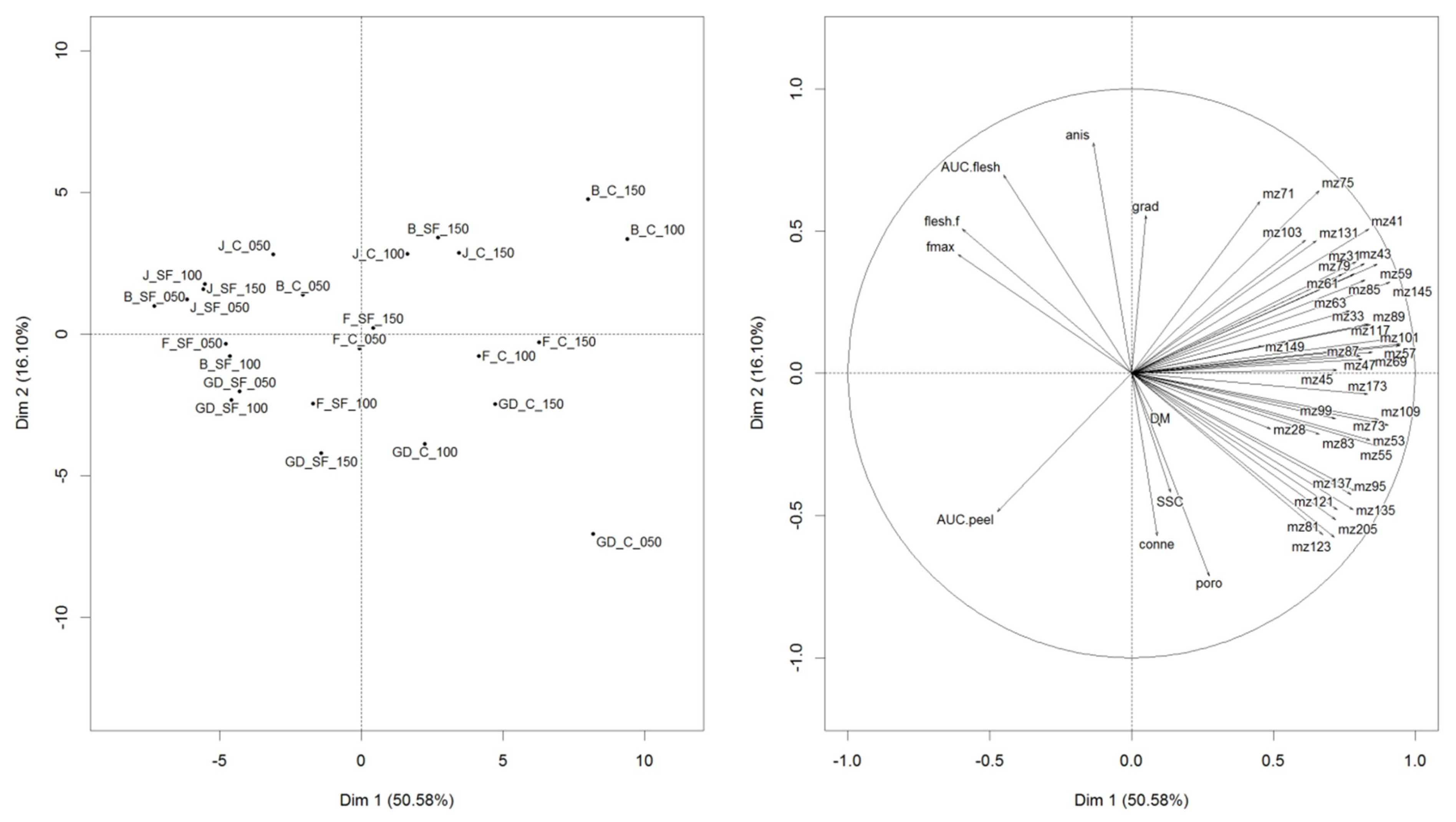
3.3. Understanding the Inter-Relationships between VOC Release, Texture, Physico-Chemical, and Morphological Properties
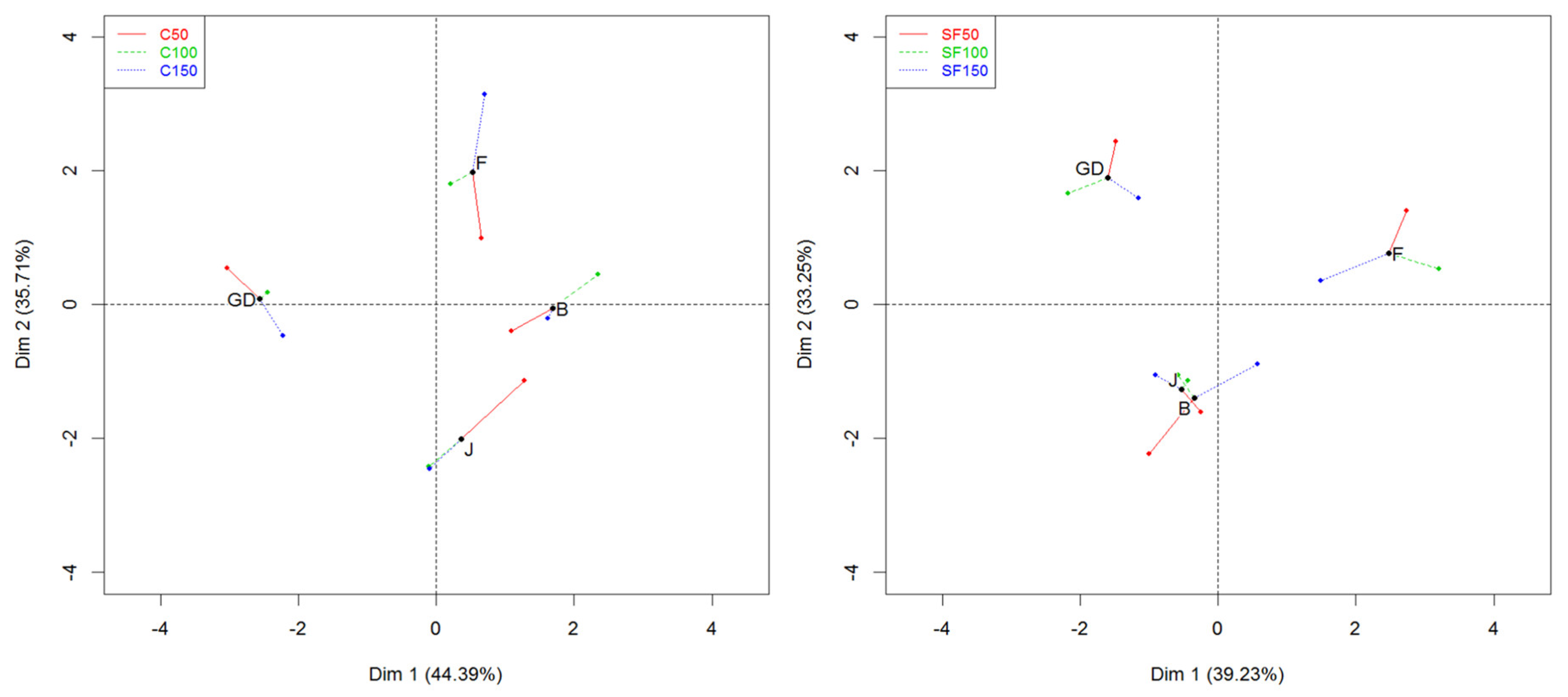
3.4. MFA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.A.T.M. Postharvest softening of apple (Malus domestica) fruit: A review. N. Z. J. Crop Hortic. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- Barry, C.; Giovannoni, J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- DeEll, J.R.; Khanizadeh, S.; Saad, F.; Ferree, D.C. Factors affecting apple fruit firmness—A review. J. Am. Pomol. Soc. 2001, 55, 8–27. [Google Scholar]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.L.; Morris, J.R. Temperature Effects on Produce Degradation. In Produce Degradation; CRC Press: Boca Raton, FL, USA, 2005; pp. 599–647. [Google Scholar]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Schaffer, R.J.; Cohen, D.; Gleave, A.P.; Crowhurst, R.N.; Janssen, B.J.; Yao, J.-L.; Newcomb, R.D.; Friel, E.N.; Souleyre, E.J.F.; Bolitho, K.; et al. Genomics approach reveals that aroma production in apple as controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Muche, B.M.; Jordan, M.; Forney, C.F.; Speers, R.A.; Rupasinghe, H.P.V. Effect of 1-methylcyclopropene (1-MCP) and storage atmosphere on the volatile aroma composition of cloudy and clear apple juices. Beverages 2022, 6, 59. [Google Scholar] [CrossRef]
- Fan, X.; Blankenship, S.M.; Mattheis, J.P. 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, M.C.; Ku, K.H. Chemical, physical, and sensory properties of 1-MCP-treated Fuji apple (Malus domestica Borkh.) fruits after long-term cold storage. Appl. Biol. Chem. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Yoo, J.; Jung, H.; Win, N.M.; Kwon, J.-G.; Cho, Y.-J.; Jung, H.-E.; Lee, D.H.; Kang, I.-K. Changes in fruit quality attributes, cell wall materials, and related hydrolases activities in 1-methylcyclopropene (1-MCP)-treated ‘Honggeum’ apples during cold storage. Sci. Technol. 2020, 38, 870–879. [Google Scholar] [CrossRef]
- Małachowska, M.; Tomala, K. Effect of preharvest and postharvest application of 1-MCP on the quality of Gala Schniga® SchniCo Red(s) apples during long-term storage. Agriculture 2022, 12, 2073. [Google Scholar] [CrossRef]
- Tomala, K.; Guzek, D.; Głabska, D.; Małachowska, M.; Widłak, Ł.; Krupa, T.; Gutkowska, K. Maintaining the quality of ‘Red Jonaprince’ apples during storage by 1-Methylcyclopropene preharvest and postharvest treatment. Agriculture 2022, 12, 1189. [Google Scholar] [CrossRef]
- Khan, A.A.; Vincent, J.F.V. Anisotropy of apple parenchyma. J. Sci. Food Agric. 1990, 52, 455–466. [Google Scholar] [CrossRef]
- Khan, A.A.; Vincent, J.F.V. Anisotropy in the fracture properties of apple flesh as investigated by crack-opening tests. J. Mat. Sci. 1993, 28, 45–51. [Google Scholar] [CrossRef]
- Harker, F.R.; Hallett, I.C. Physiological changes associated with development of mealiness of apple fruit during cool storage. HortScience 1992, 27, 1291–1294. [Google Scholar] [CrossRef]
- Ting, V.J.L.; Silcock, P.; Bremer, P.J.; Biasioli, F. X-ray micro-computer tomographic method to visualize the microstructure of different apple cultivars. J. Food Sci. 2013, 78, E1735–E1742. [Google Scholar] [CrossRef] [PubMed]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I.K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Kwon, S.I.; Watkins, C.B.; Kang, I.K. Characterization of fruit quality attributes and cell wall metabolism in 1-methylcycloprenes (1-MCP) treated “Summer King” and “Green Ball” apples during cold storage. Font. Plant Sci. 2019, 10, 1513. [Google Scholar] [CrossRef]
- International Standards for Fruit and Vegetables: Apples. 2021. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/apples_12ebba9f-en-fr (accessed on 5 November 2023).
- Dever, M.C.; Cliff, M.A.; Hall, J.W. Analysis of variation and multivariate relationships among analytical and sensory characteristics in whole apple evaluation. J. Sci. Food Agric. 1995, 69, 329–338. [Google Scholar] [CrossRef]
- Bourne, M.C. Principles of Objective Texture Measurement. In Food Texture and Viscosity, 2nd ed.; Academic Press: London, UK, 2002; pp. 107–188. [Google Scholar]
- Saei, A.; Tustin, D.S.; Zamani, Z.; Talaie, A.; Hall, A.J. Cropping effects on the loss of apple fruit firmness during storage: The relationship between texture retention and fruit dry matter concentration. Sci. Hortic. 2011, 130, 256–265. [Google Scholar] [CrossRef]
- Biasioli, F.; Gasperi, F.; Aprea, E.; Endrizzi, I.; Framondino, V.; Marini, F.; Mott, D.; Märk, T.D. Correlation of PTR-MS spectral fingerprints with sensory characterisation of flavour and odour profile of “Trentingrana” cheese. Food Qual. Prefer. 2006, 17, 63–75. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Doube, M.; Kłosowski, M.; Arganda-Carreras, I.; Cordeliéres, F.; Dougherty, R.; Jackson, J.; Schmid, B.; Hutchinson, J.; Shefelbine, S. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Cappellin, L.; Makhoul, S.; Schuhfried, E.; Romano, A.; Sanchez del Pulgar, J.; Aprea, E.; Farneti, B.; Costa, F.; Gasperi, F.; Biasioli, F. Ethylene: Absolute real-time high sensitivity detection with PTR/SRI-MS. The example of fruits, leaves and bacteria. Int. J. Mass Spectrom. 2014, 365–366, 33–41. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for Multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Soukoulis, C.; Cappellin, L.; Aprea, E.; Costa, F.; Viola, R.; Märk, T.; Gasperi, F.; Biasioli, F. PTR-ToF-MS, A novel, rapid, high sensitivity and non-invasive tool to monitor volatile compound release during fruit post-harvest storage: The case study of apple ripening. Food Bioprocess Technol. 2013, 6, 2831–2843. [Google Scholar] [CrossRef]
- Aprea, E.; Biasioli, F.; Märk, T.D.; Gasperi, F. PTR-MS study of esters in water and water/ethanol solutions: Fragmentation patterns and partition coefficients. Int. J. Mass Spectrom. 2007, 262, 114–121. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Granitto, P.M.; Schuhfried, E.; Soukoulis, C.; Costa, F.; Maek, T.D.; Gasperi, F. On data analysis in PTR-TOF-MS: From raw spectra to data mining. Sens. Actuators B Chem. 2011, 155, 183–190. [Google Scholar] [CrossRef]
- Dandekar, A.M.; Teo, G.; Defilippi, B.G.; Uratsu, S.L.; Passey, A.J.; Kader, A.A.; Stow, J.R.; Colgan, R.J.; James, D.J. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 2004, 13, 373–384. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Impact of suppression of ethylene action or biosynthesis on flavor metabolites in apple (Malus domestica Borkh) fruits. J. Agric. Food Chem. 2004, 52, 5694–5701. [Google Scholar] [CrossRef] [PubMed]
- Thewes, F.R.; Both, V.; Brackmann, A.; de Freitas Ferreira, D.; Wagnee, R. 1-methylcyclopropene effects on volatile profile and quality of ‘Royal Gala’ apples produced in Southern Brazil and stored in controlled atmosphere. Cienc. Rural 2015, 45, 2259–2266. [Google Scholar] [CrossRef][Green Version]
- Ferenczi, A.; Song, J.; Tian, M.; Vlachonasios, K.; Dilley, D.; Beaudry, R. Volatile Ester Suppression and Recovery following 1-Methylcyclopropene Application to Apple Fruit. J. Am. Soc. Hortic. Sci. 2006, 131, 691–701. [Google Scholar] [CrossRef]
- Costa, F.; Cappellin, L.; Longhi, S.; Guerra, W.; Magnago, P.; Porro, D.; Soukoulis, C.; Salvi, S.; Velasco, R.; Biasioli, F.; et al. Assessment of apple (Malus × domestica Borkh.) fruit texture by a combined acoustic-mechanical profiling strategy. Postharvest Biol. Technol. 2011, 61, 21–28. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Romano, A.; Biasioli, F.; Costa, G.; Costa, F. Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS. Metabolomics 2014, 11, 838–850. [Google Scholar] [CrossRef]
- Ting, V.J.L.; Soukoulis, C.; Silcock, P.; Cappellin, L.; Romano, A.; Aprea, E.; Bremer, P.J.; Maerk, T.D.; Gasperi, F.; Biasioli, F. In vitro and in vivo flavor release from intact and fresh-cut apple in relation with genetic, textural, and physicochemical parameters. J. Food Sci. 2012, 77, C1226–C1233. [Google Scholar] [PubMed]
- Ting, V.J.L.; Romano, A.; Silcock, P.; Bremer, P.J.; Corollaro, M.L.; Soukoulis, C.; Cappellin, L.F.G.; Biasioli, F. Apple flavor: Linking sensory perception to volatile release and textural properties. J. Sens. Stud. 2015, 30, 195–210. [Google Scholar] [CrossRef]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Song, J.; Forney, C.F. Flavour volatile production and regulation in fruit. Can. J. Plant Sci. 2008, 88, 537–550. [Google Scholar] [CrossRef]
- Lurie, S.; Pre-Aymard, C.; Ravid, U.; Larkov, O.; Fallik, E. Effect of 1-Methylcyclopropene on Volatile Emission and Aroma in Cv. Anna Apples. J. Agric. Food Chem. 2002, 50, 4251–4256. [Google Scholar] [CrossRef]
- Dixon, J. Factors affecting apple aroma/flavour volatile concentration: A Review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Flath, R.A.; Black, D.R.; Guadagni, D.G.; McFadden, W.H.; Schultz, T.H. Identification and organoleptic evaluation of compounds in Delicious apple essence. J. Agric. Food Chem. 1967, 15, 29–35. [Google Scholar] [CrossRef]
- Knee, M.; Hatfield, S.G.S. The metabolism of alcohols by apple fruit tissue. J. Sci. Food Agric. 1981, 32, 593–600. [Google Scholar] [CrossRef]
- Lee, J.; Rudell, D.; Davies, P.; Watkins, C. Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple fruit during storage. Metabolomics 2012, 8, 742–753. [Google Scholar] [CrossRef]
- Allan-Wojtas, P.; Sanford, K.A.; McRae, K.B.; Carbyn, S. An integrated microstructural and sensory approach to describe apple texture. J. Am. Soc. Hortic. Sci. 2003, 128, 381–390. [Google Scholar] [CrossRef]
- Odgaard, A.; Gundersen, H.J.G. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 1993, 14, 173–182. [Google Scholar] [CrossRef]
- Power, F.B.; Chesnut, V.K. The odourous constituents of apples. Emanation of acetaldehyde from the ripe fruit. J. Am. Chem. Soc. 1920, 42, 1509–1526. [Google Scholar] [CrossRef][Green Version]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Buchanan, D.A.; Fellman, J.K. Change in apple fruit volatiles after storage in atmospheres inducing anaerobic metabolism. J. Agric. Food Chem. 1991, 39, 1602–1605. [Google Scholar] [CrossRef]
- McGlone, V.A.; Jordan, R.B.; Seelye, R.; Clark, C.J. Dry-matter—A better predictor of the post-storage soluble solids in apples? Postharvest Biol. Technol. 2003, 28, 431–435. [Google Scholar] [CrossRef]
- White, A. Apple Tree Named ‘Scifresh’. USPP 13888P3, 17 June 2003. [Google Scholar]
- Herremans, E.; Verboven, P.; Bongaers, E.; Estrade, P.; Verlinden, B.E.; Wevers, M.; Hertog, M.L.A.T.M.; Nicolai, B.M. Characterisation of ‘Braeburn’ browning disorder by means of X-ray micro-CT. Postharvest Biol. Technol. 2013, 75, 114–124. [Google Scholar] [CrossRef]
- Jarvis, M.C. Intercellular separation forces generated by intracellular pressure. Plant Cell Environ. 1998, 21, 1307–1310. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, J.M.; Perez, A. Aroma biochemistry of fruits and vegetables. In Phytochemistry of Fruit and Vegetables; Tomás-Barberán, F.A., Robins, R.J., Eds.; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Cadena, R.S.; Cruz, A.G.; Netto, R.R.; Castro, W.F.; Faria, J.dA.F.; Bolini, H.M.A. Sensory profile and physicochemical characteristics of mango nectar sweetened with high intensity sweeteners throughout storage time. Food Res. Int. 2013, 54, 1670–1679. [Google Scholar] [CrossRef]
| Mechanical Parameters | Definition |
|---|---|
| Fmax, (N) | Maximum force required to puncture the fruit skin |
| Flesh firmness, flesh.F (N) | Averaged force measured after skin rupture |
| Gradient, grad (N/mm) | Stiffness of skin measured as a slope from the start of the curve until Fmax |
| Area under the curve, AUC peel (N⋅mm) | Mechanical work needed to reach the rupture point of the skin indicated by Fmax taken as the area under the curve |
| AUC flesh (N⋅mm) | Work measured under the curve after skin rupture |
| m/z | Tentatively Identified Compounds | Untreated (C) | Treated (SF) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Braeburn | Fuji | Golden Delicious | Jazz | p-Value | Braeburn | Fuji | Golden Delicious | Jazz | p-Value | ||
| 28 | Ethylene | 464 | 321 | 775 | 850 | 0.36 | 288 | 525 | 157 | 262 | 0.49 |
| (191) | (118) | (399) | (564) | (204) | (267) | (115) | (229) | ||||
| 31 | CH3O+ | 0.5 a | 1.3 ab | 0.8 ab | 1.4 b | 0.04 | 0.2 | 0.4 | 0.1 | 0.2 | 0.62 |
| (0.3) | (0.5) | (0.3) | (0.1) | (0.3) | (0.5) | (0.2) | (0.1) | ||||
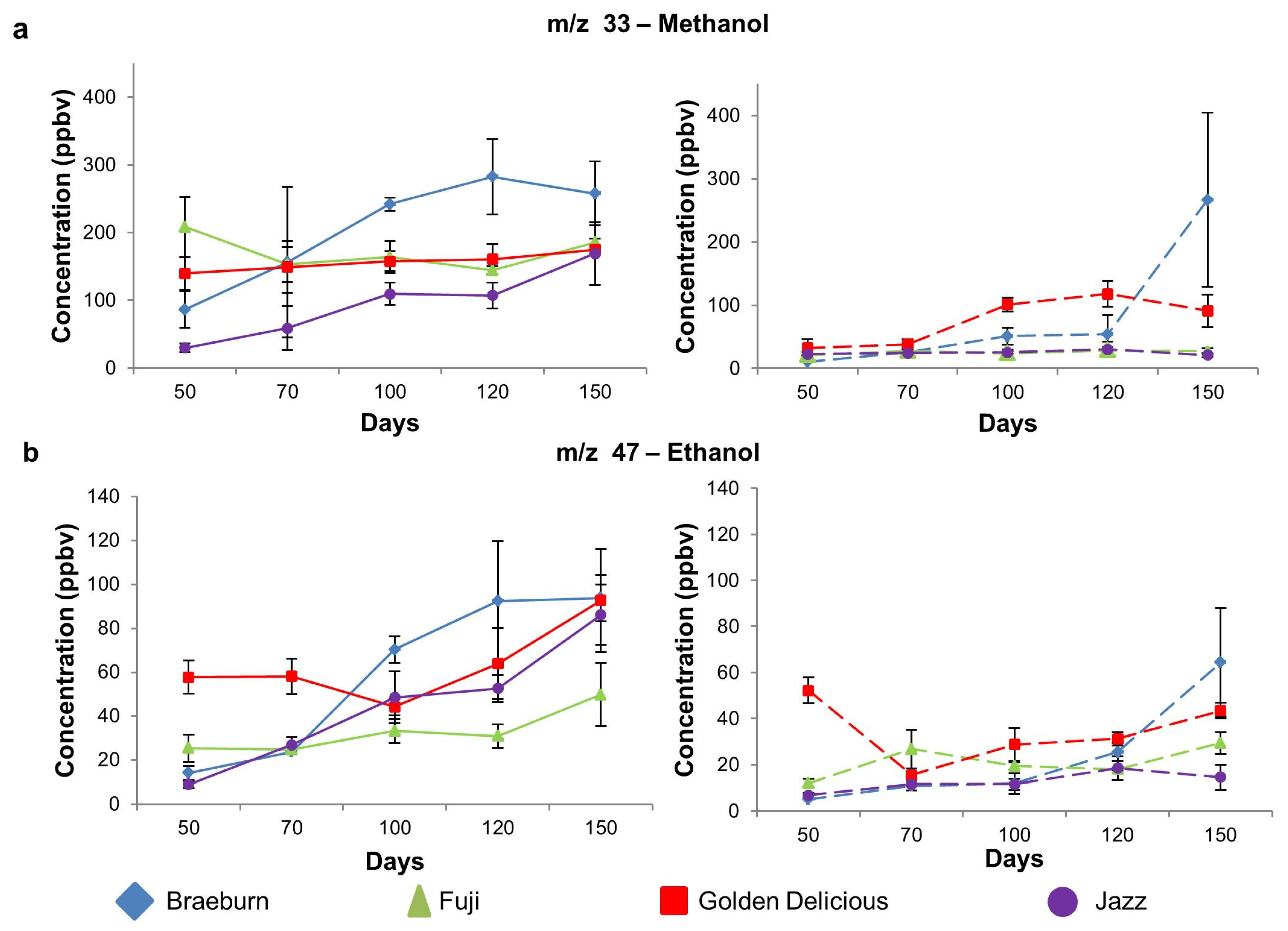
| 33 | Methanol | 87 ab | 208 c | 140 bc | 30 a | 0.00 | 11 a | 21 ab | 33 b | 23 ab | 0.03 |
| (27) | (44) | (24) | (6.1) | (0.1) | (0.4) | (13.1) | (3.7) | ||||
| 41 | Alcohol and ester frag. | 343 | 358 | 353 | 393 | 0.67 | 119 | 248 | 138 | 214 | 0.04 |
| (28) | (91) | (47) | (30) | (28) | (81) | (20) | (49) | ||||
| 43 | Alcohol and ester frag. | 1526 b | 755 a | 1938 b | 1840 b | 0.00 | 286 | 508 | 373 | 639 | 0.86 |
| (192) | (141) | (199) | (264) | (80) | (248) | (54) | (143) | ||||
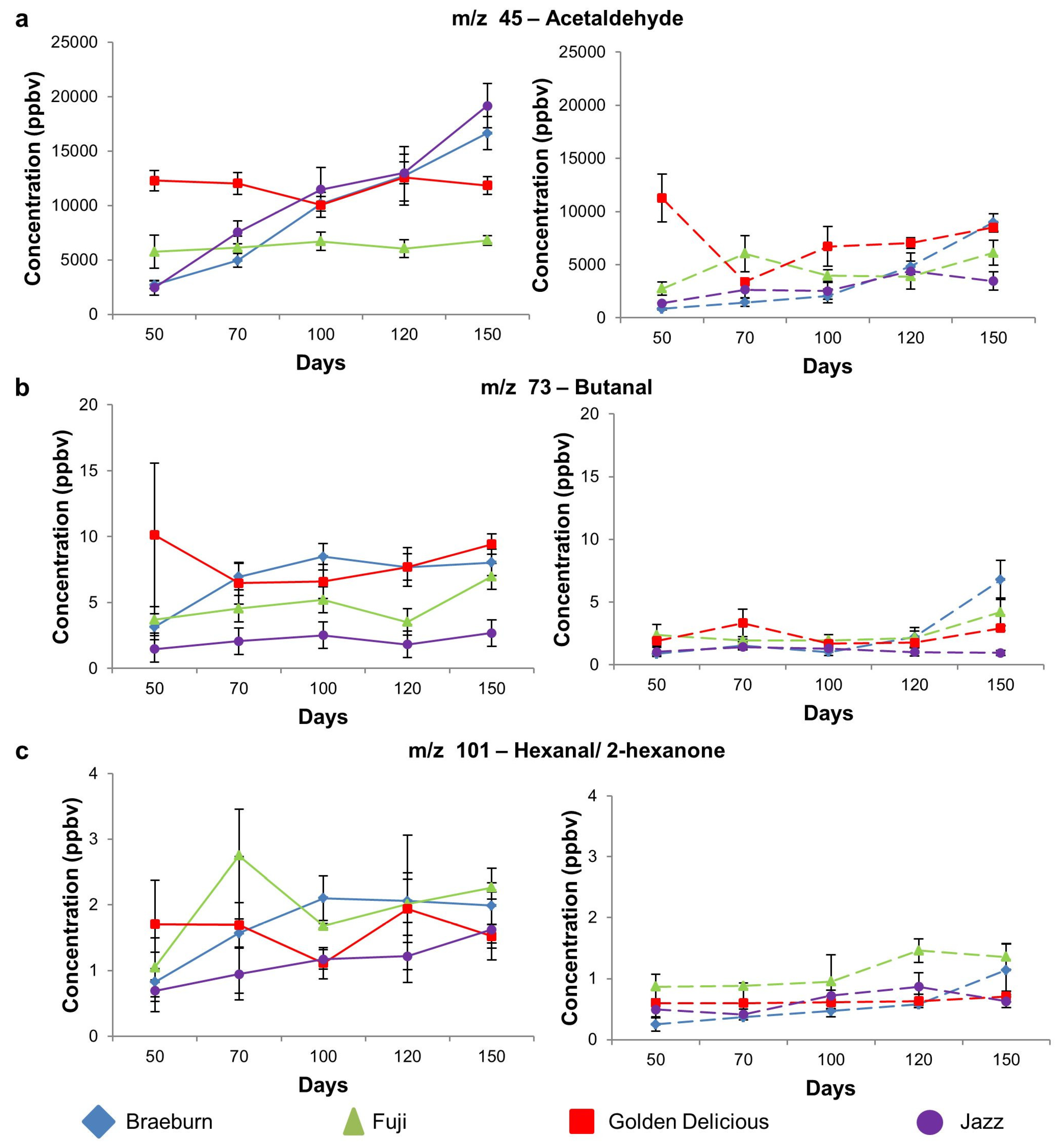
| 45 | Acetaldehyde | 2730 a | 5755 b | 12298 b | 2468 a | 0.00 | 837 a | 2732 a | 11271 b | 1388 a | 0.00 |
| (365) | (1524) | (947) | (688) | (82) | (621) | (2246) | (41) | ||||
| 47 | Ethanol | 14 ab | 25 b | 58 c | 9.0 a | 0.00 | 5.1 a | 12 a | 52 b | 6.8 a | 0.00 |
| (3.2) | (6.1) | (7.5) | (1.5) | (0.2) | (1.8) | (5.6) | (0.9) | ||||
| 53 | C4H5+ | 2.6 a | 3.8 ab | 5.4 b | 2.0 a | 0.13 | 1.0 a | 2.0 b | 1.2 a | 1.1 a | 0.01 |
| (0.5) | (0.2) | (1.9) | (0.3) | (0.1) | (0.2) | (0.5) | (0.2) | ||||
| 55 | C4H7+ | 69 a | 88 ab | 148 b | 42 a | 0.10 | 25 a | 43 b | 38 ab | 28 ab | 0.03 |
| (8.2) | (10) | (55) | (1.9) | (1.2) | (5.4) | (8.1) | (8.3) | ||||
| 57 | Alcohol and ester frag. | 207 a | 221 a | 487 b | 219 a | 0.00 | 47 a | 150 b | 116 ab | 101 ab | 0.03 |
| (28) | (35) | (77) | (13) | (11) | (53) | (30) | (27) | ||||
| 59 | Acetone | 23 a | 29 ab | 32 b | 28 ab | 0.67 | 12 | 14 | 19 | 21 | 0.07 |
| (2) | (3.9) | (4.7) | (2.8) | (0.8) | (2.2) | (1.6) | (6.9) | ||||
| 61 | Frag. of acetate esters/acetic acid | 1339 b | 509 a | 1518 b | 1638 b | 0.00 | 211 | 350 | 211 | 519 | 0.04 |
| (176) | (102) | (165) | (221) | (65) | (199) | (42) | (108) | ||||
| 63 | Ethylene glycol | 7.7 a | 6.6 a | 25 b | 9.0 a | 0.00 | 1.9 a | 4.0 a | 19 b | 4.1 a | 0.00 |
| (0.8) | (1.1) | (2.1) | (1.1) | (0.3) | (2.1) | (3.4) | (0.5) | ||||
| 69 | Isoprene | 4.1 a | 4.3 a | 8.2 b | 2.8 a | 0.00 | 1.8 a | 1.8 a | 4.1 b | 2.3 a | 0.00 |
| (0.7) | (0.8) | (1.9) | (0.3) | (0.5) | (0.2) | (0.5) | (0.4) | ||||
| 71 | Alcohol and ester frag. | 53 b | 55 b | 24 a | 42 ab | 0.00 | 27 | 41 | 25 | 36 | 0.15 |
| (3.9) | (14) | (4.1) | (0.9) | (7.3) | (11) | (1.7) | (10) | ||||
| 73 | Butanal | 3.2 ab | 3.7 ab | 10 b | 1.5 a | 0.02 | 0.9 a | 2.4 b | 1.9 ab | 1.0 a | 0.15 |
| (0.8) | (0.4) | (5.4) | (0.3) | (0.2) | (0.9) | (0.2) | (0.4) | ||||
| 75 | Butyl propanoate | 22 a | 24 a | 14 a | 49 b | 0.00 | 1.5 | 19 | 2 | 11 | 0.09 |
| (6.6) | (2.2) | (1.1) | (8.7) | (0.9) | (16) | (1.2) | (5.2) | ||||
| 79 | C2H7O3+ | 1.3 a | 0.6 a | 3.0 b | 1.5 a | 0.00 | 0.3 | 0.4 | 0.4 | 0.6 | 0.56 |
| (0.6) | (0.3) | (0.1) | (0.5) | (0.1) | (0.3) | (0.2) | (0.4) | ||||
| 81 | Terpene-related frag., aldehydes (trans-2-hexenal) | 9.8 a | 15 a | 44 b | 4.9 a | 0.00 | 5.5 ab | 11 b | 10 ab | 3.5 a | 0.02 |
| (0.8) | (2) | (11) | (1.6) | (0.9) | (2) | (4.4) | (1.9) | ||||
| 83 | Alcohols (hexanal, trans-2-hexenol, cis-2-hexenol) | 18 ab | 26 ab | 31 b | 13 a | 0.02 | 8.2 | 15 | 12 | 10 | 0.18 |
| (2.9) | (2.9) | (11) | (0.8) | (0.3) | (3) | (4.6) | (3.2) | ||||
| 85 | Alcohols (1-Hexanol, nonanol), ester frag. | 10 a | 9.7 a | 16 b | 15 b | 0.00 | 1.4 a | 5.1 b | 5.6 b | 3.5 ab | 0.00 |
| (1.5) | (1.9) | (1.7) | (1.1) | (0.7) | (0.9) | (0.6) | (1.1) | ||||
| 87 | Frag. (pentanal, 2-pentanone) | 1.1 ab | 2.4 c | 1.5 b | 0.8 a | 0.00 | 0.3 a | 0.8 b | 0.8 b | 0.5 a | 0.00 |
| (0.2) | (0.3) | (0.4) | (0.1) | (0.1) | (0.2) | (0.1) | (0.1) | ||||
| 89 | Butyrate related esters (ethyl butanoate, propyl butanoate, butyl butanoate) | 26 a | 39 ab | 59 b | 16 a | 0.00 | 2.6 | 18 | 6.1 | 5.8 | 0.05 |
| (9.5) | (9) | (15) | (2.1) | (0.7) | (12) | (1.4) | (0.7) | ||||
| 95 | Farnesene frag. | 0.9 a | 1.3 ab | 4.6 b | 0.3 a | 0.01 | 0.1 | 0.3 | 0.8 | 0.3 | 0.10 |
| (0.7) | (0.6) | (2) | (0.5) | (0.2) | (0.3) | (0.3) | (0.2) | ||||
| 99 | Aldehydes (trans-2-hexanal), esters (ethyl hexanoate, hexyl acetate) | 5.4 a | 11 b | 11 b | 3.4 a | 0.00 | 3.3 a | 7.0 b | 4.1 ab | 2.3 a | 0.00 |
| (1.1) | (1.9) | (2.6) | (0.9) | (0.8) | (0.8) | (1.8) | (0.6) | ||||
| 101 | Aldehydes(2-hexanone, hexanal) | 0.8 | 1 | 1.7 | 0.7 | 0.12 | 0.3 a | 0.9 | 0.6 ab | 0.5 ab | 0.01 |
| (1.5) | (0.4) | (0.7) | (0.2) | (0.1) | (0.2) | (0.2) | (0.1) | ||||
| 103 | Esters (isoamyl esters, propyl acetate, ethyl 2-methyl butanoate, methyl butanoate) | 18 a | 29 b | 9.7 a | 20 ab | 0.00 | 3.2 a | 17 b | 6.6 a | 8.4 a | 0.00 |
| (1.1) | (6.1) | (2.1) | (4.3) | (1) | (3.8) | (3.6) | (2.9) | ||||
| 109 | Unidentified | 1.4 a | 1.5 a | 4.1 b | 0.7 a | 0.00 | 0.7 | 0.6 | 0.8 | 0.4 | 0.34 |
| (0.3) | (0.5) | (1.1) | (0.2) | (0.1) | (0.2) | (0.4) | (0.1) | ||||
| 117 | Esters (hexanoates, ethyl 2-methyl butanoate, Isobutyl acetate, butyl acetate) | 22 a | 25 a | 44 b | 18 a | 0.00 | 2.3 a | 9.2 b | 4.0 ab | 6.6 ab | 0.02 |
| (4.3) | (2.6) | (3.7) | (3.3) | (1.1) | (3.7) | (1.3) | (1.6) | ||||
| 121 | Acetophenone | 0.7 a | 0.9 a | 3.7 b | 0.4 a | 0.00 | 0.1 | 0.5 | 0.4 | 0.3 | 0.35 |
| (0.3) | (1) | (1.2) | (0.2) | (0.1) | (0.5) | (0.2) | (0.1) | ||||
| 123 | Farnesene frag. | 0.4 a | 1.1 a | 2.3 b | 0.3 a | 0.00 | 0.3 | 0.4 | 0.5 | 0.3 | 0.58 |
| (0.3) | (0.7) | (1.4) | (0.5) | (0.3) | (0.3) | (0.2) | (0.2) | ||||
| 131 | Esters (heptanoates, methyl hexyl-esters) | 1.4 b | 3.1 c | 0.6 a | 1.2 ab | 0.00 | 0.4 | 0.9 | 0.5 | 0.6 | 0.35 |
| (0.4) | (0.1) | (0.2) | (0.3) | (0.2) | (0.6) | (0.2) | (0.1) | ||||
| 135 | farnesene frag., P-cymene | 0.5 a | 0.7 a | 3.4 b | 0.2 a | 0.00 | 0.3 | 0.4 | 0.3 | 0.1 | 0.80 |
| (0.4) | (0.4) | (0.3) | (0.4) | (0.2) | (0.6) | (0.5) | (0.1) | ||||
| 137 | Monoterpenes, farnesene frag. | 0.7 a | 0.3 a | 3.2 b | 0 a | 0.00 | 0.1 | 0 | 0.3 | 0.1 | 0.28 |
| (0.7) | (0.5) | (0.4) | (0.1) | (0.1) | (0.1) | (0.3) | (0.2) | ||||
| 145 | Esters (ethyl hexanoate, butyl butanoate) | 2.4 a | 2.9 a | 4.3 b | 3.1 ab | 0.01 | 0.3 | 1.1 | 0.4 | 0.8 | 0.20 |
| (0.3) | (0.1) | (0.9) | (0.4) | (0.3) | (0.8) | (0.2 | (0.2) | ||||
| 149 | Farnesene frag., Phenyls (estragole, anethol) | 1.9 a | 0.6 a | 5.3 b | 4.0 a | 0.01 | 0.3 | 0.7 | 0.2 | 0.3 | 0.57 |
| (0.1) | (0.4) | (1.4) | (2.1) | (0.3) | (0.6) | (0.2) | (0.4) | ||||
| 173 | Decanoates | 1.0 ab | 1.4 ab | 2.2 b | 0.8 a | 0.03 | 0.2 | 0.2 | 0.3 | 0.4 | 0.46 |
| (0.4) | (0.1) | (0.8) | (1.4) | (0.1) | (0.2) | (0.1) | (0.1) | ||||
| 205 | Alpha-farnesene | 1.4 a | 1.8 a | 7.3 b | 0.4 a | 0.00 | 0 | 0.6 | 0.3 | 0.1 | 0.02 |
| (0.3) | (1.1) | (2.3) | (0.4) | (0) | (0.1) | (0.3) | (0.1) | ||||
| Cultivar | Untreated (days) | Treated (SF) (days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 70 | 100 | 120 | 150 | 50 | 70 | 100 | 120 | 150 | ||||
| Ester | m/z 61 | Acetate-related esters | Braeburn | A | ab | ab | B | b | A | A | A | A | B |
| Fuji | A | ab | b | ab | ab | - | - | - | - | - | |||
| Golden Delicious | Ab | ab | ab | bc | c | - | - | - | - | - | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 89 | Butyrate-related esters | Braeburn | - | - | - | - | - | A | A | A | A | B | |
| Fuji | a | ab | bc | bc | c | AB | A | AB | BC | C | |||
| Golden Delicious | b | ab | ab | ab | ab | - | - | - | - | - | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 117 | Hexanoate-related esters | Braeburn | - | - | - | - | - | A | A | A | A | B | |
| Fuji | a | ab | ab | ab | b | A | AB | AB | AB | B | |||
| Golden Delicious | bc | ab | b | bc | c | A | A | A | B | B | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 145 | Ethyl hexanoate/butyl butanoate | Braeburn | a | ab | ab | b | b | A | A | A | A | B | |
| Fuji | a | ab | ab | ab | b | - | - | - | - | - | |||
| Golden Delicious | ab | a | ab | ab | b | A | A | A | B | B | |||
| Jazz | a | ab | ab | b | b | - | - | - | - | - | |||
| Aldehyde | m/z 45 | Acetaldehyde | Braeburn | a | a | b | b | c | A | A | A | B | C |
| Fuji | - | - | - | - | - | A | B | AB | AB | B | |||
| Golden Delicious | - | - | - | - | - | C | A | AB | B | BC | |||
| Jazz | a | b | bc | c | d | A | AB | AB | B | AB | |||
| m/z 73 | Butanal | Braeburn | a | ab | b | b | b | A | A | A | A | B | |
| Fuji | - | - | - | - | - | A | A | A | AB | B | |||
| Golden Delicious | - | - | - | - | - | A | B | A | A | AB | |||
| Jazz | - | - | - | - | - | - | - | - | - | - | |||
| m/z 101 | Hexanal/2-hexanone | Braeburn | a | ab | b | b | b | A | A | A | A | B | |
| Fuji | a | ab | ab | b | b | - | - | - | - | - | |||
| Golden Delicious | - | - | - | - | - | - | - | - | - | - | |||
| Jazz | a | ab | ab | ab | b | A | A | AB | B | AB | |||
| Alcohol | m/z 33 | Methanol | Braeburn | a | ab | ab | b | b | A | A | A | A | B |
| Fuji | - | - | - | - | - | A | AB | AB | B | B | |||
| Golden Delicious | - | - | - | - | - | A | A | B | B | B | |||
| Jazz | a | ab | bc | bc | c | - | - | - | - | - | |||
| m/z 47 | Ethanol | Braeburn | a | a | b | b | b | A | A | A | A | B | |
| Fuji | a | a | ab | ab | b | - | - | - | - | - | |||
| Golden Delicious | a | ab | ab | ab | b | C | A | B | B | C | |||
| Jazz | a | ab | bc | c | d | A | AB | AB | B | AB | |||
| DM | SSC | Fmax | Flesh.f | Gradient | |
|---|---|---|---|---|---|
| DM | - | ||||
| SSC | 0.789 | - | |||
| Fmax | 0.244 | 0.132 | - | ||
| Flesh.f | 0.116 | 0.029 | 0.900 | ||
| Gradient | −0.006 | 0.001 | 0.468 | 0.520 | - |
| AUC.peel | 0.316 | 0.297 | 0.189 | 0.014 | −0.713 |
| AUC.flesh | −0.123 | −0.276 | 0.773 | 0.861 | 0.497 |
| Porosity | 0.005 | 0.187 | −0.691 | −0.715 | −0.484 |
| Anisotropy | −0.156 | −0.382 | 0.435 | 0.468 | 0.284 |
| Connectivity | −0.082 | 0.170 | −0.319 | −0.257 | −0.066 |
| AUC.peel | AUC.flesh | Porosity | Anisotropy | ||
| DM | |||||
| SSC | |||||
| Fmax | |||||
| Flesh.f | |||||
| Grad | |||||
| AUC.peel | - | ||||
| AUC.flesh | −0.188 | - | |||
| Porosity | 0.183 | −0.811 | - | ||
| Anisotropy | −0.191 | 0.654 | −0.619 | - | |
| Connectivity | −0.057 | −0.356 | 0.493 | −0.745 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, V.J.L.; Silcock, P.; Biasioli, F.; Bremer, P. The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage. Foods 2023, 12, 4050. https://doi.org/10.3390/foods12224050
Ting VJL, Silcock P, Biasioli F, Bremer P. The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage. Foods. 2023; 12(22):4050. https://doi.org/10.3390/foods12224050
Chicago/Turabian StyleTing, Valentina J. L., Pat Silcock, Franco Biasioli, and Phil Bremer. 2023. "The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage" Foods 12, no. 22: 4050. https://doi.org/10.3390/foods12224050
APA StyleTing, V. J. L., Silcock, P., Biasioli, F., & Bremer, P. (2023). The Physical and Structural Effects of 1-MCP on Four Different Apple Cultivars during Storage. Foods, 12(22), 4050. https://doi.org/10.3390/foods12224050








