bglG Regulates the Heterogeneity Driven by the Acid Tolerance Response in Lacticaseibacillus paracasei L9
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
2.2. Acid Adaption and Acid Challenge
2.3. Fluorescence Labelling and FCM Analysis
2.4. Intracellular ATP Content
2.5. Transcriptomics
2.6. Quantitative Real-Time PCR
2.7. DNA Manipulation Techniques
2.8. Insertional Inactivation of bglG
2.9. Statistical Analysis
3. Results
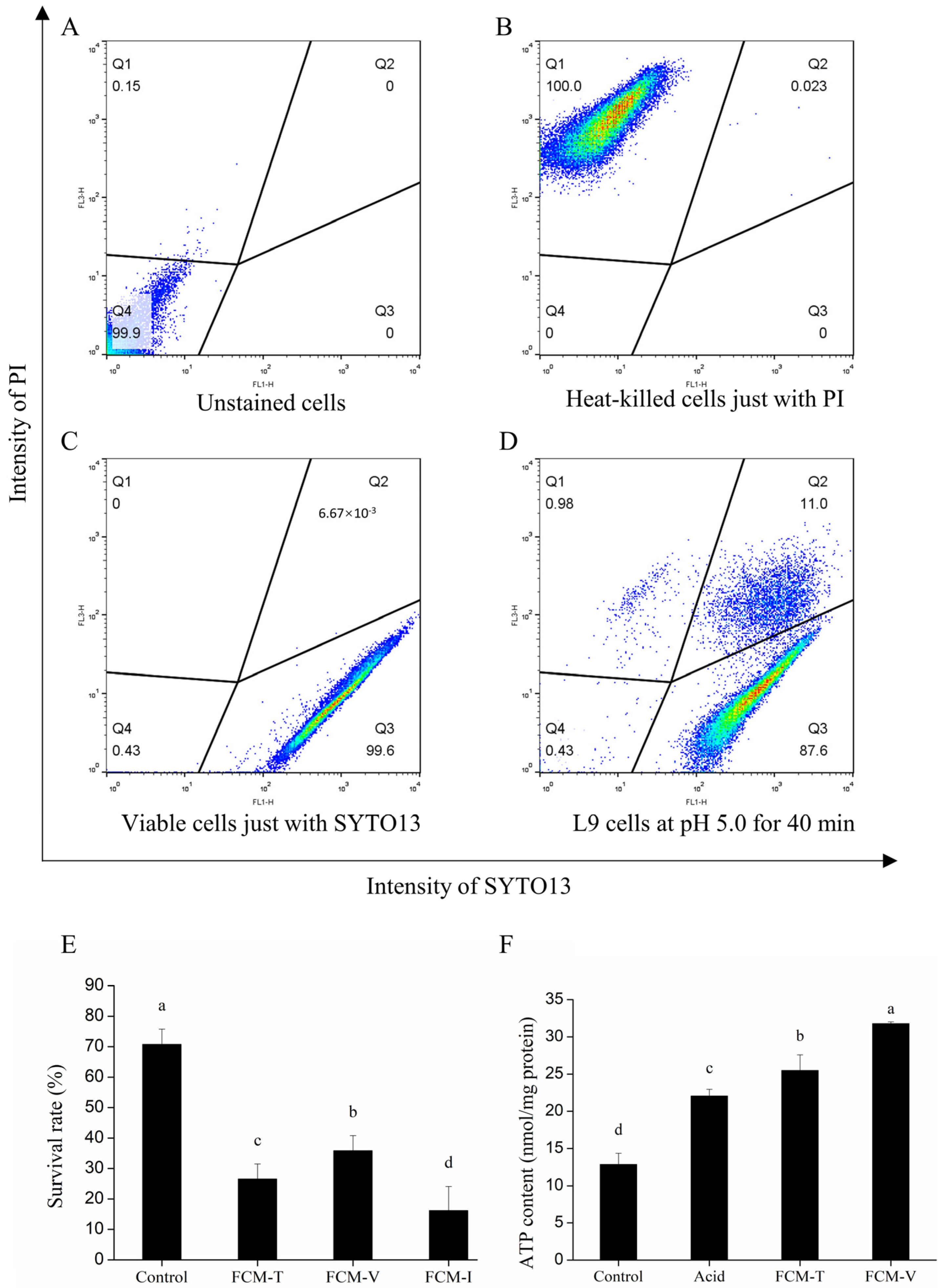
3.1. Acid Tolerance Response of Lacticaseibacillus paracasei L9
3.2. Heterogeneity Driven by the ATR of Lacticaseibacillus paracasei L9
3.3. Transcriptomic Changes in the ATR in Different Subpopulations
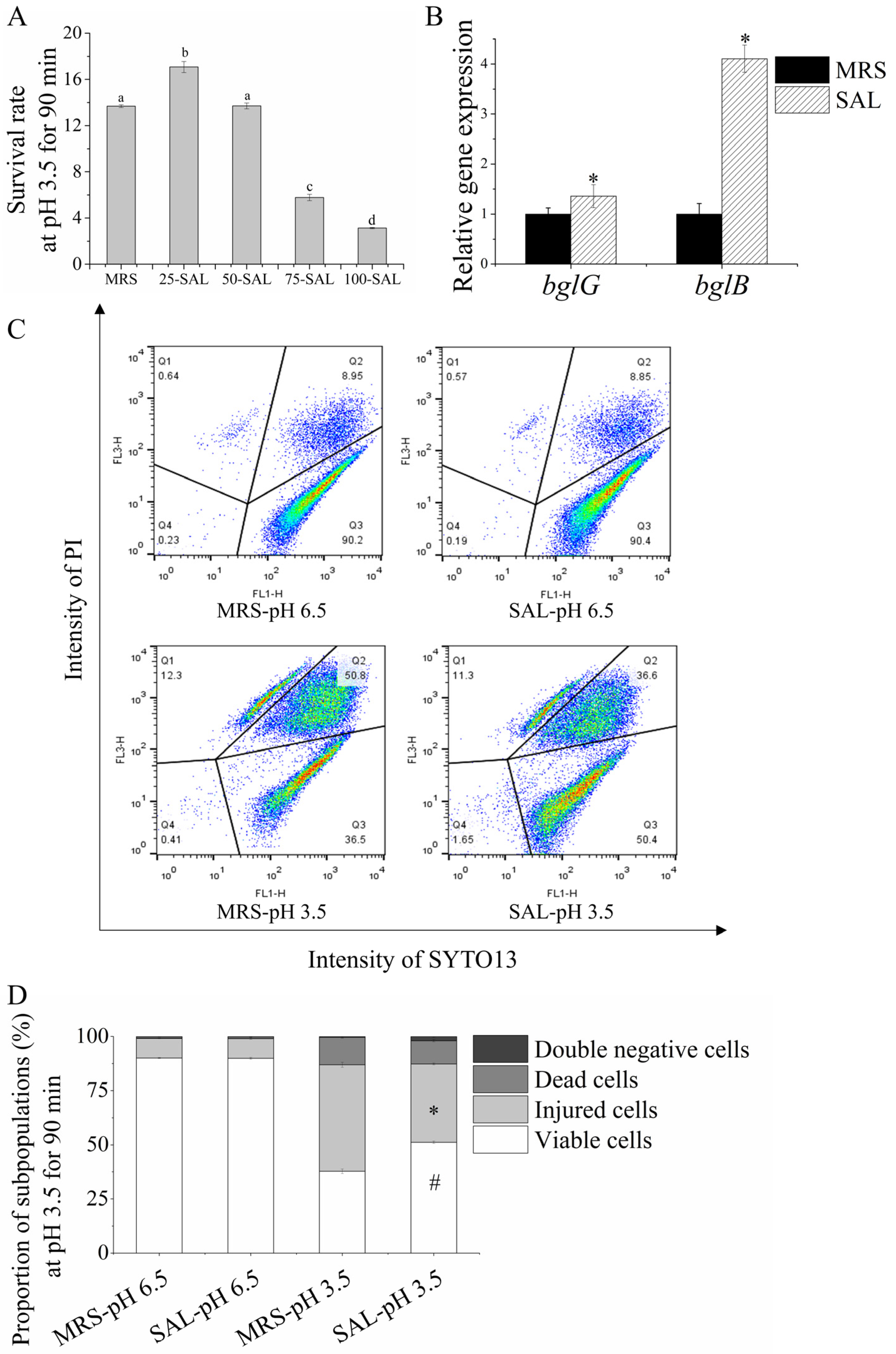
3.4. Salicin Increases Acid Tolerance in Lacticaseibacillus paracasei L9
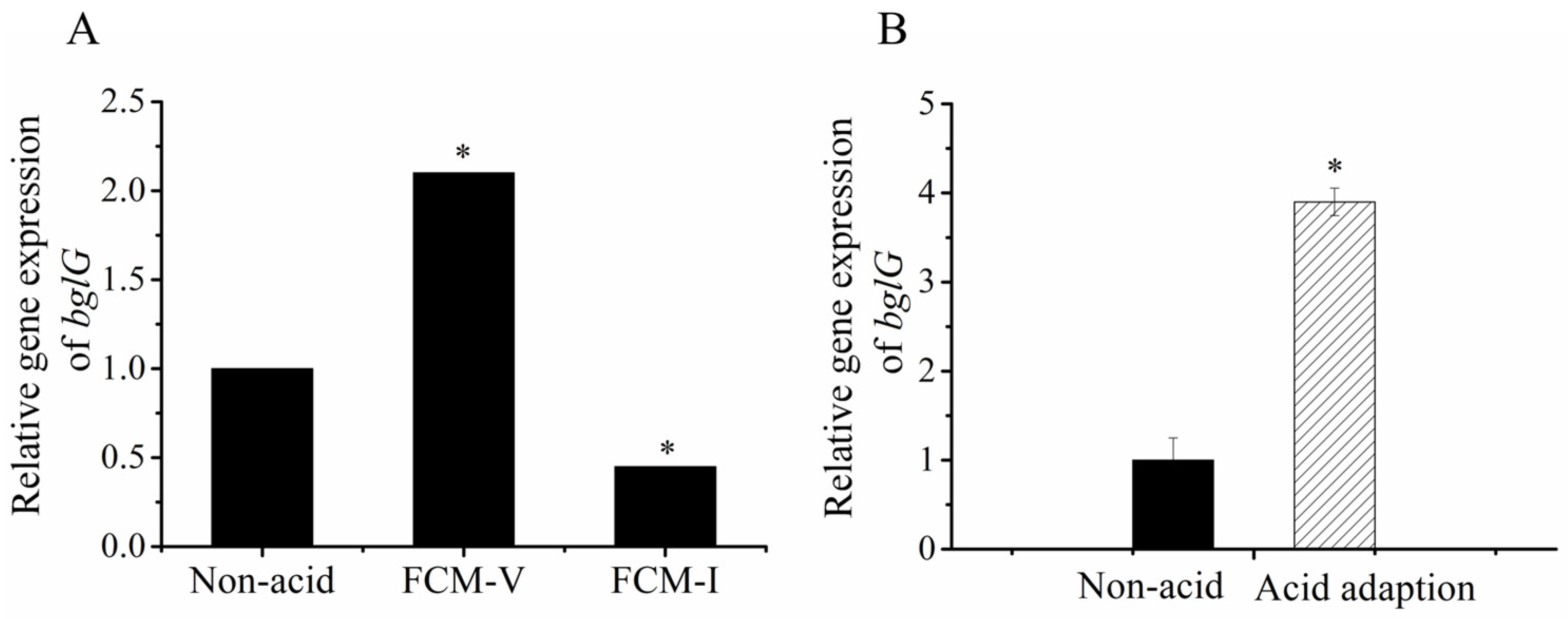
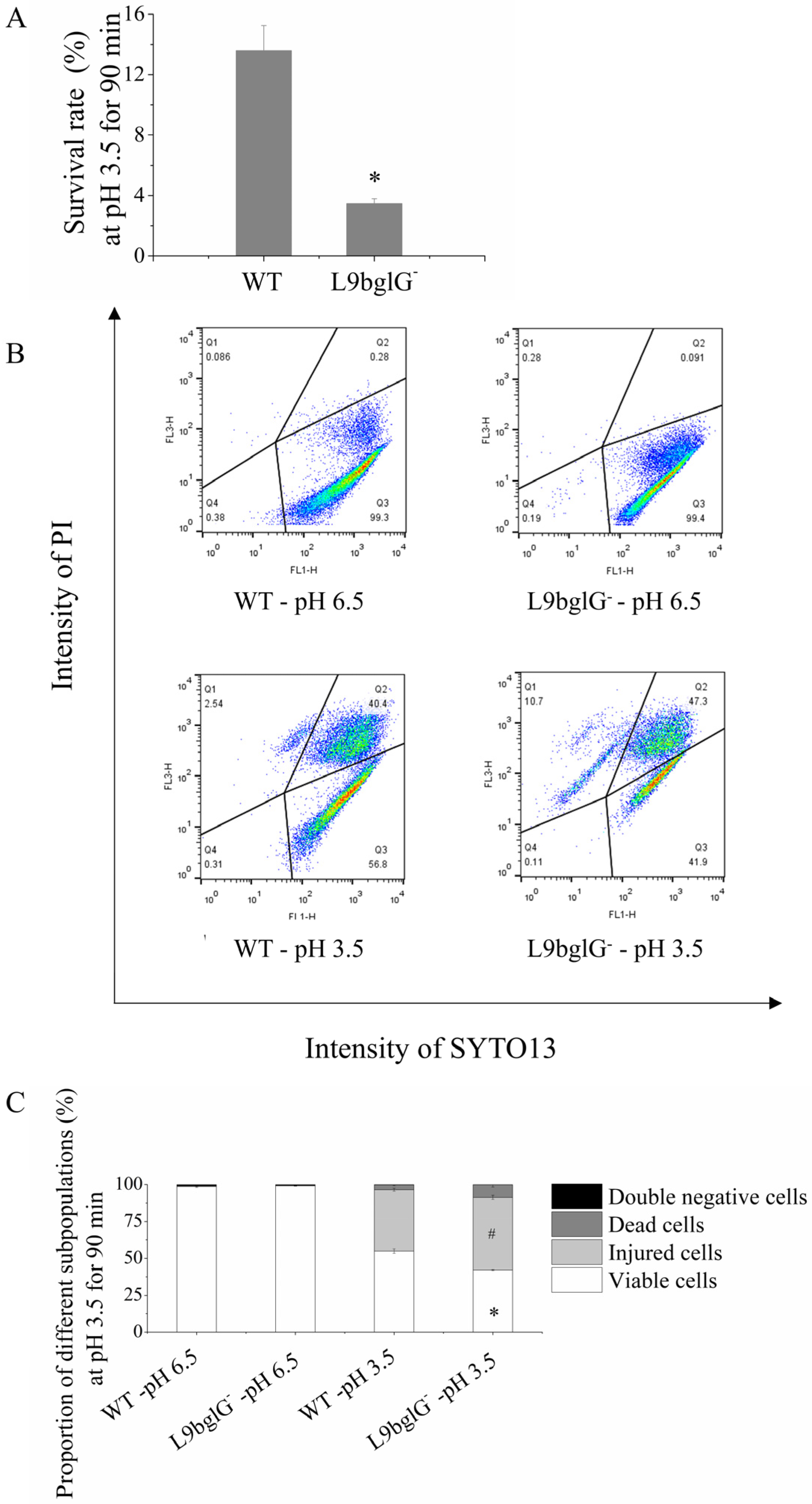
3.5. bglG Participates in the ATR Heterogeneity of Lacticaseibacillus paracasei L9
3.6. Potential Genes Regulated by bglG in Acid Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magdanova, L.A.; Golyasnaya, N.V. Heterogeneity as an adaptive trait of microbial populations. Microbiology 2013, 82, 1–10. [Google Scholar] [CrossRef]
- Gazel, D.; Erinmez, M.; Büyüktaş Manay, A.; Zer, Y. Investigation of heteroresistant vancomycin intermediate Staphylococcus aureus among MRSA isolates. J. Infect. Dev. Countr. 2021, 15, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Ko, K.S. Tigecycline heteroresistance and resistance mechanism in clinical isolates of Acinetobacter baumannii. Microbiol. Spectr. 2021, 9, e101021. [Google Scholar] [CrossRef]
- Davis, K.M.; Isberg, R.R. Defining heterogeneity within bacterial populations via single cell approaches. Bioessays 2016, 38, 782–790. [Google Scholar] [CrossRef] [PubMed]
- González-Cabaleiro, R.; Mitchell, A.M.; Smith, W.; Wipat, A.; Ofiţeru, I.D. Heterogeneity in pure microbial systems: Experimental measurements and modeling. Front. Microbiol. 2017, 8, 1813. [Google Scholar] [CrossRef] [PubMed]
- Carlquist, M.; Fernandes, R.L.; Helmark, S.; Heins, A.; Lundin, L.; Sørensen, S.J.; Gernaey, K.V.; Lantz, A.E. Physiological heterogeneities in microbial populations and implications for physical stress tolerance. Microb. Cell Fact. 2012, 11, 94. [Google Scholar] [CrossRef]
- Lidstrom, M.E.; Konopka, M.C. The role of physiological heterogeneity in microbial population behavior. Nat. Chem. Biol. 2010, 6, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Microbiol. 2015, 13, 497–508. [Google Scholar] [CrossRef]
- Dewachter, L.; Fauvart, M.; Michiels, J. Bacterial heterogeneity and antibiotic survival: Understanding and combatting persistence and heteroresistance. Mol. Cell 2019, 76, 255–267. [Google Scholar] [CrossRef]
- Rainey, P.B.; Beaumont, H.J.; Ferguson, G.C.; Gallie, J.; Kost, C.; Libby, E.; Zhang, X.X. The evolutionary emergence of stochastic phenotype switching in bacteria. Microb. Cell Fact. 2011, 10 (Suppl. 1), S14. [Google Scholar] [CrossRef]
- Newman, J.R.S.; Ghaemmaghami, S.; Ihmels, J.; Breslow, D.K.; Noble, M.; DeRisi, J.L.; Weissman, J.S. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 2006, 441, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Kuchina, A.; Brettner, L.M.; Paleologu, L.; Roco, C.M.; Rosenberg, A.B.; Carignano, A.; Kibler, R.; Hirano, M.; Depaolo, R.W.; Seelig, G. Microbial single-cell RNA sequencing by split-pool barcoding. Science 2021, 371, eaba5257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Knøchel, S.; Siegumfeldt, H. Heterogeneity between and within strains of Lactobacillus brevis exposed to beer compounds. Front. Microbiol. 2017, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Pratsinis, H.; Nebe-von-Caron, G.; Kletsas, D.; Tsakalidou, E. Rapid assessment of the physiological status of Streptococcus macedonicus by flow cytometry and fluorescence probes. Int. J. Food Microbiol. 2006, 111, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Herrero, M.; Rendueles, M.; Díaz, M. Physiological heterogeneity in Lactobacillus casei fermentations on residual yoghurt whey. Process Biochem. 2014, 49, 732–739. [Google Scholar] [CrossRef]
- Hansen, G.; Johansen, C.L.; Marten, G.; Wilmes, J.; Jespersen, L.; Arneborg, N. Influence of extracellular pH on growth, viability, cell size, acidification activity, and intracellular pH of Lactococcus lactis in batch fermentations. Appl. Microbiol. Biot. 2016, 100, 5965–5976. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Kocot, A.M.; Laniewska-Trokenheim, L. Physiological functions at single-cell level of Lactobacillus spp. isolated from traditionally fermented cabbage in response to different pH conditions. J. Biotechnol. 2015, 200, 19–26. [Google Scholar] [CrossRef]
- Yang, J.; Ren, F.; Zhang, H.; Jiang, L.; Hao, Y.; Luo, X. Induction of regulatory dendritic cells by Lactobacillus paracasei L9 prevents allergic sensitization to bovine β-lactoglobulin in mice. J. Microbiol. Biotechnol. 2015, 25, 1687–1696. [Google Scholar] [CrossRef]
- Zhai, Z.; Douillard, F.P.; An, H.; Wang, G.; Guo, X.; Luo, Y.; Hao, Y. Proteomic characterization of the acid tolerance response in Lactobacillus delbrueckii subsp. bulgaricus CAUH1 and functional identification of a novel acid stress-related transcriptional regulator Ldb0677. Environ. Microbiol. 2014, 16, 1524–1537. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Siegumfeldt, H.; Larsen, N.; Jespersen, L. Diversity in NaCl tolerance of Lactococcus lactis strains from DL-starter cultures for production of semi-hard cheeses. Int. Dairy. J. 2020, 105, 104673. [Google Scholar] [CrossRef]
- Fu, H.; Yang, L.; Zhang, H.; Wang, J. Deciphering of the mannitol metabolism pathway in Clostridium tyrobutyricum ATCC 25755 by comparative transcriptome analysis. Appl. Biochem. Biotechnol. 2023, 195, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pu, Y.; Li, J.Y.; Yin, X.; Liao, Y.H. Isolation and identification of lactic acid bacteria from mud of Baijiu and comparison of fermented volatile flavor compounds. J. Food Sci. Technol. 2020, 38, 26–35. [Google Scholar]
- Mukerji, M.; Mahadevan, S. Cryptic genes: Evolutionary puzzles. J. Genet. 1997, 76, 147–159. [Google Scholar] [CrossRef]
- Prasad, I.; Schaefler, S. Regulation of the beta-glucoside system in Escherchia coli K-12. J. Bacteriol. 1974, 120, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Raghunand, T.R.; Mahadevan, S. Mutational analysis of β-glucoside utilization in Klebsiella aerogenes: Evidence for the presence of multiple genetic systems. J. Genet. 2004, 83, 285–289. [Google Scholar] [CrossRef]
- Dole, S.; Kühn, S.; Schnetz, K. Post-transcriptional enhancement of Escherichia coli bgl operon silencing by limitation of BglG-mediated antitermination at low transcription rates. Mol. Microbiol. 2002, 43, 217–226. [Google Scholar] [CrossRef]
- Harwani, D. Regulation of gene expression: Cryptic beta-glucoside (bgl) operon of Escherichia coli as a paradigm. Braz. J. Microbiol. 2014, 45, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.; Moorthy, S.; Mahadevan, S. Enhanced expression of the bgl operon of Escherichia coli in the stationary phase. FEMS Microbiol. Lett. 2008, 288, 131–139. [Google Scholar] [CrossRef][Green Version]
- Fox, C.F.; Wilson, G. The role of a phosphoenolpyruvate-dependent kinase system in beta-glucoside catabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 1968, 59, 988–995. [Google Scholar] [CrossRef]
- Dole, S.; Klingen, Y.; Nagarajavel, V.; Schnetz, K. The protease Lon and the RNA-binding protein Hfq reduce silencing of the Escherichia coli bgl operon by H-NS. J. Bacteriol. 2004, 2708–2716. [Google Scholar] [CrossRef]
- Mukerji, M.; Mahadevan, S. Characterization of the negative elements involved in silencing the bgl operon of Escherichia coli: Possible roles for DNA gyrase, H-NS, and CRP-cAMP in regulation. Mol. Microbiol. 1997, 24, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Ueguchi, C.; Mizuno, T. rpoS function is essential for bgl silencing caused by C-terminally truncated H-NS in Escherichia coli. J. Bacteriol. 1999, 181, 6278–6283. [Google Scholar] [CrossRef]
- Comas, I.; Borrell, S.; Roetzer, A.; Rose, G.; Malla, B.; Kato-Maeda, M.; Galagan, J.; Niemann, S.; Gagneux, S. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat. Genet. 2012, 44, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Eilertson, B.; Maruri, F.; Blackman, A.; Herrera, M.; Samuels, D.C.; Sterling, T.R. High proportion of heteroresistance in gyrA and gyrB in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob. Agents Chemother. 2014, 58, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.H. Single-Cell Gene Expression Analysis of Transcriptional Heterogeneity in Monoculture and Dual-Culture of Desulfovibrio vulgaris. Doctoral Thesis, Tianjin University, Tianjin, China, 2016. [Google Scholar]
- Esen, A. β-Glucosidases: Biochemistry and Molecular Biology; American Chemical Society: Washington, DC, USA, 1993; pp. 1–13. [Google Scholar]
- Madan, R.; Kolter, R.; Mahadevan, S. Mutations that activate the silent bgl operon of Escherichia coli confer a growth advantage in stationary phase. J. Bacteriol. 2005, 187, 7912–7917. [Google Scholar] [CrossRef]
- Harwani, D.; Zangoui, P.; Mahadevan, S. The β-glucoside (bgl) operon of Escherichia coli is involved in the regulation of oppA, encoding an oligopeptide transporter. J. Bacteriol. 2012, 194, 90–99. [Google Scholar] [CrossRef]
- Houman, F.; Diaz-Torres, M.R.; Wright, A. Transcriptional antitermination in the bgl operon of E. coli is modulated by a specific RNA binding protein. Cell 1990, 62, 1153–1163. [Google Scholar] [CrossRef]
- Gulati, A.; Mahadevan, S. The Escherichia coli antiterminator protein BglG stabilizes the 5’region of the bgl mRNA. J. Biosci. 2001, 26, 193–203. [Google Scholar] [CrossRef]
- Gusev, N.B.; Bukach, O.V.; Marston, S.B. Structure, properties, and probable physiological role of small heat shock protein with molecular mass 20 kD (Hsp20, HspB6). Biochemistry 2005, 6, 629–637. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Zhang, Z.; Fitzgerald, G.F.; van Sinderen, D. Molecular characterization of hsp20, encoding a small heat shock protein of Bifidobacterium breve UCC2003. Appl. Environ. Microb. 2007, 73, 4695–4703. [Google Scholar] [CrossRef]
- Wu, R.; Xu, X.; Meng, L.; Zou, T.; Tang, X.; Wu, J.; Yue, X. Identification of salt stress responsive protein in Lactobacillus paracasei LN-1 using SDS-PAGE. IERI Procedia 2014, 8, 60–65. [Google Scholar] [CrossRef]
- Yang, X.; Teng, K.; Zhang, J.; Wang, F.; Zhang, T.; Ai, G.; Han, P.; Bai, F.; Zhong, J. Transcriptome responses of Lactobacillus acetotolerans F28 to a short and long term ethanol stress. Sci. Rep. 2017, 7, 2650. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.A.; Previato-Mello, M.; Barroso, K.C.M.; Koide, T.; Da Silva Neto, J.F. The MarR family regulator OsbR controls oxidative stress response, anaerobic nitrate respiration, and biofilm formation in Chromobacterium violaceum. BMC Microbiol. 2021, 21, 304. [Google Scholar] [CrossRef] [PubMed]


| No. | Gene ID | Gene Name | log2FC (FCM-V/Non-Acid) | log2FC (FCM-I/Non-Acid) | Annotated Function |
|---|---|---|---|---|---|
| 1 | gene2198 | LPL9_RS10370 | 2.0377 ↑ | −1.0162 ↓ | Amino acid ABC transporter permease |
| 2 | gene2284 | LPL9_RS10760 | 1.6958 ↑ | −1.3129 ↓ | PTS beta-glucoside transporter subunit IIBCA |
| 3 | gene807 | LPL9_RS03780 | 1.275 ↑ | −1.0363 ↓ | Iron–sulfur cluster biosynthesis family protein |
| 4 | gene66 | LPL9_RS00305 | 1.2064 ↑ | −1.0832 ↓ | ABC transporter permease |
| 5 | gene3139 | LPL9_RS14735 | 1.0483 ↑ | −1.1387 ↓ | BglG family transcriptional antiterminator |
| 6 | gene59 | LPL9_RS00270 | −3.3762 ↓ | 1.0407 ↑ | Transcriptional regulator |
| 7 | gene1874 | LPL9_RS08830 | −2.8654 ↓ | 1.156 ↑ | Hypothetical protein |
| 8 | gene2556 | LPL9_RS12000 | −2.7301 ↓ | 1.2204 ↑ | Glutamate 5-kinase |
| 9 | gene780 | LPL9_RS03660 | −1.9645 ↓ | 1.2441 ↑ | Hsp20/alpha-crystallin family protein |
| 10 | gene3020 | LPL9_RS14160 | −1.7589 ↓ | 1.0555 ↑ | NUDIX domain-containing protein |
| 11 | gene3055 | LPL9_RS14325 | −1.5069 ↓ | 1.2373 ↑ | Hsp20/alpha-crystallin family protein |
| 12 | gene1995 | LPL9_RS09420 | −1.4664 ↓ | 1.1811 ↑ | N-acetyltransferase |
| 13 | gene1535 | LPL9_RS07225 | −1.4 ↓ | 1.0644 ↑ | Hypothetical protein |
| 14 | gene1231 | LPL9_RS05775 | −1.2073 ↓ | 2.3676 ↑ | Flavodoxin |
| 15 | gene1741 | LPL9_RS08205 | −1.1713 ↓ | 1.0484 ↑ | Zinc-containing alcohol dehydrogenase/quinone oxidoreductase[NADPH] |
| 16 | gene28 | LPL9_RS00140 | −1.1 ↓ | 1.9574 ↑ | Hypothetical protein |
| 17 | gene1239 | LPL9_RS05810 | −1.0844 ↓ | 1.3583 ↑ | DEAD/DEAH box helicase |
| Gene ID | Gene Description | Log2FC (SAL_ACID/SAL) | Log2FC (WT_ACID/WT) | |
|---|---|---|---|---|
| LPL9_RS03660 | Hsp20/alpha-crystallin family protein | 2.11 | > | 1.99 |
| LPL9_RS05270 | Type II secretion system F family protein | 1.61 | > | 1.29 |
| LPL9_RS08570 | Hypothetical protein | 1.21 | > | 1.13 |
| LPL9_RS10845 | MarR family transcriptional regulator | 1.12 | > | 1.04 |
| LPL9_RS08520 | 30S ribosomal protein S2 | 1.1 | > | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Lin, L.; Zhai, Z.; Liang, J.; Chen, L.; Hao, Y.; Zhao, L. bglG Regulates the Heterogeneity Driven by the Acid Tolerance Response in Lacticaseibacillus paracasei L9. Foods 2023, 12, 3971. https://doi.org/10.3390/foods12213971
Shen Z, Lin L, Zhai Z, Liang J, Chen L, Hao Y, Zhao L. bglG Regulates the Heterogeneity Driven by the Acid Tolerance Response in Lacticaseibacillus paracasei L9. Foods. 2023; 12(21):3971. https://doi.org/10.3390/foods12213971
Chicago/Turabian StyleShen, Zhichao, Li Lin, Zhengyuan Zhai, Jingjing Liang, Long Chen, Yanling Hao, and Liang Zhao. 2023. "bglG Regulates the Heterogeneity Driven by the Acid Tolerance Response in Lacticaseibacillus paracasei L9" Foods 12, no. 21: 3971. https://doi.org/10.3390/foods12213971
APA StyleShen, Z., Lin, L., Zhai, Z., Liang, J., Chen, L., Hao, Y., & Zhao, L. (2023). bglG Regulates the Heterogeneity Driven by the Acid Tolerance Response in Lacticaseibacillus paracasei L9. Foods, 12(21), 3971. https://doi.org/10.3390/foods12213971








