Investigation of 60Co Irradiation on the Volatile Organic Compounds from Finger Citron (Citri Sarcodactylis Fructus) Using GC–IMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. 60Co Radiation
2.2.2. The Extraction Process of Finger Citron Volatile Oils
2.2.3. GC-IMS Analysis
2.2.4. Statistical Analysis
3. Results
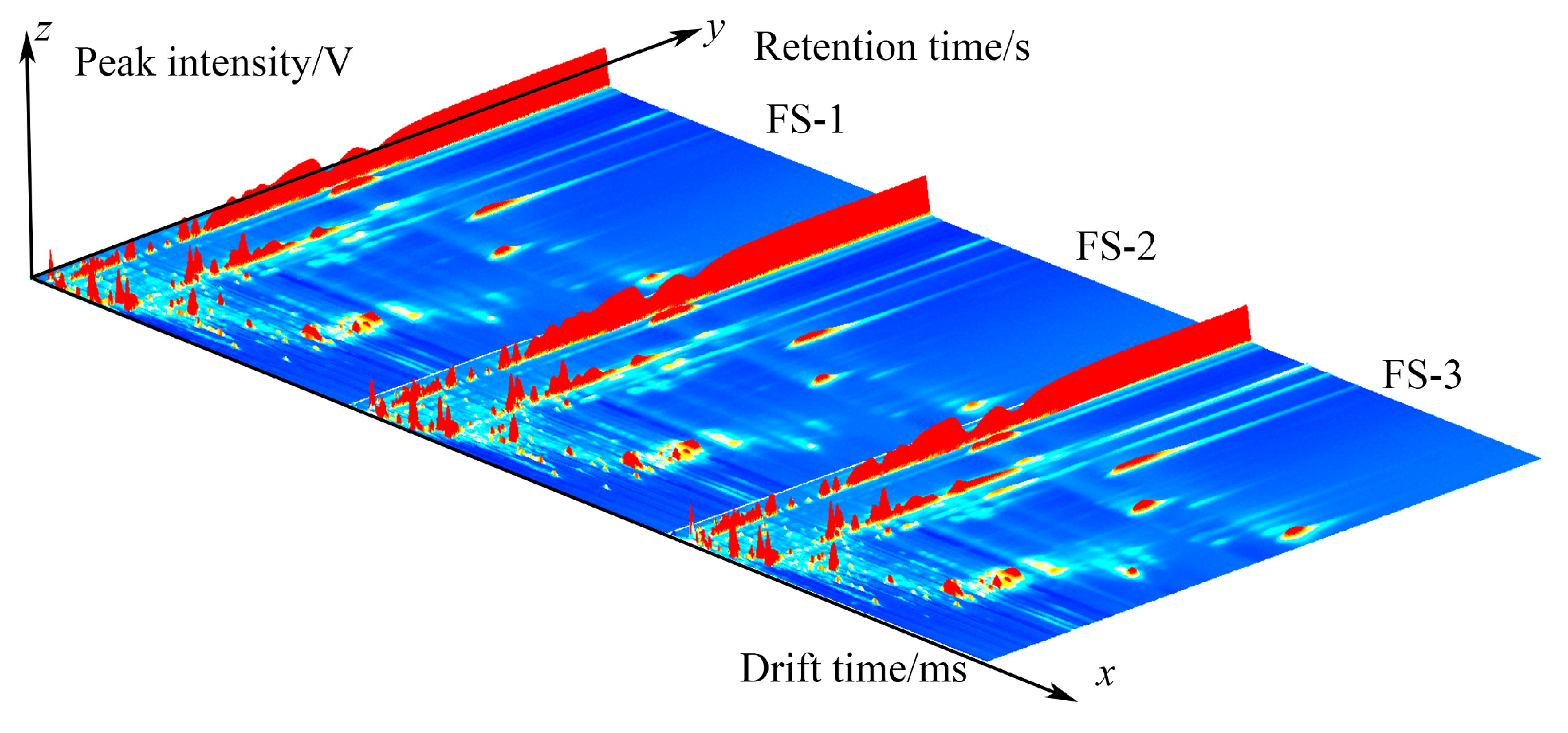
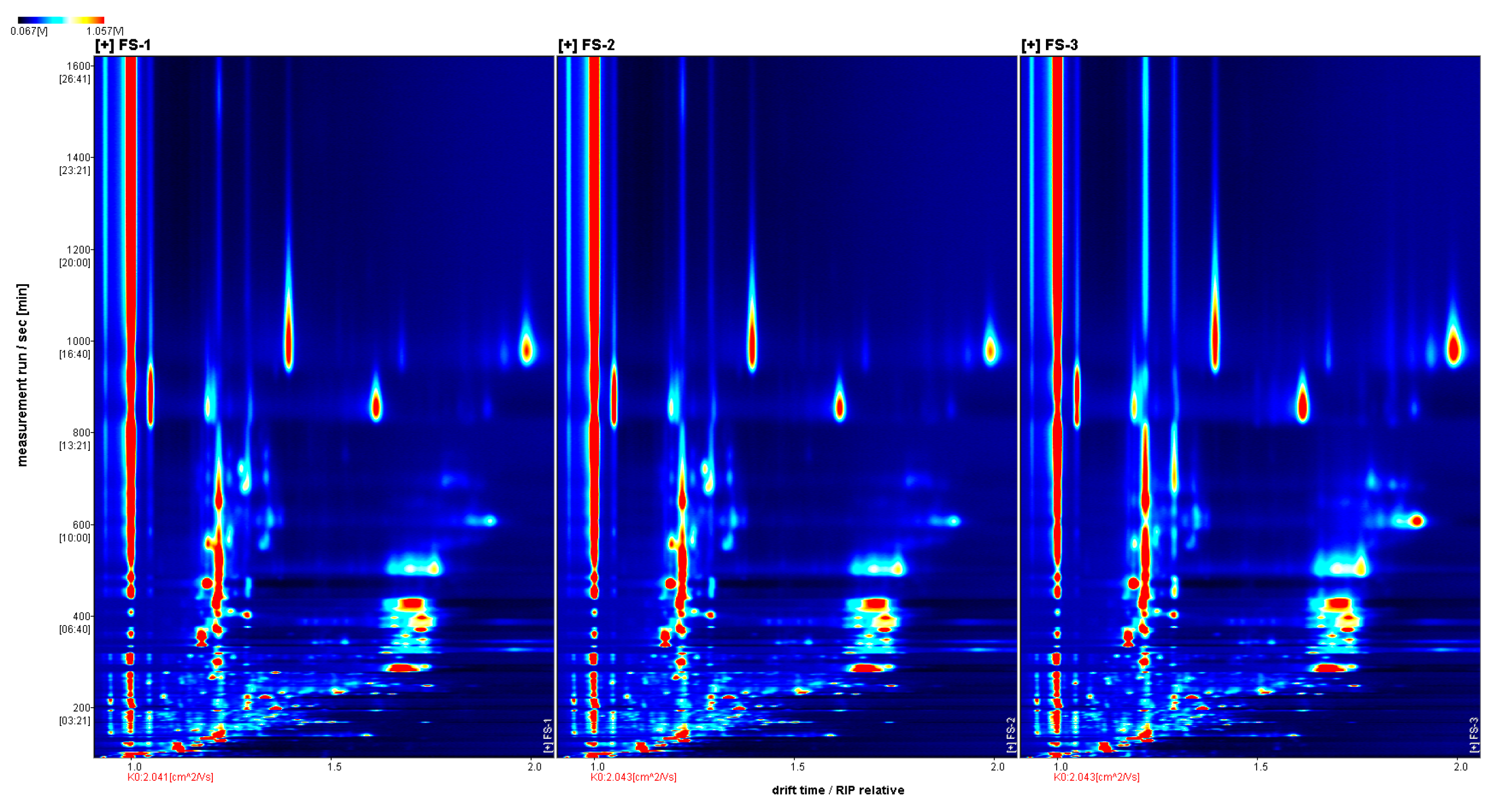
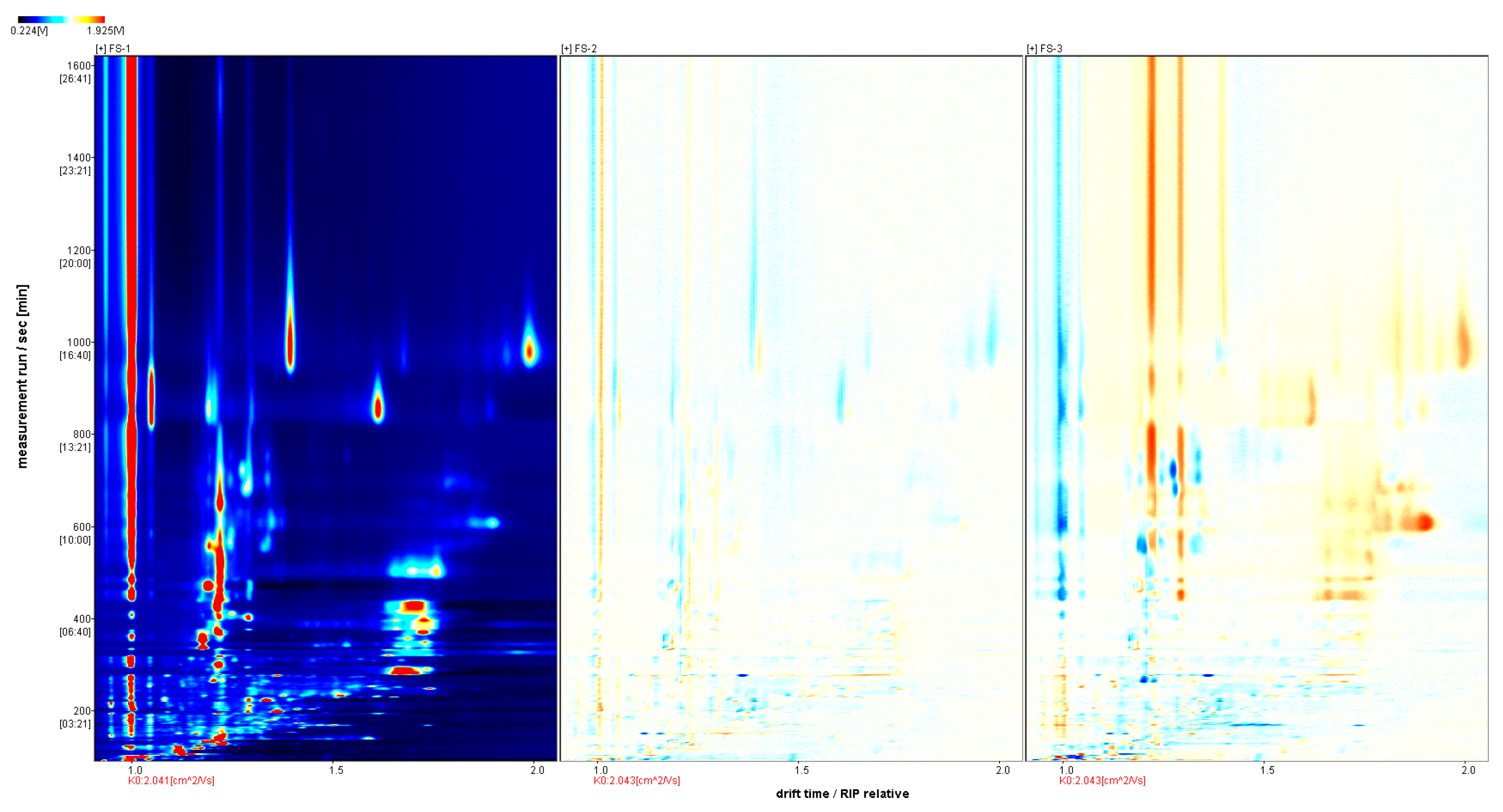
3.1. GC-IMS Profile of Finger Citron at Different Irradiation Doses
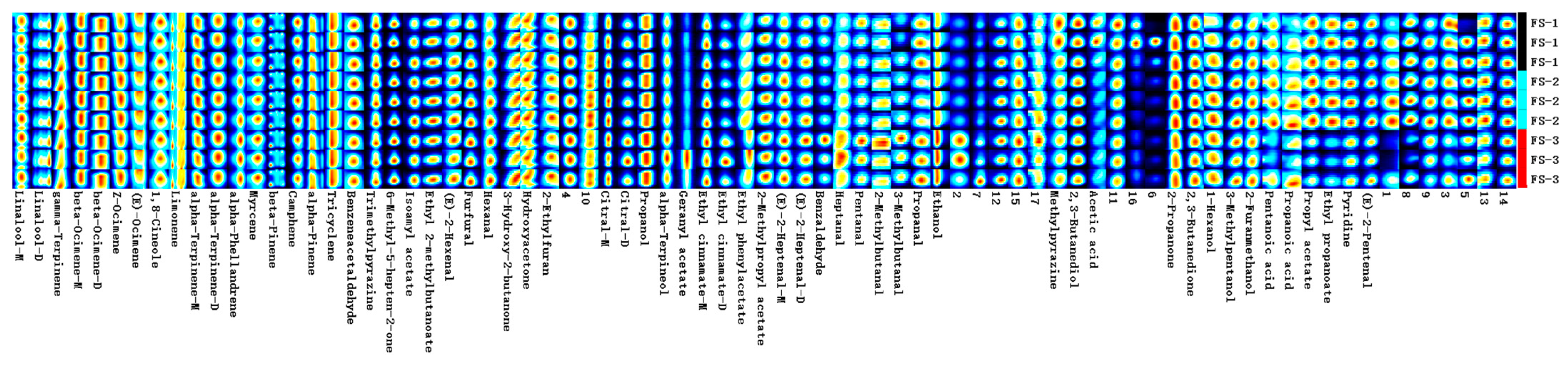
3.2. Qualitative Analysis of Volatile Organic Compounds in Finger Citron (GC × IMS Library Search)
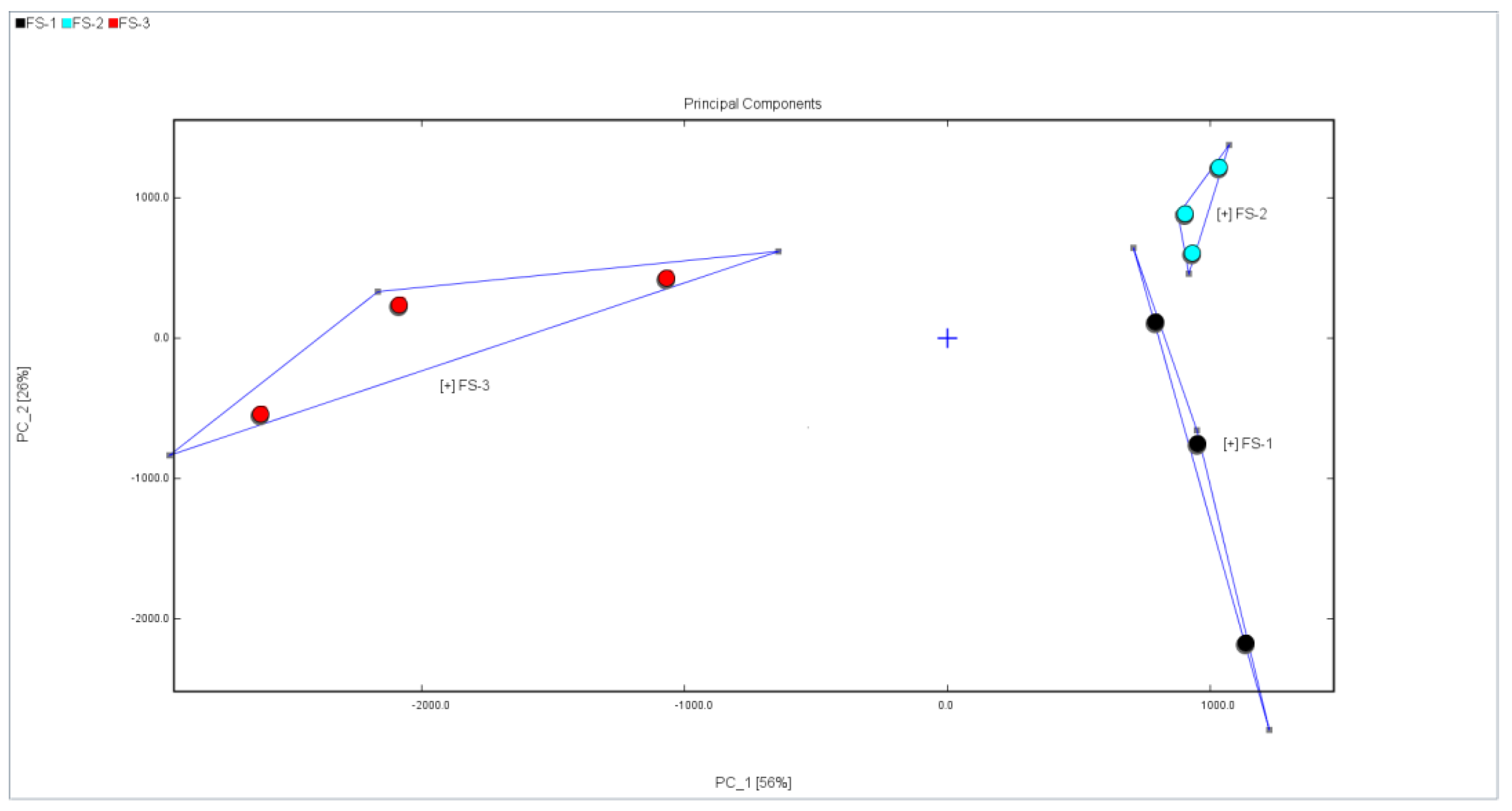
3.3. Principal Component Analysis (PCA) of Volatile Organic Compounds of Finger Citron Samples at 3 Irradiation Doses
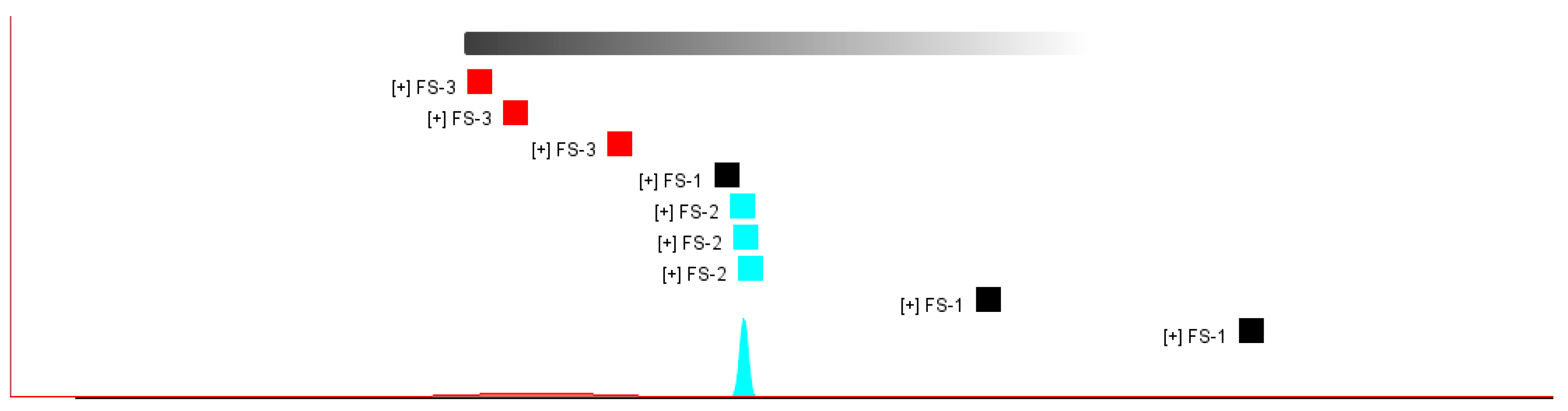
3.4. Fingerprint Analysis of Volatile Organic Compounds of Finger Citron at 3 Irradiation Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, S.; Zhao, D.; Ma, Y.; Zhang, K. Simultaneous determination of 6 chemical components in Citrus medica from different areas by quantitative analysis of multi-components with single marker method. Cent. South Pharm. 2022, 20, 1167–1172. [Google Scholar]
- Guo, W.D.; Zheng, J.S.; Deng, G. Study on the bacteriostatic activity of Citri Sarcodactylis Fructus volatile oils. J. Chin. Cereals Oils Assoc. 2009, 24, 103–107. [Google Scholar]
- O’Neill, A.J.; Chopra, I. Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opin. Investig. Drugs 2004, 13, 1045–1063. [Google Scholar] [CrossRef] [PubMed]
- Seukep, J.A.; Fankam, A.G.; Djeussi, D.E.; Voukeng, I.K.; Tankeo, S.B.; Noumdem, J.A.; Kuete, A.H.; Kuete, V. Antibacterial activities of the methanol extracts of seven Cameroonian dietary plants against bacteria expressing MDR phenotypes. Springerplus 2013, 2, 363. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.; Xu, X.; Gan, R.; Zhang, Y.; Xia, E.; Li, H. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Su, D.M.; Yao, F.H. 60Co—Optimal irradiation dose selection of proprietary Chinese medicines sterilized by γ irradiation (2). J. Pharm. Anal. 1992, 12, 4. [Google Scholar]
- Li, Z.W.; Zhang, S.M. Preliminary study of ointment γ irradiation sterilization. Acta Laser Biol. Sin. 1994, 3, 51–60. [Google Scholar]
- Zhao, X.L.; Jiang, H. 60Co-γ irradiation sterilization study of hospital preparations. Zhejiang Med. J. 1998, 20, 448. [Google Scholar]
- Luo, D.; Zhao, S.; Tang, Y.; Wang, Q.; Liu, H.; Ma, S. Analysis of the Effect of 60Co-γ Irradiation Sterilization Technology on the Chemical Composition of Saffron Using UPLC and UPLC/Q-TOF-MS. J. Anal. Methods Chem. 2018, 2018, 2402676. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhao, X.J.; Sun, Z.M.; Chen, H.; Sun, G.L.; Li, X.Y. Radiation decontamination of mycelium powder of sinensis by 60Co γ-rays. J. Radiat. Res. Radiat. Process. 2005, 32, 122. [Google Scholar]
- Xu, F.; Wang, Y.J.; Sun, Y.; Liang, Y.; Li, M.Z.; Pu, S.H.; An, T.Z. Effect of 60Co-γ Ray Irradiation on the Effective Components of Radix Trichosanthis. China Pharm. 2019, 4, 355–358. [Google Scholar]
- Zhao, X.J.; Shi, J.J.; Sun, Z.M.; Zhu, B.Q. Decontamination of Fermental Powder of Cepha/osporium Sinensis and Influence on Its Main Components by Irradiation. Sci. Agric. Sin. 2003, 36, 5. [Google Scholar]
- Wang, E.B.; Zhao, Z.B.; Qu, T.L. Effect of 60Co irradiation sterilization on the main components of anti-rinse oral liquid. Chin. Tradit. Pat. Med. 2013, 35, 3. [Google Scholar]
- Chen, D.J.; Zhang, M.G.; Nei, X.B.; Jiang, P.H.; Zhang, Y.H.; Zhang, C.F.; Ren, F. Quality Detection of Turbot (Scophtalmus marimus) Treated with Electrostatic Field Using Gas Chromatography-Ion Mobility Spectrometry. Food Sci. 2019, 40, 7. [Google Scholar]
- Moura, P.C.; Santos, F.; Fujão, C.; Vassilenko, V. In Situ Indoor Air Volatile Organic Compounds Assessment in a Car Factory Painting Line. Processes 2023, 11, 2259. [Google Scholar] [CrossRef]
- Fu, J.; Sun, Y.; Cui, M.; Zhang, E.; Dong, L.; Wang, Y.; Wang, W.; Li, Z.; Yang, J. Analysis of Volatile Compounds and Flavor Fingerprint Using Gas Chromatography–Ion Mobility Spectrometry (GC-IMS) on Crassostrea gigas with Different Ploidy and Gender. Molecules 2023, 28, 4475. [Google Scholar] [CrossRef]
- He, Y.; Yin, L.; Zhou, W.; Wan, H.; Lei, C.; Li, S.; Huang, D. Evaluation of 60Co Irradiation on Volatile organic compounds of Turmeric (Curcumae Longae Rhizoma) Volatile Oil with GC–IMS. Foods 2023, 12, 2489. [Google Scholar] [CrossRef]
- Lei, C.; Liu, J.; Zhou, W.; Zhou, W.; Li, S.; Huang, D. Influence of 60Co-Irradiation on the Components of Essential Oil of Curcuma. Molecules 2023, 28, 5877. [Google Scholar] [CrossRef]
- Freire, R.; Fernandez, L.; Mallafré-Muro, C.; Martín-Gómez, A.; Madrid-Gambin, F.; Oliveira, L.; Pardo, A.; Arce, L.; Marco, S. Full Workflows for the Analysis of Gas Chromatography—Ion Mobility Spectrometry in Foodomics: Application to the Analysis of Iberian Ham Aroma. Sensors 2021, 21, 6156. [Google Scholar] [CrossRef]
- Gabelica, V.; Marklund, E. Fundamentals of ion mobility spectrometry. Curr. Opin. Chem. Biol. 2018, 42, 51–59. [Google Scholar] [CrossRef]
- Vautz, W.; Seifert, L.; Mohammadi, M.; Klinkenberg, I.A.G.; Liedtke, S. Detection of axillary perspiration metabolites using ion mobility spectrometry coupled to rapid gas chromatography. Anal. Bioanal. Chem. 2020, 412, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ruzsanyi, V.; Mochalski, P.; Schmid, A.; Wiesenhofer, H.; Klieber, M.; Hinterhuber, H.; Amann, A. Ion mobility spectrometry for detection of skin volatiles. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 911, 84–92. [Google Scholar] [CrossRef]
- Puton, J.; Holopainen, S.I.; Mäkinen, M.A.; Sillanpää, M.E. Quantitative response of IMS detector for mixtures containing two active components. Anal. Chem. 2012, 84, 9131–9138. [Google Scholar] [CrossRef]
- Li, F.; Xie, Z.; Schmidt, H.; Sielemann, S.; Baumbach, J. Ion mobility spectrometer for online monitoring of trace compounds. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 1563–1574. [Google Scholar] [CrossRef]
- Mayer, T.; Borsdorf, H. Accuracy of ion mobility measurements dependent on the influence of humidity. Anal. Chem. 2014, 86, 5069–5076. [Google Scholar] [CrossRef]
- Moura, P.C.; Fernandes, J.M.; Diniz, M.S.; Fetter, V.; Vassilenko, V. Differentiation of the Organoleptic Volatile Organic Compound Profile of Three Edible Seaweeds. Metabolites 2023, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Yan, W. Research progress on chemical constituents and pharmacological effects of Formosante. J. Pract. Tradit. Chin. Med. 2015, 31, 3. [Google Scholar]
- Jin, X.L.; Xu, L.S.; Zheng, X.H. Chemical Compositions of Essential Oil from Beregamot. J. Instrum. Anal. 2000, 19, 3. [Google Scholar]
- Wang, R.; Zhu, F.F.; Sun, Y.L.; Hu, H.F. Analysis of fingerprint differences in volatile substances in leaves of different walnut varieties by GC-IMS. J. Fruit Sci. 2021, 38, 1930–1941. [Google Scholar]
- Christmann, J.; Rohn, S.; Weller, P. GC-IMS data on the discrimination between geographic origins of olive oils. Data Brief 2022, 45, 108730. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Xu, X.; Zhao, Y. Influence of thermal processing on flavor and sensory profile of sturgeon meat. Food Chem. 2022, 374, 131689. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, B.; Dong, X.; Wei, Y.; Li, H.; Tang, B. Differentiation of Goat Meat Freshness Using Gas Chromatography with Ion Mobility Spectrometry. Molecules 2023, 28, 3874. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, L.; Liu, J.; Zhang, X.; Lu, Y. Analysis of the Volatile Flavor Compounds of Pomegranate Seeds at Different Processing Temperatures by GC-IMS. Molecules 2023, 28, 2717. [Google Scholar] [CrossRef]
- Guo, X.; Schwab, W.; Ho, C.T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2021, 376, 131933. [Google Scholar] [CrossRef]
- Li, J.P. Discussion on the application of γ-ray irradiation sterilization in traditional Chinese medicine and its preparations and related problems. China J. Chin. Mater. Med. 2007, 32, 2078–2081. [Google Scholar]
- Huang, C.H.; Lee, S.C.; Chen, Y.S.; Yao, C.H. Evaluation of 60Co-gamma radiosterilization on Chinese medicines with HPLC/FTIR. Biomed. Chromatogr. 2010, 24, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.T.; Liu, C.Q.; Yu, G.; Zhao, Y.F.; Ji, P.; Jin, J.; Gu, G.Q. Effect of Irradiation Decontamination on the Qualities of Green Tea. J. Nucl. Agric. Sci. 2005, 19, 363–366. [Google Scholar]
- Wang, C.; Li, X.T.; Tao, H.; Meng, X.J. Effect of 60Co γ-Iradiation on Storage Quality and Membrane Lipid Peroxidation of Blueberry Fruits during Cold Storage. Food Sci. 2016, 37, 318–323. [Google Scholar]
- Wang, F.; Tan, Z.; Xu, Q.; Gao, Y.; Wu, J.F.; Li, Y.P.; Zeng, X.X. Study on effects of Baoji Pills’ 60Co irradiation sterilization on contents of puerarin, hesperidin and naringine and intestinal propulsion. J. Guangdong Pharm. Univ. 2017, 33, 326–330. [Google Scholar]
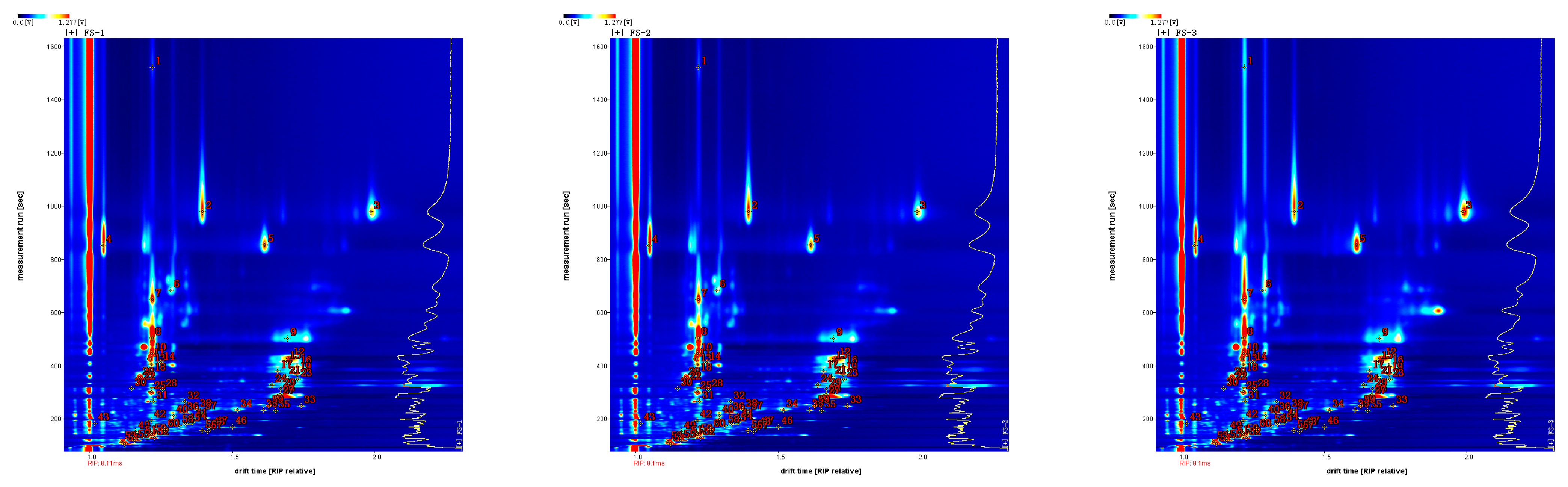
| Count | Compound | CAS | Molecular Formula | RI | Rt/s | Dt/ms (RIPrel) | Comment |
|---|---|---|---|---|---|---|---|
| 1 | Geranyl acetate | C105873 | C12H20O2 | 1815.8 | 1521.204 | 1.22204 | - |
| 2 | Ethyl cinnamate | C103366 | C11H12O2 | 1438.3 | 979.259 | 1.39763 | Monomers |
| 3 | Ethyl cinnamate | C103366 | C11H12O2 | 1438.3 | 979.259 | 1.9898 | Dimers |
| 4 | Citral | C5392405 | C10H16O | 1350.0 | 852.464 | 1.04831 | Monomers |
| 5 | Citral | C5392405 | C10H16O | 1351.4 | 854.509 | 1.61619 | Dimers |
| 6 | Ethyl phenylacetate | C101973 | C10H12O2 | 1232.0 | 683.103 | 1.28529 | - |
| 7 | alpha-Terpineol | C98555 | C10H18O | 1208.0 | 648.582 | 1.22095 | - |
| 8 | Linalool | C78706 | C10H18O | 1107.4 | 504.171 | 1.22218 | Monomers |
| 9 | Linalool | C78706 | C10H18O | 1106.2 | 502.483 | 1.69512 | Dimers |
| 10 | gamma-Terpinene | C99854 | C10H16 | 1066.8 | 445.944 | 1.21965 | - |
| 11 | beta-Ocimene | C13877913 | C10H16 | 1052.3 | 425.051 | 1.21421 | Monomers |
| 12 | beta-Ocimene | C13877913 | C10H16 | 1054.1 | 427.662 | 1.70437 | Dimers |
| 13 | Z-Ocimene | C3338554 | C10H16 | 1038.2 | 404.81 | 1.21829 | - |
| 14 | Benzeneacetaldehyde | C122781 | C8H8O | 1042.3 | 410.686 | 1.25097 | - |
| 15 | (E)-Ocimene | C3779611 | C10H16 | 1045.0 | 414.604 | 1.69211 | - |
| 16 | 1,8-Cineole | C470826 | C10H18O | 1033.7 | 398.281 | 1.73024 | - |
| 17 | Limonene | C138863 | C10H16 | 1021.4 | 380.653 | 1.6608 | - |
| 18 | alpha-Terpinene | C99865 | C10H16 | 1014.6 | 370.859 | 1.21693 | Monomers |
| 19 | alpha-Terpinene | C99865 | C10H16 | 1015.0 | 371.512 | 1.72751 | Dimers |
| 20 | Trimethylpyrazine | C14667551 | C7H10N2 | 1003.6 | 355.189 | 1.17608 | - |
| 21 | alpha-Phellandrene | C99832 | C10H16 | 1006.4 | 359.107 | 1.68939 | - |
| 22 | 6-Methyl-5-hepten-2-one | C110930 | C8H14O | 988.6 | 338.213 | 1.18017 | - |
| 23 | Myrcene | C123353 | C10H16 | 999.5 | 349.313 | 1.7316 | - |
| 24 | beta-Pinene | C127913 | C10H16 | 976.2 | 327.767 | 1.64037 | - |
| 25 | Camphene | C79925 | C10H16 | 946.9 | 302.956 | 1.21693 | - |
| 26 | alpha-Pinene | C80568 | C10H16 | 932.2 | 290.551 | 1.67033 | - |
| 27 | Tricyclene | C508327 | C10H16 | 923.7 | 283.369 | 1.66761 | - |
| 28 | (E)-2-Heptenal | C18829555 | C7H12O | 955.4 | 310.138 | 1.25778 | Monomers |
| 29 | (E)-2-Heptenal | C18829555 | C7H12O | 957.2 | 311.703 | 1.67193 | Dimers |
| 30 | Benzaldehyde | C100527 | C7H6O | 961.1 | 315.009 | 1.14929 | - |
| 31 | Pentanoic acid | C109524 | C5H10O2 | 903.0 | 265.829 | 1.22569 | - |
| 32 | Heptanal | C111717 | C7H14O | 902.0 | 265.002 | 1.33482 | - |
| 33 | Isoamyl acetate | C123922 | C7H14O2 | 877.2 | 248.884 | 1.74347 | - |
| 34 | (E)-2-Hexenal | C6728263 | C6H10O | 847.8 | 233.593 | 1.52035 | - |
| 35 | Ethyl 2-methylbutanoate | C7452791 | C7H14O2 | 843.0 | 231.113 | 1.65374 | - |
| 36 | Furfural | C98011 | C5H4O2 | 827.9 | 223.26 | 1.33361 | - |
| 37 | Methylpyrazine | C109080 | C5H6N2 | 832.7 | 225.74 | 1.39545 | - |
| 38 | 2-Furanmethanol | C98000 | C5H6O2 | 849.4 | 234.419 | 1.37848 | - |
| 39 | 3-Methylpentanol | C589355 | C6H14O | 846.2 | 232.766 | 1.6113 | - |
| 40 | Hexanal | C66251 | C6H12O | 805.6 | 211.688 | 1.29481 | - |
| 41 | 2,3-Butanediol | C513859 | C4H10O2 | 781.1 | 199.29 | 1.36392 | - |
| 42 | 2-Methylpropyl acetate | C110190 | C6H12O2 | 771.3 | 195.306 | 1.21788 | - |
| 43 | Pyridine | C110861 | C5H5N | 742.8 | 183.678 | 1.01998 | - |
| 44 | (E)-2-Pentenal | C1576870 | C5H8O | 748.2 | 185.905 | 1.36201 | - |
| 45 | 3-Hydroxy-2-butanone | C513860 | C4H8O2 | 734.2 | 180.215 | 1.33799 | - |
| 46 | Propyl acetate | C109604 | C5H10O2 | 708.7 | 169.825 | 1.50272 | - |
| 47 | Ethyl propanoate | C105373 | C5H10O2 | 706.9 | 169.082 | 1.43637 | - |
| 48 | Pentanal | C110623 | C5H10O | 696.0 | 164.629 | 1.42493 | - |
| 49 | Acetic acid | C64197 | C2H4O2 | 651.9 | 151.937 | 1.16181 | - |
| 50 | Hydroxyacetone | C116096 | C3H6O2 | 608.7 | 140.528 | 1.22029 | - |
| 51 | 2,3-Butanedione | C431038 | C4H6O2 | 585.8 | 134.474 | 1.18545 | - |
| 52 | Propanol | C71238 | C3H8O | 564.6 | 128.886 | 1.22775 | - |
| 53 | 2-Propanone | C67641 | C3H6O | 503.8 | 112.821 | 1.11577 | - |
| 54 | Ethanol | C64175 | C2H6O | 475.6 | 105.37 | 1.12323 | - |
| 55 | Propanal | C123386 | C3H6O | 526.7 | 118.874 | 1.15932 | - |
| 56 | 2-Methylbutanal | C96173 | C5H10O | 663.4 | 154.964 | 1.39573 | - |
| 57 | 3-Methylbutanal | C590863 | C5H10O | 651.9 | 151.937 | 1.41564 | - |
| 58 | 2-Ethylfuran | C3208160 | C6H8O | 720.3 | 174.522 | 1.31485 | - |
| 59 | 1-Hexanol | C111273 | C6H14O | 875.7 | 248.098 | 1.6309 | - |
| 60 | Propanoic acid | C79094 | C3H6O2 | 682.8 | 160.086 | 1.26384 | - |
| Count | Compound | CAS | Molecular Formula | Comment | [+] FS-1 | [+] FS-2 | [+] FS-3 |
|---|---|---|---|---|---|---|---|
| 1 | Geranyl acetate | C105873 | C12H20O2 | - | 2174.20 | 2809.97 | 3242.02 |
| 2 | Ethyl cinnamate | C103366 | C11H12O2 | Monomers | 21,007.74 | 22,228.10 | 25,490.10 |
| 3 | Ethyl cinnamate | C103366 | C11H12O2 | Dimers | 10,415.91 | 10,665.09 | 15,509.94 |
| 4 | Citral | C5392405 | C10H16O | Monomers | 17,817.99 | 19,651.39 | 20,557.82 |
| 5 | Citral | C5392405 | C10H16O | Dimers | 10,485.74 | 10,952.11 | 14,868.86 |
| 6 | Ethyl phenylacetate | C101973 | C10H12O2 | - | 1801.01 | 2259.74 | 1868.36 |
| 7 | alpha-Terpineol | C98555 | C10H18O | - | 6645.98 | 7106.53 | 8627.20 |
| 8 | Linalool | C78706 | C10H18O | Monomers | 14,479.82 | 15,034.59 | 15,002.15 |
| 9 | Linalool | C78706 | C10H18O | Dimers | 18,387.31 | 17,681.03 | 19,107.66 |
| 10 | gamma-Terpinene | C99854 | C10H16 | - | 4928.03 | 5324.99 | 5441.35 |
| 11 | beta-Ocimene | C13877913 | C10H16 | Monomers | 6569.52 | 6771.70 | 6558.95 |
| 12 | beta-Ocimene | C13877913 | C10H16 | Dimers | 10,060.84 | 10,008.38 | 10,162.43 |
| 13 | Z-Ocimene | C3338554 | C10H16 | - | 1540.19 | 1642.53 | 1585.68 |
| 14 | Benzeneacetaldehyde | C122781 | C8H8O | - | 1117.73 | 1117.20 | 1082.82 |
| 15 | (E)-Ocimene | C3779611 | C10H16 | - | 5078.11 | 5200.52 | 5338.61 |
| 16 | 1,8-Cineole | C470826 | C10H18O | - | 1305.46 | 1319.25 | 1309.89 |
| 17 | Limonene | C138863 | C10H16 | - | 5690.72 | 5756.64 | 5768.12 |
| 18 | alpha-Terpinene | C99865 | C10H16 | Monomers | 4689.79 | 4884.76 | 4749.27 |
| 19 | alpha-Terpinene | C99865 | C10H16 | Dimers | 2514.61 | 2567.02 | 2700.57 |
| 20 | Trimethylpyrazine | C14667551 | C7H10N2 | - | 7641.59 | 7333.87 | 7059.73 |
| 21 | alpha-Phellandrene | C99832 | C10H16 | - | 1296.22 | 1333.11 | 1453.98 |
| 22 | 6-Methyl-5-hepten-2-one | C110930 | C8H14O | - | 4046.45 | 4175.84 | 4391.30 |
| 23 | Myrcene | C123353 | C10H16 | - | 1333.17 | 1307.14 | 1426.65 |
| 24 | beta-Pinene | C127913 | C10H16 | - | 6194.19 | 6336.64 | 6305.77 |
| 25 | Camphene | C79925 | C10H16 | - | 4119.98 | 4157.74 | 4010.99 |
| 26 | alpha-Pinene | C80568 | C10H16 | - | 6692.03 | 6853.91 | 6727.11 |
| 27 | Tricyclene | C508327 | C10H16 | - | 6656.68 | 6753.87 | 6696.71 |
| 28 | (E)-2-Heptenal | C18829555 | C7H12O | Monomers | 325.31 | 345.18 | 353.34 |
| 29 | (E)-2-Heptenal | C18829555 | C7H12O | Dimers | 181.60 | 198.59 | 242.31 |
| 30 | Benzaldehyde | C100527 | C7H6O | - | 450.69 | 293.64 | 461.70 |
| 31 | Pentanoic acid | C109524 | C5H10O2 | - | 659.09 | 738.71 | 466.59 |
| 32 | Heptanal | C111717 | C7H14O | - | 130.78 | 198.43 | 226.07 |
| 33 | Isoamyl acetate | C123922 | C7H14O2 | - | 611.47 | 798.39 | 808.40 |
| 34 | (E)-2-Hexenal | C6728263 | C6H10O | - | 636.96 | 874.07 | 904.80 |
| 35 | Ethyl 2-methylbutanoate | C7452791 | C7H14O2 | - | 236.78 | 266.03 | 264.10 |
| 36 | Furfural | C98011 | C5H4O2 | - | 2210.18 | 2478.32 | 2603.42 |
| 37 | Methylpyrazine | C109080 | C5H6N2 | - | 312.63 | 259.44 | 254.56 |
| 38 | 2-Furanmethanol | C98000 | C5H6O2 | - | 465.39 | 510.06 | 502.23 |
| 39 | 3-Methylpentanol | C589355 | C6H14O | - | 312.24 | 356.44 | 355.38 |
| 40 | Hexanal | C66251 | C6H12O | - | 516.35 | 566.50 | 571.58 |
| 41 | 2,3-Butanediol | C513859 | C4H10O2 | - | 3883.92 | 3213.42 | 3119.87 |
| 42 | 2-Methylpropyl acetate | C110190 | C6H12O2 | - | 422.88 | 494.24 | 543.61 |
| 43 | Pyridine | C110861 | C5H5N | - | 94.87 | 95.94 | 84.64 |
| 44 | (E)-2-Pentenal | C1576870 | C5H8O | - | 262.16 | 251.43 | 222.98 |
| 45 | 3-Hydroxy-2-butanone | C513860 | C4H8O2 | - | 190.57 | 196.83 | 201.22 |
| 46 | Propyl acetate | C109604 | C5H10O2 | - | 450.26 | 447.78 | 253.98 |
| 47 | Ethyl propanoate | C105373 | C5H10O2 | - | 233.85 | 237.53 | 120.04 |
| 48 | Pentanal | C110623 | C5H10O | - | 232.90 | 186.91 | 261.11 |
| 49 | Acetic acid | C64197 | C2H4O2 | - | 382.69 | 273.02 | 303.89 |
| 50 | Hydroxyacetone | C116096 | C3H6O2 | - | 3736.68 | 3757.55 | 3624.16 |
| 51 | 2,3-Butanedione | C431038 | C4H6O2 | - | 3617.12 | 3562.79 | 3445.12 |
| 52 | Propanol | C71238 | C3H8O | - | 3481.21 | 3628.86 | 3843.26 |
| 53 | 2-Propanone | C67641 | C3H6O | - | 7010.10 | 6963.47 | 7286.70 |
| 54 | Ethanol | C64175 | C2H6O | - | 2742.83 | 2898.58 | 2672.49 |
| 55 | Propanal | C123386 | C3H6O | - | 472.26 | 422.22 | 485.95 |
| 56 | 2-Methylbutanal | C96173 | C5H10O | - | 78.53 | 81.57 | 102.75 |
| 57 | 3-Methylbutanal | C590863 | C5H10O | - | 53.87 | 69.16 | 102.84 |
| 58 | 2-Ethylfuran | C3208160 | C6H8O | - | 193.64 | 200.94 | 200.05 |
| 59 | 1-Hexanol | C111273 | C6H14O | - | 293.47 | 383.70 | 377.24 |
| 60 | Propanoic acid | C79094 | C3H6O2 | - | 146.73 | 173.43 | 170.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Lei, C.; Hu, G.; Zhou, W.; Li, Y.; Huang, D. Investigation of 60Co Irradiation on the Volatile Organic Compounds from Finger Citron (Citri Sarcodactylis Fructus) Using GC–IMS. Foods 2023, 12, 3543. https://doi.org/10.3390/foods12193543
Xiang Y, Lei C, Hu G, Zhou W, Li Y, Huang D. Investigation of 60Co Irradiation on the Volatile Organic Compounds from Finger Citron (Citri Sarcodactylis Fructus) Using GC–IMS. Foods. 2023; 12(19):3543. https://doi.org/10.3390/foods12193543
Chicago/Turabian StyleXiang, Yun, Chang Lei, Ge Hu, Wei Zhou, Ya Li, and Dan Huang. 2023. "Investigation of 60Co Irradiation on the Volatile Organic Compounds from Finger Citron (Citri Sarcodactylis Fructus) Using GC–IMS" Foods 12, no. 19: 3543. https://doi.org/10.3390/foods12193543
APA StyleXiang, Y., Lei, C., Hu, G., Zhou, W., Li, Y., & Huang, D. (2023). Investigation of 60Co Irradiation on the Volatile Organic Compounds from Finger Citron (Citri Sarcodactylis Fructus) Using GC–IMS. Foods, 12(19), 3543. https://doi.org/10.3390/foods12193543






