Inhibition of IEC-6 Cell Proliferation and the Mechanism of Ulcerative Colitis in C57BL/6 Mice by Dandelion Root Polysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Extraction, Isolation and Chemical Composition of DP
2.1.2. Determination of Contents of Carbohydrate and Protein
2.2. Animals and Grouping
2.2.1. H&E Staining Assay
2.2.2. Transmission Electron Microscopy (TEM) Experiments
2.2.3. TUNEL Assay
2.2.4. Western Blot Analysis
2.3. Iron Ion Detection
2.3.1. Immunohistochemical Experiment
2.3.2. ELISA Assay
2.3.3. RT-qPCR Assay
2.3.4. Immunofluorescence Assay
2.3.5. Fecal Microbiota 16S rRNA Analysis
2.3.6. Determination of Short-Chain Fatty Acid Content in Stool
2.3.7. Cell Culture
2.3.8. CCK-8 Assay
2.3.9. Apoptosis Assay
2.4. Statistical Analysis
3. Results
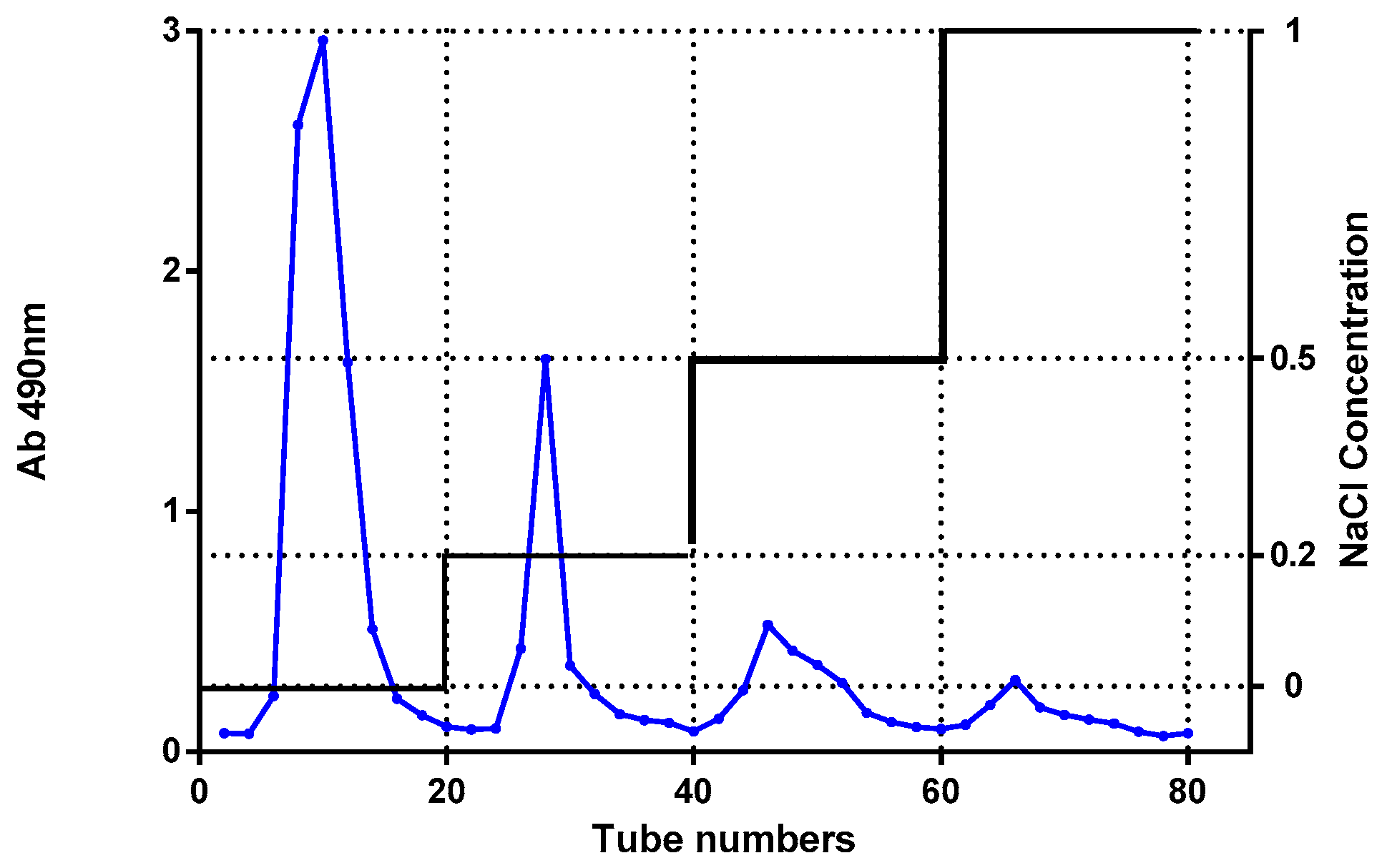
3.1. Separation and Purification of DP
3.2. Analysis of Polysaccharide and Protein Contents
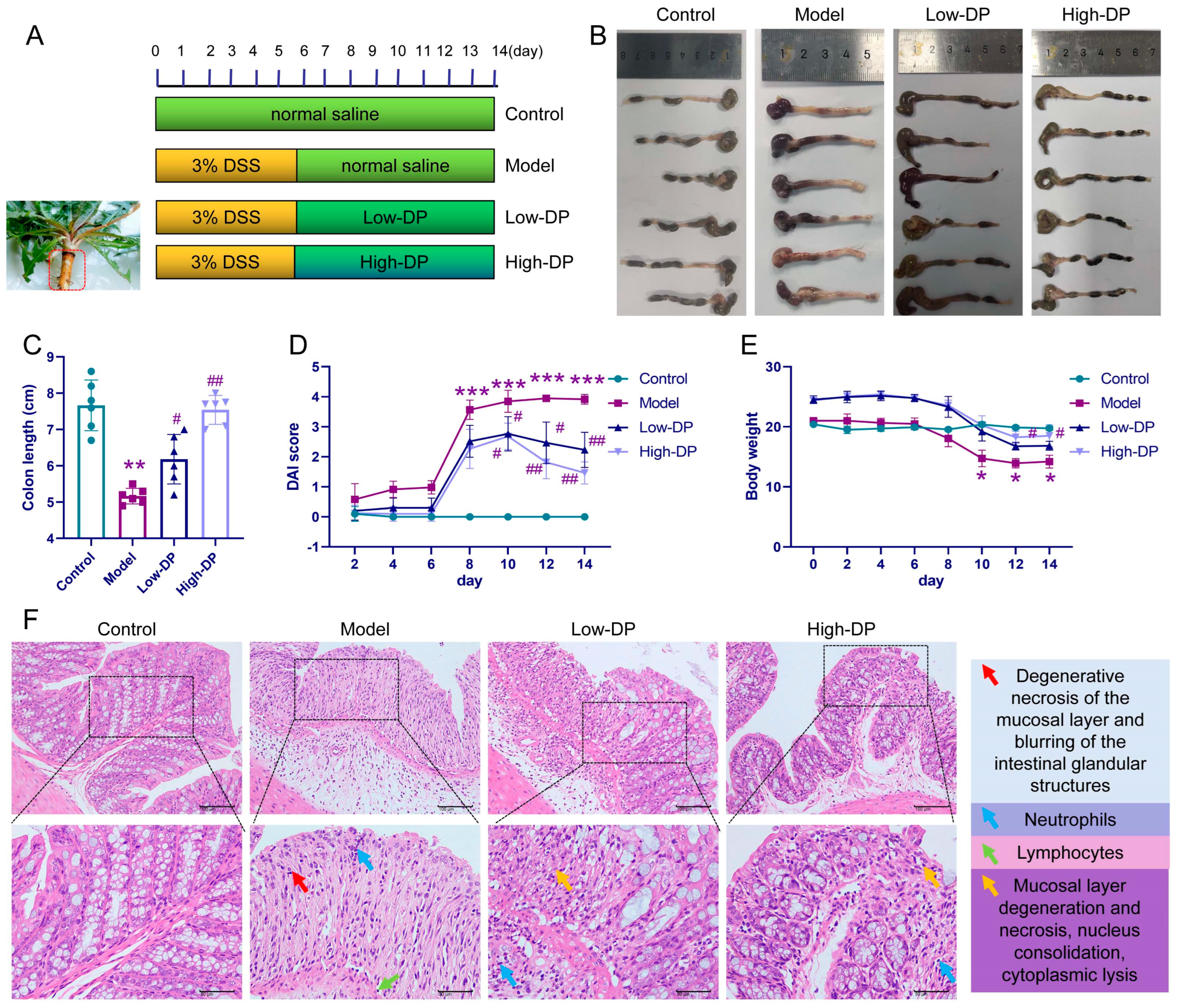
3.3. Protective Effect of DP on DSS-Induced UC Colitis Mice
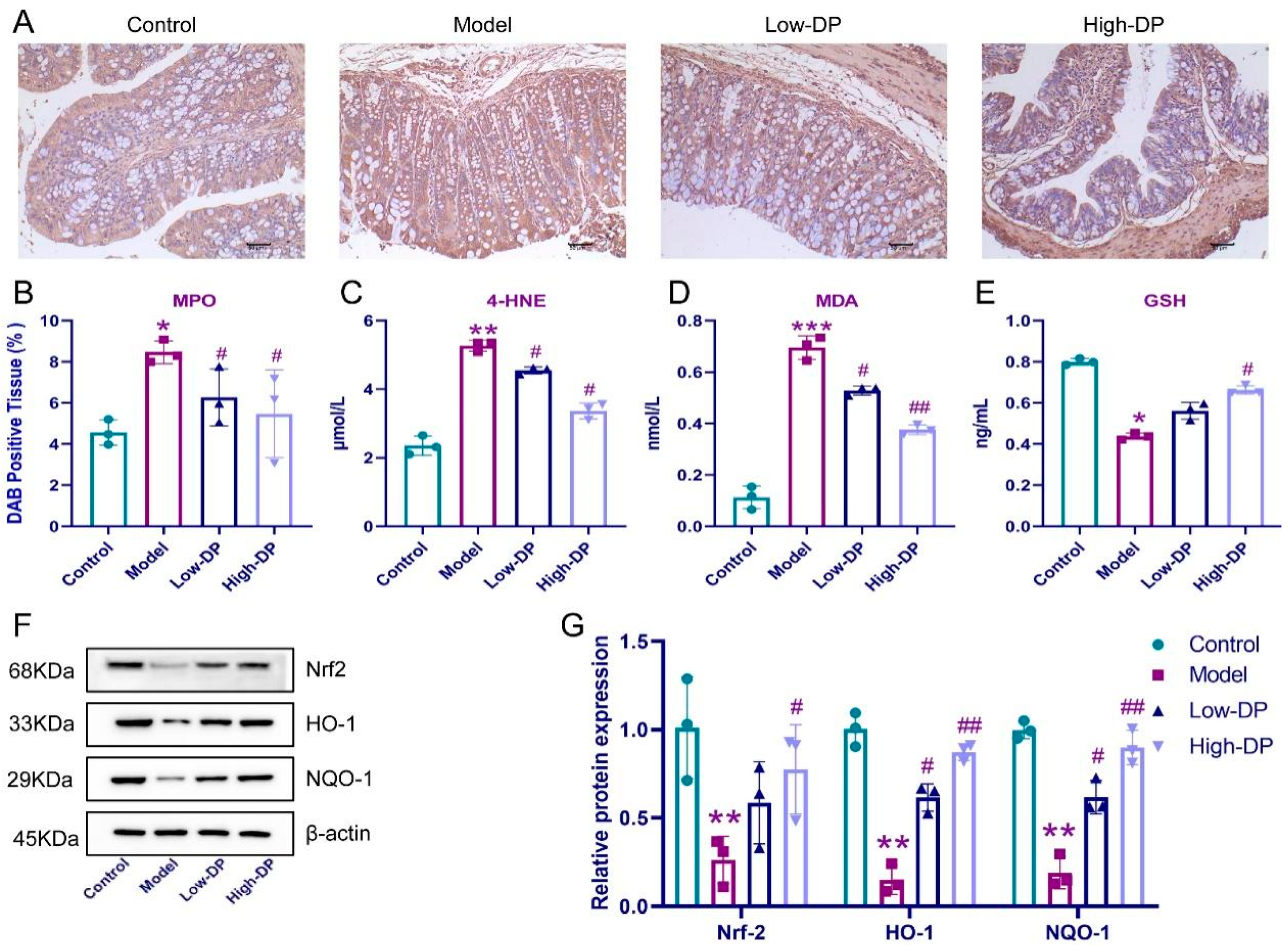
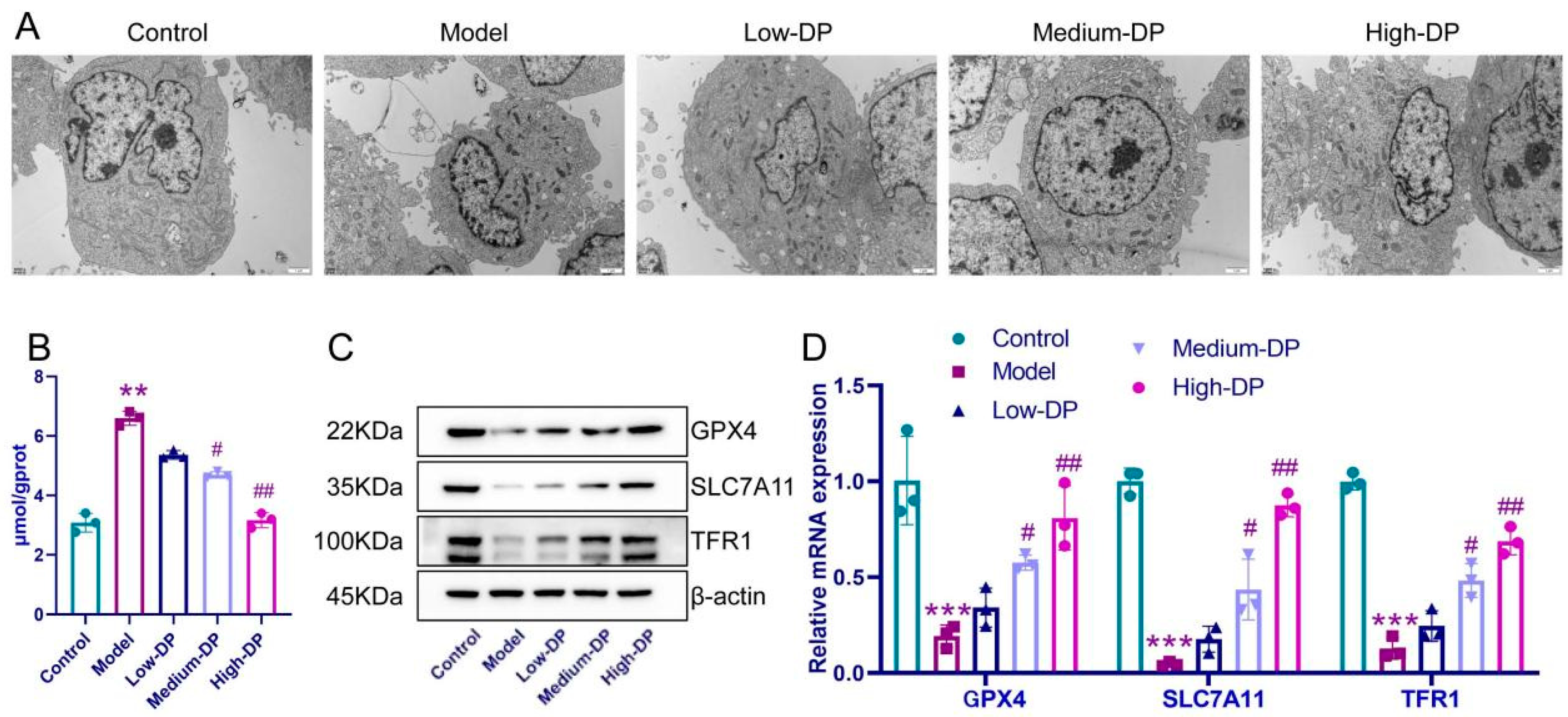
3.4. Inhibitory of DP on Ferroptosis in Colonic Epithelial Cells of UC Mice
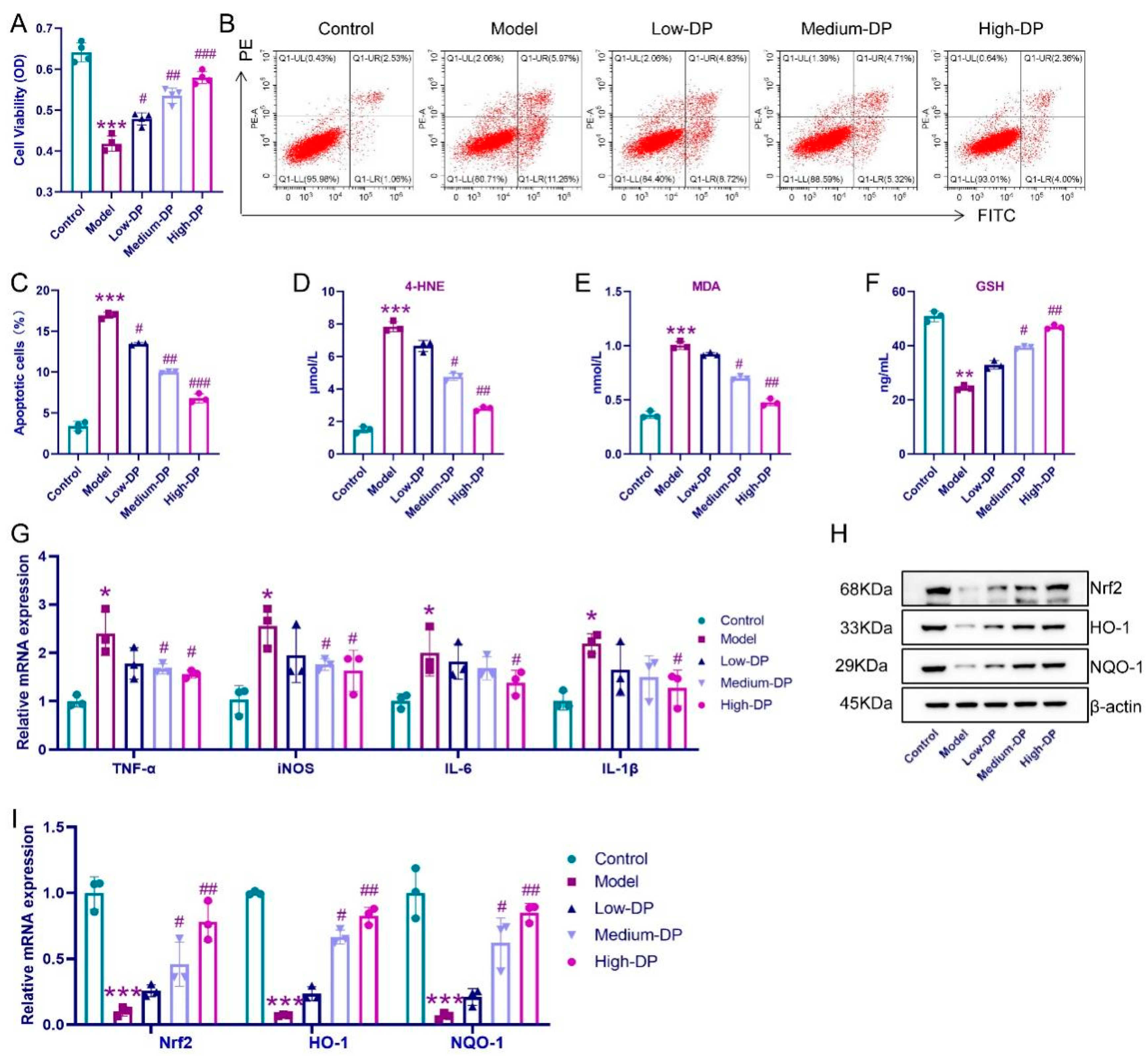
3.5. DP Enhance Antioxidant Defense in Colon of UC Mice
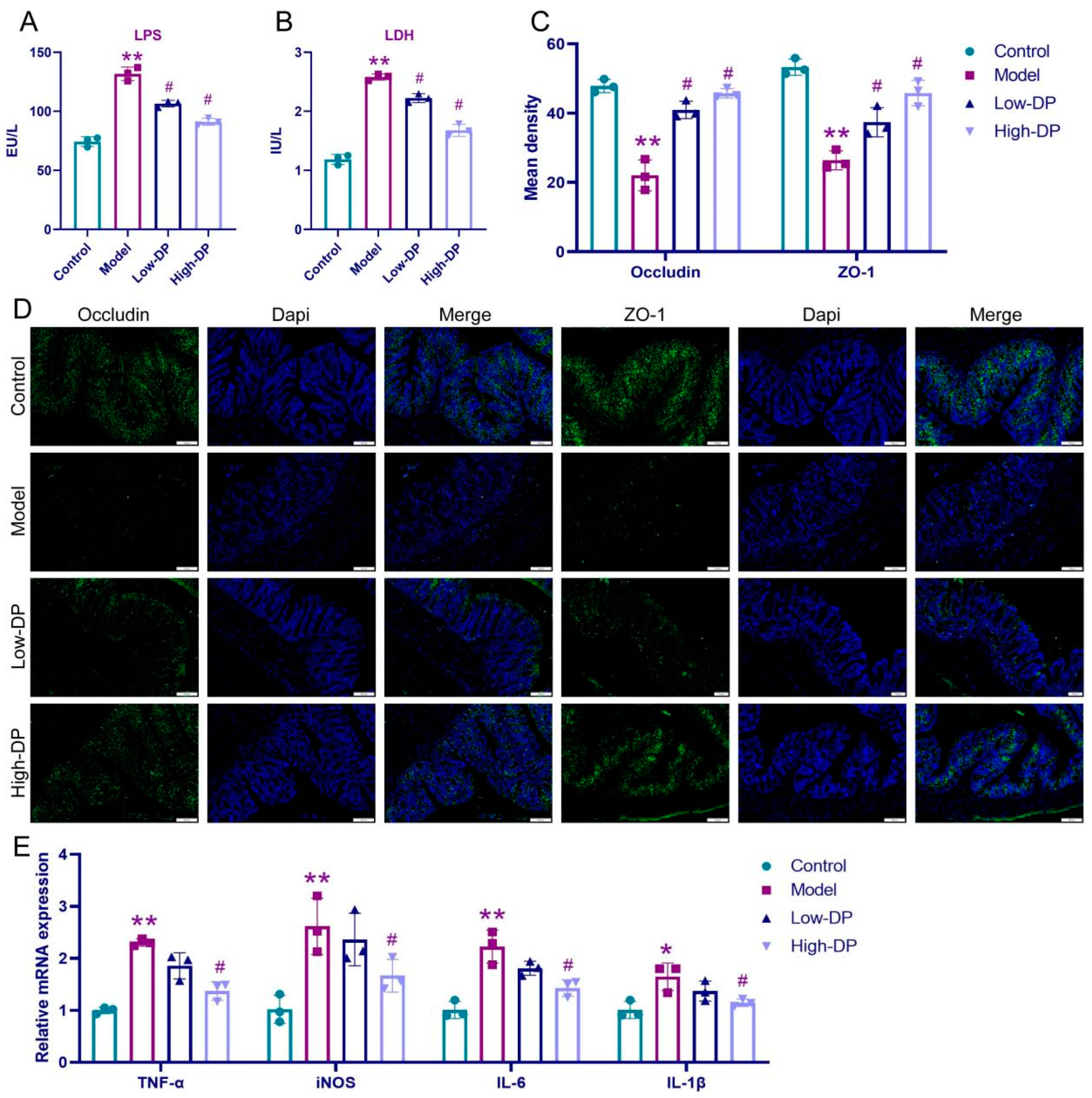
3.6. DP Repairs Intestinal Barrier and Inhibits Inflammation in UC Mice
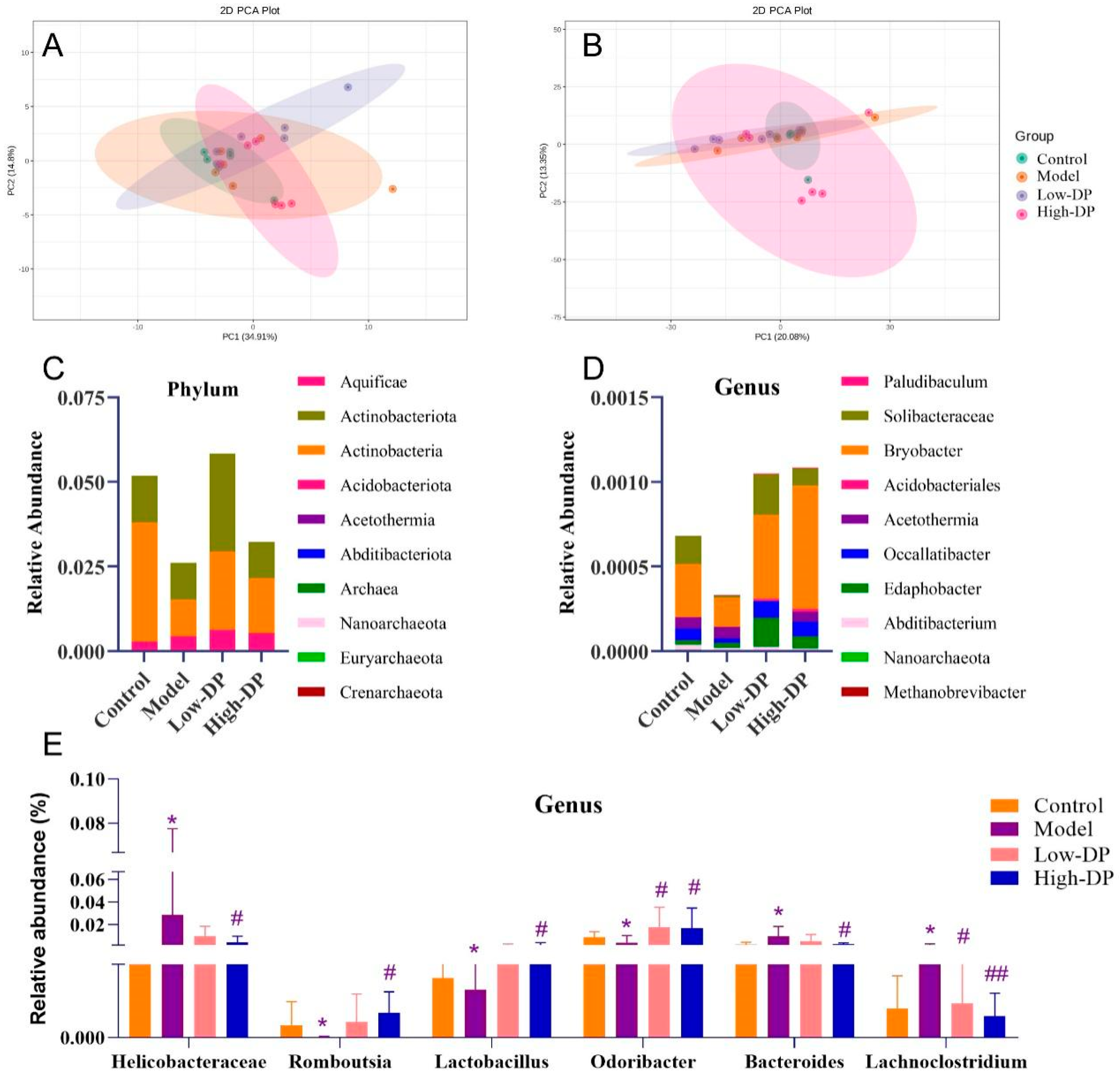
3.7. DP Adjustment of Intestinal Flora
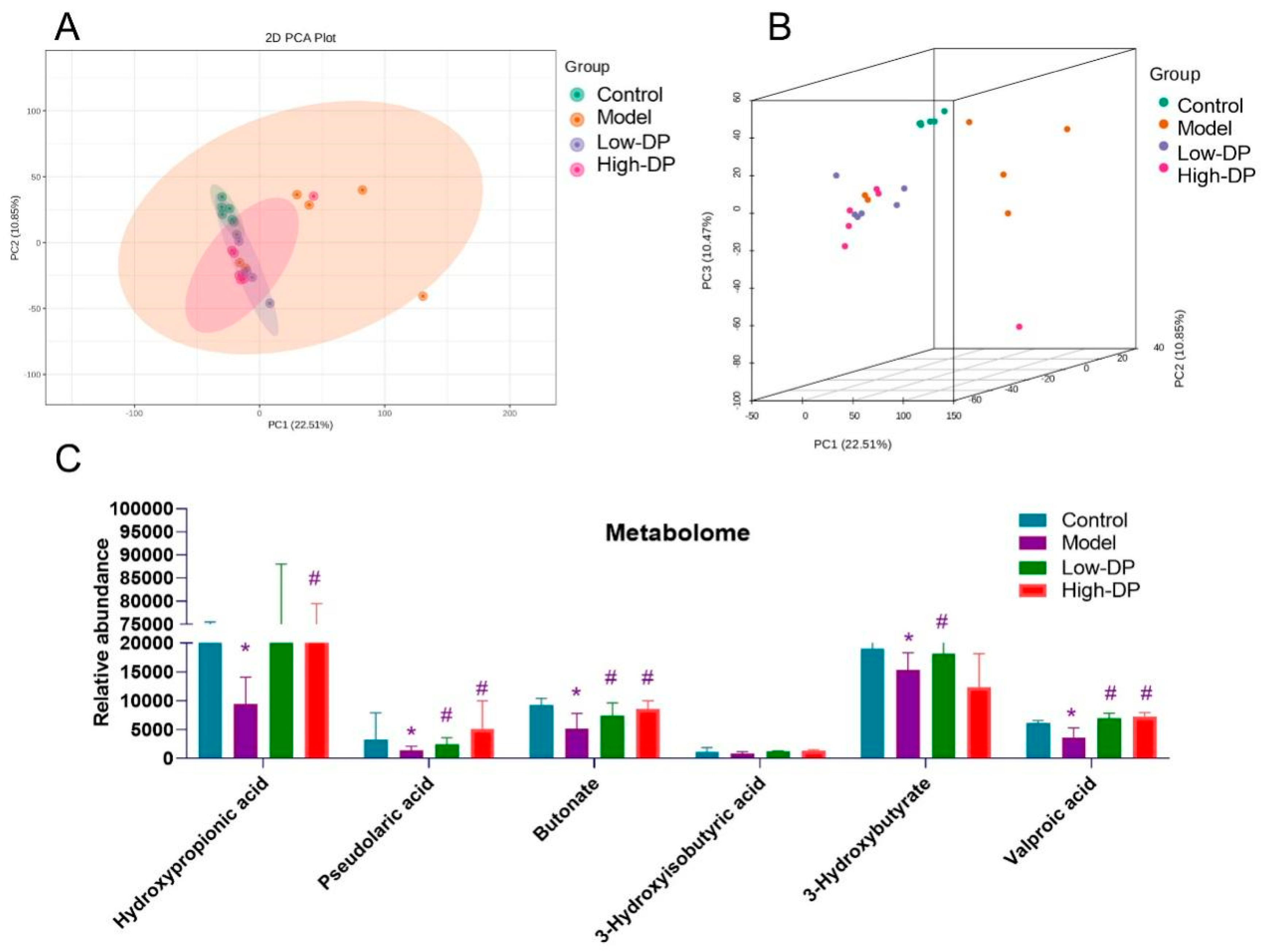
3.8. Metabolome Study of Short-Chain Fatty Acids in Feces
3.9. Anti-Inflammatory and Antioxidant Effects of DP on IEC-6 Cells
3.10. DP Inhibits Iron Death of IEC-6 Cells
4. Discussion
Future Improvement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- You, Y.; Yoo, S.; Yoon, H.G.; Park, J.; Lee, Y.H.; Kim, S.; Oh, K.T.; Lee, J.; Cho, H.Y.; Jun, W. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B. Cellulase-assisted extraction and antibacterial activity of polysaccharides from the dandelion Taraxacum officinale. Carbohydr. Polym. 2014, 103, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Schutz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef]
- Park, C.M.; Cho, C.W.; Song, Y.S. TOP 1 and 2, polysaccharides from Taraxacum officinale, inhibit NFkappaB-mediated inflammation and accelerate Nrf2-induced antioxidative potential through the modulation of PI3K-Akt signaling pathway in RAW 264.7 cells. Food Chem. Toxicol. 2014, 66, 56–64. [Google Scholar] [CrossRef]
- Berends, S.E.; Strik, A.S.; Löwenberg, M.; D’Haens, G.R.; Mathôt, R.A.A. Clinical Pharmacokinetic and Pharmacodynamic Considerations in the Treatment of Ulcerative Colitis. Clin. Pharmacokinet. 2019, 58, 15–37. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, M.; Liu, K. Colon-targeted drug delivery of polysaccharide-based nanocarriers for synergistic treatment of inflammatory bowel disease: A review. Carbohydr. Polym. 2021, 272, 118530. [Google Scholar] [CrossRef]
- Armuzzi, A.; Liguori, G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: A narrative review. Dig. Liver Dis. 2021, 53, 803–808. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Shamoon, M.; Martin, N.M.; O’brien, C.L. Recent advances in gut Microbiota mediated therapeutic targets in inflammatory bowel diseases: Emerging modalities for future pharmacological implications. Pharmacol. Res. 2019, 148, 104344. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Velayos, F.S. Day-by-Day Management of the Inpatient with Moderate to Severe Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2020, 16, 449–457. [Google Scholar]
- Sutherland, L.; Macdonald, J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2006, 2006, Cd000544. [Google Scholar]
- Yang, X.; Geng, J.; Meng, H. Glucocorticoid receptor modulates dendritic cell function in ulcerative colitis. Histol. Histopathol. 2020, 35, 1379–1389. [Google Scholar]
- Christophorou, D.; Funakoshi, N.; Duny, Y.; Valats, J.C.; Bismuth, M.; Pineton, D.C.G.; Daures, J.P.; Blanc, P. Systematic review with meta-analysis: Infliximab and immunosuppressant therapy vs. infliximab alone for active ulcerative colitis. Aliment. Pharmacol. Ther. 2015, 41, 603–612. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Osterman, M.T. Biologic Therapy for Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 717–729. [Google Scholar] [CrossRef]
- Magro, F.; Cordeiro, G.; Dias, A.M.; Estevinho, M.M. Inflammatory Bowel Disease—Non-biological treatment. Pharmacol. Res. 2020, 160, 105075. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, J.; Zhang, L.; Qin, N.B.; Zhu, B.; Xia, X. Jellyfish skin polysaccharides enhance intestinal barrier function and modulate the gut microbiota in mice with DSS-induced colitis. Food Funct. 2021, 12, 10121–10135. [Google Scholar] [CrossRef]
- Shao, X.; Sun, C.; Tang, X.; Zhang, X.; Han, D.; Liang, S.; Qu, R.; Hui, X.; Shan, Y.; Hu, L.; et al. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of Jinxiang Garlic (Allium sativum L.) Polysaccharides toward Dextran Sodium Sulfate-Induced Colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The impacts of natural polysaccharides on intestinal microbiota and immune responses—A review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef]
- Pope, J.L.; Bhat, A.A.; Sharma, A.; Ahmad, R.; Krishnan, M.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 2014, 63, 622–634. [Google Scholar] [CrossRef]
- Chen, W.; Fan, H.; Liang, R.; Zhang, R.; Zhang, J.; Zhu, J. Taraxacum officinale extract ameliorates dextran sodium sulphate-induced colitis by regulating fatty acid degradation and microbial dysbiosis. J. Cell Mol. Med. 2019, 23, 8161–8172. [Google Scholar] [CrossRef]
- Stojanović, O.; Altirriba, J.; Rigo, D.; Martina, S.; Emilien, E.; Benedek, R.; Salvatore, F.; Nicola, Z.; Pierre, M.; Françoise, R.J.; et al. Dietary excess regulates absorption and surface of gut epithelium through intestinal PPARα. Nat. Commun. 2021, 12, 7031. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Li, C.; Lei, L.; Duraiarasan, S.; Haiying, C. Antibacterial activity of PEO nanofibers incorporating polysaccharide from dandelion and its derivative. Carbohydr. Polym. 2018, 198, 225–232. [Google Scholar] [CrossRef]
- Cai, L.L.; Wan, D.W.; Yi, F.L.; Libiao, L. Purification, preliminary characterization and hepatoprotective effects of polysaccharides from dandelion root. Molecules 2017, 22, 1409. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Katharina, G.; Benno, W.; Stefan, F.F.; Markus, F.N. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, N.; Shim, Y.K.; Yoon, J.C.; Ryoung, H.N.; Yoon, J.C.; Min, H.H.; Ji, H.S.; Sun, M.L.; Chang, M.L.; et al. Adequate Dextran Sodium Sulfate-induced Colitis Model in Mice and Effective Outcome Measurement Method. J. Cancer Prev. 2015, 20, 260–267. [Google Scholar] [CrossRef]
- Wiegand, S.; Zakrzewski, S.S.; Eichner, M.; Emanuel, S.; Dorothee, G.; Robert, P.; Rita, R.; Christian, B.; André, B.; Ulrich, D.; et al. Zinc treatment is efficient against Escherichia coli α-haemolysin-induced intestinal leakage in mice. Sci. Rep. 2017, 7, 45649. [Google Scholar] [CrossRef]
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020, 11, 86. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Jiang, L.; Siwei, T.; Huiling, L.; Jie, J.; Fengping, Z.; Bin, W. Neuroprotective effects of morroniside from Cornus officinalis sieb. Et zucc against Parkinson’s disease via inhibiting oxidative stress and ferroptosis. BMC Complement. Med. Ther. 2023, 23, 218. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Liu, P.; Feng, Y.; Li, H.; Chen, X.; Wang, G.; Xu, S.; Li, Y.; Zhao, L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol. Biol. Lett. 2020, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.S.; Jiang, W.Y.; Park, P.H.; Dong, H.S.; Jae, H.C.; Sung, H.L. Hirsutenone reduces deterioration of tight junction proteins through EGFR/Akt and ERK1/2 pathway both converging to HO-1 induction. Biochem. Pharmacol. 2014, 90, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.; Wang, L.; Yang, D.; Bu, W.; Gou, L.; Huang, J.; Duan, X.; Pan, Y.; Cao, S.; et al. Troxerutin Improves Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2021, 69, 2729–2744. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, W.; Luo, C.; Zhang, X.; Huang, M. Atractylenolide III Ameliorates TNBS-Induced Intestinal Inflammation in Mice by Reducing Oxidative Stress and Regulating Intestinal Flora. Chem. Biodivers. 2021, 18, e2001001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Liu, J.; Qian, C.; Jin, C. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Hansen, J.J.; Holt, L.; Sartor, R.B. Gene expression patterns in experimental colitis in IL-10-deficient mice. Inflamm. Bowel Dis. 2009, 15, 890–899. [Google Scholar] [CrossRef]
- Krndija, D.; EL Marjou, F.; Guirao, B.; Richon, S.; Leroy, O.; Bellaiche, Y.; Hannezo, E.; Matic Vignjevic, D. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 2019, 365, 705–710. [Google Scholar] [CrossRef]
- Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; Santana-Filho, A.P.; Watanabe, P.S.; Ana, D.M.G.S.; Luciano, F.B.; Bocate, K.C.P.; Wijngaard, R.M.; Werner, M.F.P.; et al. Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human Caco-2 cells. Sci. Rep. 2018, 8, 12261. [Google Scholar] [CrossRef]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, S.; Liu, Y.; Wang, Y. The combined effect of food additive titanium dioxide and lipopolysaccharide on mouse intestinal barrier function after chronic exposure of titanium dioxide-contained feedstuffs. Part. Fibre Toxicol. 2021, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Gersemann, M.; Becker, S.; Kübler, I.; Koslowski, M.; Wang, G.; Herrlinger, K.R.; Griger, J.; Fritz, P.; Fellermann, K.; Schwab, M.; et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 2009, 77, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Huang, C.; Tang, Y.; Zhang, D.; Wu, Z.; Chen, X. Effect of Bacillus subtilis on Aeromonas hydrophila-induced intestinal mucosal barrier function damage and inflammation in grass carp (Ctenopharyngodon idella). Sci. Rep. 2017, 7, 1588. [Google Scholar] [CrossRef]
- Pickert, G.; Wirtz, S.; Matzner, J.; Muhammad, A.K.; Rosario, H.; Sebastian, R.; Dorothe, T.; Rambabu, S.; Dirk, E.; Jan, W.; et al. Wheat Consumption Aggravates Colitis in Mice via Amylase Trypsin Inhibitor-mediated Dysbiosis. Gastroenterology 2020, 159, 257–272.e17. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Sergeant, M.; Kay, G.; Tariq, I.; Jacqueline, C.; Chrystala, C.; Palak, T.; James, F.; David, H.A.; Mark, P.; et al. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut 2017, 66, 386–388. [Google Scholar] [CrossRef]
- Wan, P.; Peng, Y.; Chen, G.; Xie, M.; Dai, Z.; Huang, K.; Dong, W.; Zeng, X.; Sun, Y. Modulation of gut microbiota by Ilex kudingcha improves dextran sulfate sodium-induced colitis. Food Res. Int. 2019, 126, 108595. [Google Scholar] [CrossRef]
- Li, A.L.; Ni, W.W.; Zhang, Q.M.; Li, Y.; Zhang, X.; Wu, H.; Du, P.; Hou, J.; Zhang, Y. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol. 2020, 64, 23–32. [Google Scholar] [CrossRef]
- Lin, M.Y.; De Zoete, M.R.; Van Putten, J.P.; Strijbis, K. Redirection of Epithelial Immune Responses by Short-Chain Fatty Acids through Inhibition of Histone Deacetylases. Front. Immunol. 2015, 6, 554. [Google Scholar] [CrossRef]
- Salim, S.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Smitham, J.; Barrett, K.E. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G153–G162. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Higashikubo, A.; Tanaka, N.; Noda, N.; Maeda, I.; Yagi, K.; Mizoguchi, T.; Nanri, H. Increase in thioredoxin activity of intestinal epithelial cells mediated by oxidative stress. Biol. Pharm. Bull. 1999, 22, 900. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shi, X.; Yang, J.; Zhao, Y.; Xue, L.; Xu, L.; Cai, J. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell 2021, 12, 346–359. [Google Scholar] [CrossRef]
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2020, 13, 135. [Google Scholar] [CrossRef] [PubMed]

| Primer | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin (mous) | CTACCTCATGAAGATCCTGACC | CACAGCTTCTCTTTGATGTCAC |
| IL-1β (mous) | TCGCAGCAGCACATCAACAAGAG | AGGTCCACGGGAAAGACACAGG |
| IL-6 (mous) | CTCCCAACAGACCTGTCTATAC | CCATTGCACAACTCTTTTCTCA |
| iNOS (mous) | ATCTTGGAGCGAGTTGTGGATTGTC | TAGGTGAGGGCTTGGCTGAGTG |
| TNF-α (mous) | ATGTCTCAGCCTCTTCTCATTC | GCTTGTCACTCGAATTTTGAGA |
| β-actin (rat) | GGGAAATCGTGCGTGACATT | GCGGCAGTGGCCATCTC |
| IL-1β (rat) | ATCCTCTCCAGTCAGGCTTCCTTGTG | AGCTCTTGTCGAGATGCTGCTGTGA |
| IL-6 (rat) | ACTTCCAGCCAGTTGCCTTCTTG | TGGTCTGTTGTGGGTGGTATCCTC |
| iNOS (rat) | AAATCCTACCAAGGTGACCTGAAAGAG | CCTGTGTTGTTGGGCTGGGAATAG |
| TNF-α (rat) | ATGGGCTCCCTCTCATCAGTTCC | CCTCCGCTTGGTGGTTTGCTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Yin, L.; Dong, R. Inhibition of IEC-6 Cell Proliferation and the Mechanism of Ulcerative Colitis in C57BL/6 Mice by Dandelion Root Polysaccharides. Foods 2023, 12, 3800. https://doi.org/10.3390/foods12203800
Yan S, Yin L, Dong R. Inhibition of IEC-6 Cell Proliferation and the Mechanism of Ulcerative Colitis in C57BL/6 Mice by Dandelion Root Polysaccharides. Foods. 2023; 12(20):3800. https://doi.org/10.3390/foods12203800
Chicago/Turabian StyleYan, Shengkun, Lijun Yin, and Rong Dong. 2023. "Inhibition of IEC-6 Cell Proliferation and the Mechanism of Ulcerative Colitis in C57BL/6 Mice by Dandelion Root Polysaccharides" Foods 12, no. 20: 3800. https://doi.org/10.3390/foods12203800
APA StyleYan, S., Yin, L., & Dong, R. (2023). Inhibition of IEC-6 Cell Proliferation and the Mechanism of Ulcerative Colitis in C57BL/6 Mice by Dandelion Root Polysaccharides. Foods, 12(20), 3800. https://doi.org/10.3390/foods12203800





