Nanoscale Materials Applying for the Detection of Mycotoxins in Foods
Abstract
:1. Introduction
2. Nanoscale Materials for Instrumental Analysis of Mycotoxins
2.1. Absorbent for SPE
2.2. Absorbent for SPME
3. Nanoscale Materials for Rapid Detection and Screening
3.1. Carrier for Biometric Molecules
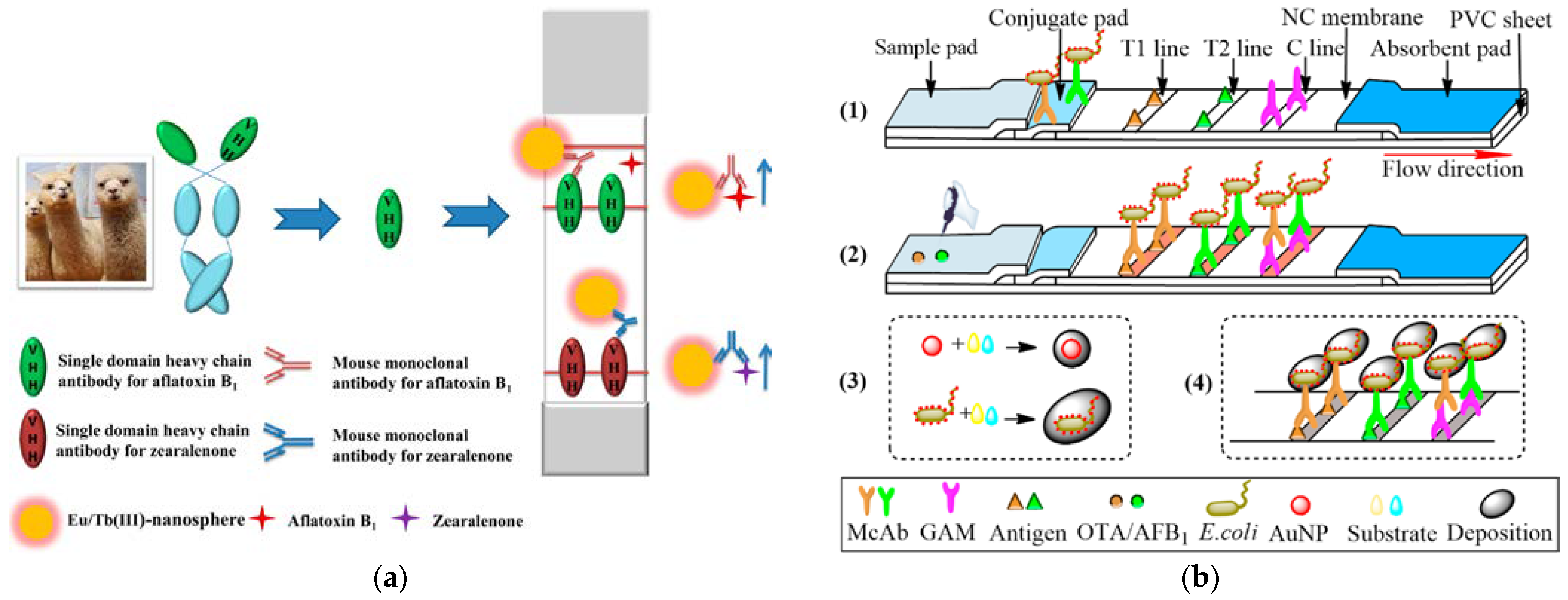
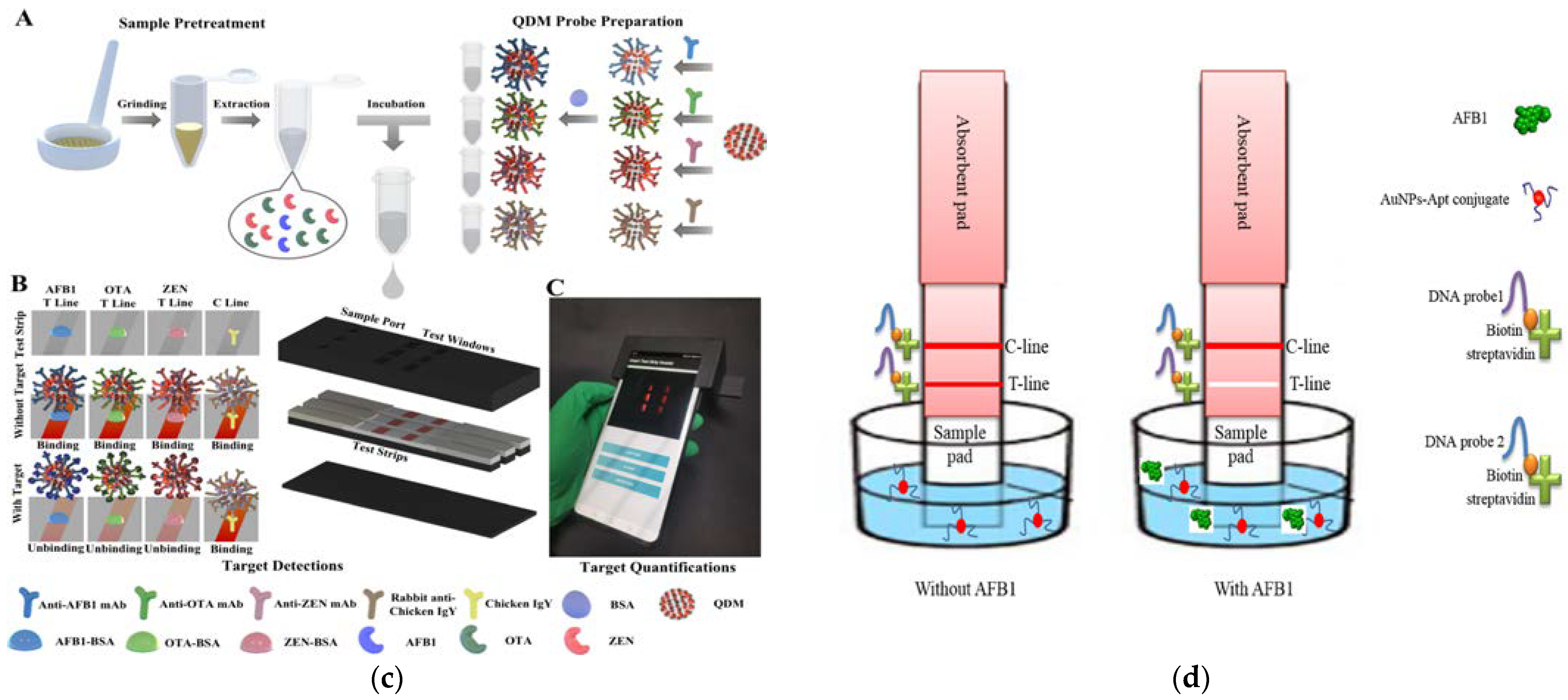
3.2. Signal Source for Rapid Detection
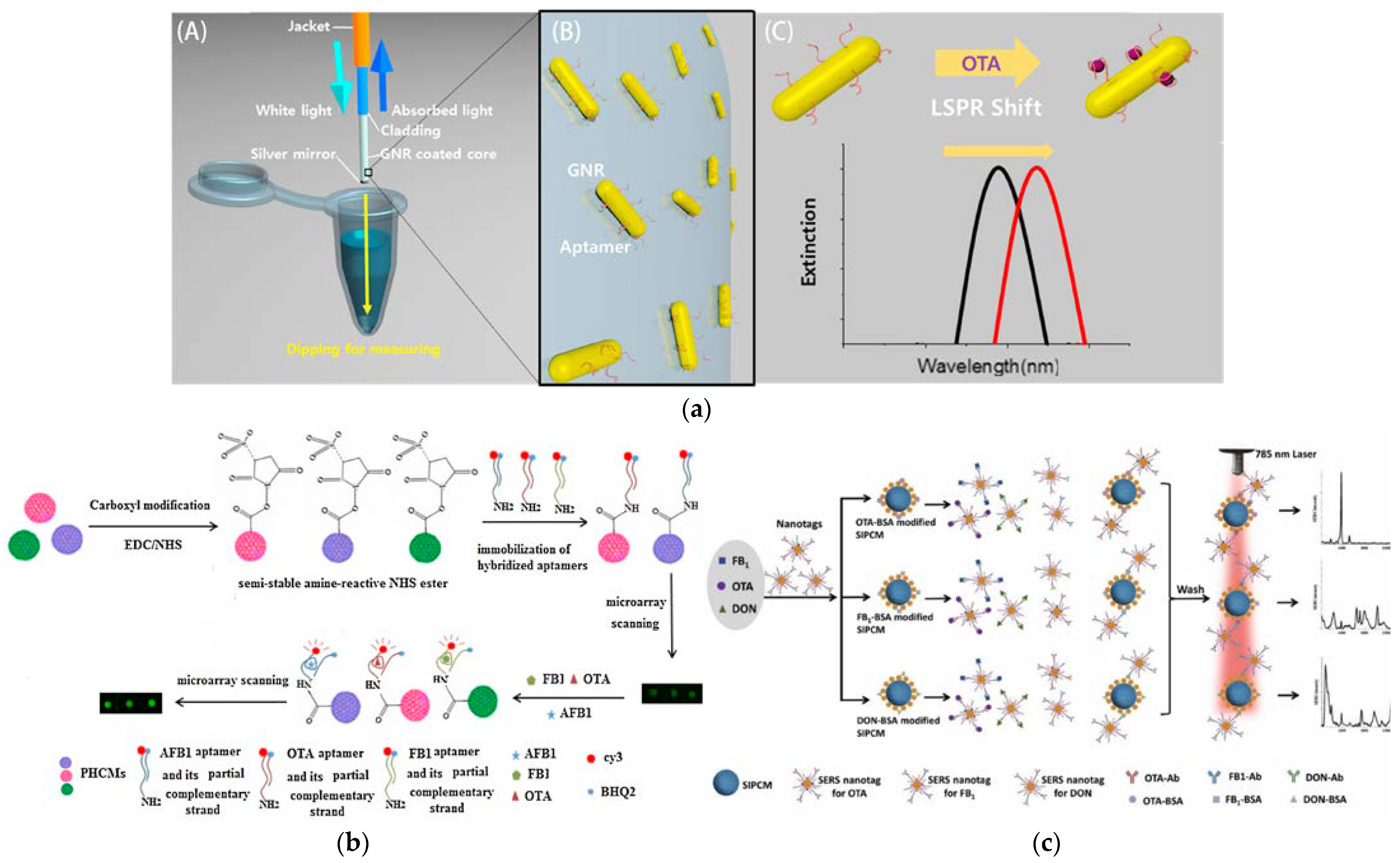
3.3. Other Functions for Mycotoxin Detection

4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Fu, Y.H.; Yin, S.T.; Zhao, C.; Fan, L.H.; Hu, H.B. Combined toxicity of food-borne mycotoxins and heavy metals or pesticides. Toxicon 2022, 217, 148–154. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Sharma, V.; Patial, V. Food mycotoxins: Dietary interventions implicated in the prevention of mycotoxicosis. ACS Food Sci. Technol. 2021, 1, 1719–1739. [Google Scholar] [CrossRef]
- Haque, A.; Wang, Y.H.; Shen, Z.Q.; Li, X.H.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Ben Amara, A.; Mehrez, A.; Ragoubi, C.; Romero-Gonzalez, R.; Frenich, A.G.; Landoulsi, A.; Maatouk, I. Fungal mycotoxins reduction by gamma irradiation in naturally contaminated sorghum. J. Food Process. Preserv. 2022, 46, e16345. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, J.; Cai, M.; Liu, Y.; Yang, K. Detoxification of mycotoxins in agricultural products by non-thermal physical technologies: A review of the past five years. Crit. Rev. Food Sci. Nutr. 2022, 12, 2095554. [Google Scholar] [CrossRef]
- Jing, G.X.; Wang, Y.Y.; Wu, M.P.; Liu, W.J.; Xiong, S.F.; Yu, J.N.; Li, W.S.; Liu, W.; Jiang, Y.M. Photocatalytic degradation and pathway from mycotoxins in food: A review. Food Rev. Int. 2023, 17, 2166062. [Google Scholar] [CrossRef]
- Kepinska-Pacelik, J.; Biel, W. Alimentary risk of mycotoxins for humans and animals. Toxins 2021, 13, 822. [Google Scholar] [CrossRef]
- Skrzydlewski, P.; Twaruzek, M.; Grajewski, J. Cytotoxicity of mycotoxins and their combinations on different cell lines: A review. Toxins 2022, 14, 244. [Google Scholar] [CrossRef]
- Guo, H.Y.; Ji, J.; Wang, J.S.; Sun, X.L. Co-contamination and interaction of fungal toxins and other environmental toxins. Trends Food Sci. Technol. 2020, 103, 162–178. [Google Scholar] [CrossRef]
- Jacobs, M. The adoption of AI in the core scientific cycle of feed research. J. Anim. Sci. 2021, 99, 42–43. [Google Scholar] [CrossRef]
- Eskola, M.; Altieri, A.; Galobart, J. Overview of the activities of the European food safety authority on mycotoxins in food and feed. World Mycotoxin J. 2018, 11, 277–289. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Morales-de la Pena, M.; Welti-Chanes, J.; Martin-Belloso, O. Novel technologies to improve food safety and quality. Curr. Opin. Food Sci. 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Shkembi, X.; Svobodova, M.; Skouridou, V.; Bashammakh, A.S.; Alyoubi, A.O.; O’Sullivan, C.K. Aptasensors for mycotoxin detection: A review. Anal. Biochem. 2022, 644, 114156. [Google Scholar] [CrossRef]
- Han, A.L.; Hao, S.J.; Yang, Y.Y.; Li, X.; Luo, X.Y.; Fang, G.Z.; Liu, J.F.; Wang, S. Perspective on recent developments of nanomaterial based fluorescent sensors: Applications in safety and quality control of food and beverages. J. Food Drug Anal. 2020, 28, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, X.; Zhang, W.; Meng, F.; Wang, X.; Qin, Y.; Zhang, M. Carbon-based nanocomposite smart sensors for the rapid detection of mycotoxins. Nanomaterials 2021, 11, 2851. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Geng, J.H.; Kou, X.H.; Xin, Z.H.; Yang, D.Y. Engineering nanomaterials-based biosensors for food safety detection. Biosen. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.J.; Li, J.M.; Liu, N.X.; Zhang, M.; Le, T. Advances in photocatalysis for mycotoxins elimination: Engineering strategies in photocatalyst designing, practical applications and future prospects. J. Alloy. Compd. 2023, 955, 170234. [Google Scholar] [CrossRef]
- Zuo, J.S.; Yan, T.T.; Tang, X.Q.; Zhang, Q.; Li, P.W. Dual-modal immunosensor made with the multifunction nanobody for fluorescent/colorimetric sensitive detection of aflatoxin B-1 in maize. ACS Appl. Mater. Interfaces 2023, 15, 2771–2780. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, G.L.; Wu, D.; Liu, J.H.; Wu, Y.N. Recent advances on emerging nanomaterials for controlling the mycotoxin contamination: From detection to elimination. Food Front. 2020, 1, 360–381. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, C.M.; Lan, L.Y.; Ping, J.F.; Ye, Z.Z.; Ying, Y.B. Nanomaterial-based biosensors for agro-product safety. TrAC Trends Anal. Chem. 2021, 143, 116369. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A review: Sample preparation and chromatographic technologies for detection of aflatoxins in foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef]
- Medina, M.L.J.; Lafarga, T.; Frenich, A.G.; Romero-Gonzalez, R. Natural occurrence, legislation, and determination of aflatoxins using chromatographic methods in food: A review (from 2010 to 2019). Food Rev. Int. 2021, 37, 244–275. [Google Scholar] [CrossRef]
- Woo, S.Y.; Ok, H.E.; Lee, S.Y.; Jeong, A.Y.; Jeong, T.K.; Chun, H.S. Simple chromatographic determination of aflatoxins in Korean fermented soybean products doenjang, ganjang, and gochujang, with comparison of derivatization methods. Food Sci. Biotechnol. 2022, 31, 475–482. [Google Scholar] [CrossRef]
- Bi, S.Y.; Xu, J.B.; Yang, X.S.; Zhang, P.; Lian, K.Q.; Ma, L. An HPLC-MS/MS method using a multitoxin clean up column for analysis of seven mycotoxins in aquafeeds. J. AOAC Int. 2022, 105, 107–114. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Liu, F.J.; He, W.Z.; Qin, Q.M.; Hu, D.Q.; Wu, A.B.; Jiang, W.B.; Wang, C. Screening of multi-mycotoxins in fruits by ultra-performance liquid chromatography coupled to ion mobility quadrupole time-of-flight mass spectrometry. Food Chem. 2022, 368, 130858. [Google Scholar] [CrossRef] [PubMed]
- Nualkaw, K.; Poapolathep, S.; Zhang, Z.W.; Zhang, Q.; Giorgi, M.; Li, P.W.; Logrieco, A.F.; Poapolathep, A. Simultaneous determination of multiple mycotoxins in swine, poultry and dairy feeds using ultra high performance liquid chromatography-tandem mass spectrometry. Toxins 2020, 12, 253. [Google Scholar] [CrossRef]
- Chen, H.; Huang, C.H.; Zhang, W.M.; Ding, Q.Q.; Gao, J.; Zhang, L. Ultrastable nitrogen-doped carbon nanotube encapsulated cobalt nanoparticles for magnetic solid-phase extraction of okadaic acid from aquatic samples. J. Chromatogr. A 2019, 1608, 460404. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Brunkhorst, J.; Cramer, B.; DeRosa, M.C.; Lattanzio, V.M.T.; Malone, R.; Maragos, C.; Stranska, M.; Sumarah, M.W. Developments in mycotoxin analysis: An update for 2019-2020. World Mycotoxin J. 2021, 14, 3–26. [Google Scholar] [CrossRef]
- Garcia-Nicolas, M.; Arroyo-Manzanares, N.; Campillo, N.; Reyes-Palomo, C.; Sanz-Fernandez, S.; Fenoll, J.; Rodriguez-Estevez, V.; Vinas, P. Use of polypyrrole ferrite microparticles and liquid chromatography-mass spectrometry for testing natural grass contamination by multiclass mycotoxins. Microchim. Acta 2023, 190, 178. [Google Scholar] [CrossRef]
- Yang, H.; Dai, H.; Wan, X.; Shan, D.; Zhang, Q.; Li, J.; Xu, Q.; Wang, C. Simultaneous determination of multiple mycotoxins in corn and wheat by high efficiency extraction and purification based on polydopamine and ionic liquid bifunctional nanofiber mat. Anal. Chim. Acta 2023, 1267, 341361. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Q.W.; Guo, W.B.; Guo, D.K.; Han, Z.; Nie, D.X. Fe3O4@COF(TAPT-DHTA) nanocomposites as magnetic solid-phase extraction adsorbents for simultaneous determination of 9 mycotoxins in fruits by UHPLC-MS/MS. Toxins 2023, 15, 117. [Google Scholar] [CrossRef]
- Xu, H.; Sun, J.; Wang, H.; Zhang, Y.; Sun, X. Adsorption of aflatoxins and ochratoxins in edible vegetable oils with dopamine-coated magnetic multi-walled carbon nanotubes. Food Chem. 2021, 365, 130409. [Google Scholar] [CrossRef]
- Jiang, K.Q.; Huang, Q.W.; Fan, K.; Wu, L.D.; Nie, D.X.; Guo, W.B.; Wu, Y.J.; Han, Z. Reduced graphene oxide and gold nanoparticle composite-based solid-phase extraction coupled with ultra-high-performance liquid chromatography-tandem mass spectrometry for the determination of 9 mycotoxins in milk. Food Chem. 2018, 264, 218–225. [Google Scholar] [CrossRef]
- Guo, D.K.; Huang, Q.W.; Zhao, R.; Guo, W.B.; Fan, K.; Han, Z.; Zhao, Z.H.; Nie, D.X. MIL-101(Cr)@Fe3O4 nanocomposites as magnetic solid-phase extraction adsorbent for the determination of multiple mycotoxins in agricultural products by ultra-high-performance liquid chromatography tandem mass spectrometry. Food Control 2023, 146, 109540. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Xu, C.; Tian, H.Y.; Shao, K.; Song, Y.N.; Yang, X.; Che, Z.M.; Huang, Y.K. Determination of aflatoxin B1 in Pixian Douban based on aptamer magnetic solid-phase extraction. RSC Adv. 2022, 12, 19528–19536. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, L.X.; Ma, F.; Li, P.W. Simultaneous determination of aflatoxins and benzo(a)pyrene in vegetable oils using humic acid-bonded silica SPE HPLC-PHRED-FLD. Toxins 2022, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.T.; He, J.; Huang, Z.P.; Li, H.Y.; Zhang, Y.X.; Wang, H.G.; Rui, C.F.; Li, Y.Y.; You, L.Q.; Li, K.; et al. An amino-functionalized zirconium-based metal-organic framework of type UiO-66-NH2 covered with a molecularly imprinted polymer as a sorbent for the extraction of aflatoxins AFB1, AFB2, AFG1 and AFG2 from grain. Microchim. Acta 2020, 187, 32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Wei, D.Z.; Wang, L.Q.; Ma, S.; Du, Y.F.; Wang, M. Multiwalled carbon nanotube for one-step cleanup of 21 mycotoxins in corn and wheat prior to ultraperformance liquid chromatography-tandem mass spectrometry analysis. Toxins 2018, 10, 409. [Google Scholar] [CrossRef]
- Wang, H.G.; He, J.; Song, L.X.; Zhang, Y.X.; Xu, M.H.; Huang, Z.P.; Jin, L.B.; Ba, X.; Li, Y.N.; You, L.Q.; et al. Etching of halloysite nanotubes hollow imprinted materials as adsorbent for extracting of zearalenone from grain samples. Microchem. J. 2020, 157, 104953. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Yang, Y.Q.; Zhang, C.B.; Zheng, Y.G.; Wu, Y.; Wang, X.Y. Development of an aptamer-functionalized capillary monolithic column for the highly-selective and highly-efficient recognition of patulin. Food Control 2021, 119, 107461. [Google Scholar] [CrossRef]
- Wu, F.L.; Xu, C.S.; Jiang, N.; Wang, J.B.; Ding, C.F. Poly (methacrylic acid-co-diethenyl-benzene) monolithic microextraction column and its application to simultaneous enrichment and analysis of mycotoxins. Talanta 2018, 178, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Masrournia, M.; Pordel, M. Simultaneous determination of four aflatoxins using dispersive micro solid phase extraction with magnetic bimetallic MOFs composite as a sorbent and high-performance liquid chromatography with fluorescence detection. Microchem. J. 2023, 189, 108506. [Google Scholar] [CrossRef]
- Mohebbi, A.; Nemati, M.; Farajzadeh, M.A.; Mogaddam, M.R.A.; Lotfipour, F. High performance liquid chromatography-tandem mass spectrometry determination of patulin and ochratoxin a in commercial fruit juices after their extraction with a green synthesized metal organic framework-based dispersive micro solid phase extraction procedure. Microchem. J. 2022, 179, 107558. [Google Scholar] [CrossRef]
- Mohebbi, A.; Nemati, M.; Mogaddam, M.R.A.; Farajzadeh, M.A.; Lotfipour, F. Dispersive micro-solid-phase extraction of aflatoxins from commercial soy milk samples using a green vitamin-based metal-organic framework as an efficient sorbent followed by high performance liquid chromatography-tandem mass spectrometry determination. J. Chromatogr. A 2022, 1673, 463099. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Recent developments in selective materials for solid phase extraction. Chromatographia 2019, 82, 1171–1189. [Google Scholar] [CrossRef]
- Hu, T.L.; Chen, R.; Wang, Q.; He, C.Y.; Liu, S.R. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef]
- Khatibi, S.A.; Hamidi, S.; Siahi-Shadbad, M.R. Current trends in sample preparation by solid-phase extraction techniques for the determination of antibiotic residues in foodstuffs: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3361–3382. [Google Scholar] [CrossRef]
- Tang, Z.T.; Liu, F.; Fang, F.; Ding, X.L.; Han, Q.R.; Tan, Y.Z.; Peng, C. Solid-phase extraction techniques based on nanomaterials for mycotoxin analysis: An overview for food and agricultural products. J. Sep. Sci. 2022, 45, 2273–2300. [Google Scholar] [CrossRef] [PubMed]
- Er, E.O.; Bozyigit, G.D.; Buyukpinar, C.; Bakirdere, S. Magnetic nanoparticles based solid phase extraction methods for the determination of trace elements. Crit. Rev. Anal. Chem. 2022, 52, 231–249. [Google Scholar] [CrossRef]
- Chen, B.H.; Inbaraj, B.S. Recent trends in analysis of mycotoxins in food using carbon-based nanomaterials. J. Food Drug Anal. 2022, 30, 562–589. [Google Scholar] [CrossRef]
- Rajabi, M.; Rahimi, M.; Hemmati, M.; Najafi, F. Chemically functionalized silica nanoparticles-based solid-phase extraction for effective pre-concentration of highly toxic metal ions from food and water samples. Appl. Organomet. Chem. 2018, 32, e4012. [Google Scholar] [CrossRef]
- Chao, Y.H.; Pang, J.Y.; Bai, Y.; Wu, P.W.; Luo, J.; He, J.; Jin, Y.; Li, X.W.; Xiong, J.; Li, H.M.; et al. Graphene-like BN@SiO2 nanocomposites as efficient sorbents for solid-phase extraction of rhodamine B and rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2018, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.Y.; Shi, H.X.; Han, Y.R.; Yang, Y.Y.; Wang, R.X.; Men, J.Y. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Bui, B.T.S.; Auroy, T.; Haupt, K. Fighting antibiotic-resistant bacteria: Promising strategies orchestrated by molecularly imprinted polymers. Angew. Chem. Int. Edit. 2022, 61. [Google Scholar] [CrossRef]
- Ashley, J.; Shahbazi, M.A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef]
- Villa, C.C.; Sanchez, L.T.; Valencia, G.A.; Ahmed, S.; Gutierrez, T.J. Molecularly imprinted polymers for food applications: A review. Trends Food Sci. Technol. 2021, 111, 642–669. [Google Scholar] [CrossRef]
- Song, Z.H.; Li, J.H.; Lu, W.H.; Li, B.W.; Yang, G.Q.; Bi, Y.; Arabi, M.; Wang, X.Y.; Ma, J.P.; Chen, L.X. Molecularly imprinted polymers based materials and their applications in chromatographic and electrophoretic separations. TrAC Trends Anal. Chem. 2022, 146, 116504. [Google Scholar] [CrossRef]
- Lhotska, I.; Gajdosova, B.; Solich, P.; Satinsky, D. Molecularly imprinted vs. reversed-phase extraction for the determination of zearalenone: A method development and critical comparison of sample clean-up efficiency achieved in an on-line coupled SPE chromatography system. Anal. Bioanal. Chem. 2018, 410, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.A.A.; Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Hu, M.L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar] [CrossRef]
- Hu, Y.L.; Dai, L.M.; Liu, D.H.; Du, W.; Wang, Y.J. Progress & prospect of metal-organic frameworks (MOFs) for enzyme immobilization (enzyme/MOFs). Renew. Sust. Energ. Rev. 2018, 91, 793–801. [Google Scholar] [CrossRef]
- Wang, D.G.; Liang, Z.B.; Gao, S.; Qu, C.; Zou, R.G. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Barreto, I.S.; Andrade, S.I.E.; Cunha, F.A.S.; Lima, M.B.; Araujo, M.C.U.; Almeida, L.F. A robotic magnetic nanoparticle solid phase extraction system coupled to flow-batch analyzer and GFAAS for determination of trace cadmium in edible oils without external pretreatment. Talanta 2018, 178, 384–391. [Google Scholar] [CrossRef]
- Hamidi, S. Recent advances in solid-phase extraction as a platform for sample preparation in biomarker assay. Crit. Rev. Anal. Chem. 2023, 53, 199–210. [Google Scholar] [CrossRef]
- Maya, F.; Cabello, C.P.; Frizzarin, R.M.; Estela, J.M.; Palomino, G.T.; Cerda, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TrAC Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Soylak, M.; Ozalp, O.; Uzcan, F. Magnetic nanomaterials for the removal, separation and preconcentration of organic and inorganic pollutants at trace levels and their practical applications: A review. Trends Environ. Anal. Chem. 2021, 29, e00109. [Google Scholar] [CrossRef]
- Salve, S.; Bahiram, Y.; Jadhav, A.; Rathod, R.; Tekade, R.K. Nanoplatform-integrated miniaturized solid-phase extraction techniques: A critical review. Crit. Rev. Anal. Chem. 2023, 53, 46–68. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Tran, Q.N.; Lee, H.J.; Tran, N. Covalent organic frameworks: From structures to applications. Polymers 2023, 15, 1279. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.L.; Guo, W.; Zhang, Y.; Feng, X.S.; Zhang, F. Synthesis of a magnetic covalent organic framework as sorbents for solid-phase extraction of aflatoxins in food prior to quantification by liquid chromatography-mass spectrometry. Food Chem. 2022, 387, 132821. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Pan, A.; Zhang, C.; Guo, M.; Lou, C.Y.; Zhang, J.; Wu, H.Z.; Wang, X. Fast extraction of aflatoxins, ochratoxins and enniatins from maize with magnetic covalent organic framework prior to HPLC-MS/MS detection. Food Chem. 2023, 404, 134464. [Google Scholar] [CrossRef]
- Liu, S.Q.; Huang, Y.Q.; Qian, C.Y.; Xiang, Z.M.; Ouyang, G.F. Physical assistive technologies of solid-phase microextraction: Recent trends and future perspectives. TrAC Trends Anal. Chem. 2020, 128, 115916. [Google Scholar] [CrossRef]
- Delinska, K.; Rakowska, P.W.; Kloskowski, A. Porous material-based sorbent coatings in solid-phase microextraction technique: Recent trends and future perspectives. TrAC Trends Anal. Chem. 2021, 143, 116386. [Google Scholar] [CrossRef]
- Zambonin, C.; Aresta, A. Recent applications of solid phase microextraction coupled to liquid chromatography. Separations 2021, 8, 34. [Google Scholar] [CrossRef]
- Reinholds, I.; Jansons, M.; Pugajeva, I.; Bartkevics, V. Recent applications of carbonaceous nanosorbents in solid phase extraction for the determination of pesticides in food samples. Crit. Rev. Anal. Chem. 2019, 49, 439–458. [Google Scholar] [CrossRef]
- Dal Bosco, C.; De Cesaris, M.G.; Felli, N.; Lucci, E.; Fanali, S.; Gentili, A. Carbon nanomaterial-based membranes in solid-phase extraction. Microchim. Acta 2023, 190, 175. [Google Scholar] [CrossRef]
- Hou, F.Y.; Chang, Q.Y.; Wan, N.N.; Li, J.; Zang, X.H.; Zhang, S.H.; Wang, C.; Wang, Z. A novel porphyrin-based conjugated microporous nanomaterial for solid-phase microextraction of phthalate esters residues in children’s food. Food Chem. 2022, 388, 133015. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, K.; Zhao, G.H. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.H.; Xianyu, Y.L. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Hou, S.L.; Ma, J.J.; Cheng, Y.Q.; Wang, Z.F.; Yan, Y.X. Overview-gold nanoparticles-based sensitive nanosensors in mycotoxins detection. Crit. Rev. Food Sci. Nutr. 2022, 16, 2095973. [Google Scholar] [CrossRef] [PubMed]
- Justyna, P.-W.; Natalia, S.; de la Miguel, G.; Jacek, N. Miniaturized solid-phase extraction techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Ghorbani, M.; Aghamohammadhassan, M.; Chamsaz, M.; Akhlaghi, H.; Pedramrad, T. Dispersive solid phase microextraction. TrAC Trends Anal. Chem. 2019, 118, 793–808. [Google Scholar] [CrossRef]
- Soares da Silva Burato, J.; Vargas Medina, D.A.; de Toffoli, A.L.; Vasconcelos Soares Maciel, E.; Mauro Lanças, F. Recent advances and trends in miniaturized sample preparation techniques. J. Sep. Sci. 2019, 43, 202–225. [Google Scholar] [CrossRef]
- Vállez-Gomis, V.; Grau, J.; Benedé, J.L.; Giokas, D.L.; Chisvert, A.; Salvador, A. Fundamentals and applications of stir bar sorptive dispersive microextraction: A tutorial review. Anal. Chim. Acta 2021, 1153, 338271. [Google Scholar] [CrossRef]
- Yu-Xin, G.; Tian-Ci, Y.; Zi-Xuan, Y.; Fang-Ming, L.; Jun, C.; Li-Hong, Y. Recent developments and applications in the microextraction and separation technology of harmful substances in a complex matrix. Microchem. J. 2022, 176, 107241. [Google Scholar] [CrossRef]
- Xu, F.; Gong, B.; Xu, Z.; Wang, J. Reverse-phase/phenylboronic-acid-type magnetic microspheres to eliminate the matrix effects in amatoxin and phallotoxin determination via ultrahigh-performance liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 332, 127394. [Google Scholar] [CrossRef]
- Gonzalez-Salamo, J.; Socas-Rodriguez, B.; Hernandez-Borges, J.; Rodriguez-Delgado, M.A. Core-shell poly(dopamine) magnetic nanoparticles for the extraction of estrogenic mycotoxins from milk and yogurt prior to LC-MS analysis. Food Chem. 2017, 215, 362–368. [Google Scholar] [CrossRef]
- Mingxia, S.; Juanjuan, F.; Yang, F.; Xubo, X.; Yali, D.; Min, S. Preparation of ionic covalent organic frameworks and their applications in solid-phase extraction. TrAC Trends Anal. Chem. 2022, 157, 116829. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Ihara, H.; Guan, M.; Qiu, H. Preparation of porous carbon nanomaterials and their application in sample preparation: A review. TrAC Trends Anal. Chem. 2021, 143, 116421. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhou, S.; Hu, Y.; Zheng, J.; Ouyang, G. Research progress on the application of derived porous carbon materials in solid-phase microextraction. Chin. J. Chromatorg. 2022, 40, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Atapattu, S.N.; Poole, C.F. Recent advances in analytical methods for the determination of citrinin in food matrices. J. Chromatogr. A 2020, 1627, 461399. [Google Scholar] [CrossRef] [PubMed]
- Rezaeefar, A.; Nemati, M.; Farajzadeh, M.A.; Mogaddam, M.R.A.; Lotfipour, F. Development of N and S doped carbon sorbent-based dispersive micro solid phase extraction method combined with dispersive liquid-liquid microextraction for selected mycotoxins from soymilk samples. Microchem. J. 2022, 173, 107039. [Google Scholar] [CrossRef]
- Hou, X.D.; Tang, S.; Wang, J. Recent advances and applications of graphene-based extraction materials in food safety. TrAC Trends Anal. Chem. 2019, 119, 115603. [Google Scholar] [CrossRef]
- Kori, A.H.; Jagirani, M.S.; Soylak, M. Graphene-based nanomaterials: A sustainable material for solid-phase microextraction (SPME) for environmental applications. Anal. Lett. 2023, 56, 2385–2400. [Google Scholar] [CrossRef]
- Tanveer, Z.I.; Huang, Q.W.; Liu, L.; Jiang, K.Q.; Nie, D.X.; Pan, H.Y.; Chen, Y.; Liu, X.S.; Luan, L.J.; Han, Z.; et al. Reduced graphene oxide-zinc oxide nanocomposite as dispersive solid-phase extraction sorbent for simultaneous enrichment and purification of multiple mycotoxins in coptidis rhizoma (Huanglian) and analysis by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2020, 1630, 461515. [Google Scholar] [CrossRef]
- Wang, S.W.; Shao, R.; Li, W.W.; Li, X.; Sun, J.L.; Jiao, S.S.; Dai, S.J.; Dou, M.H.; Xu, R.M.; Li, Q.J.; et al. Three-dimensional ordered macroporous magnetic inverse photonic crystal microsphere-based molecularly imprinted polymer for selective capture of aflatoxin B-1. ACS Appl. Mater. Interfaces 2022, 14, 18845–18853. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Li, G.; Liu, S.; Zhang, X.; Liu, J.; Su, Z.; Wu, Y. Emerging nanolabels-based immunoassays: Principle and applications in food safety. TrAC Trends Anal. Chem. 2021, 145, 116462. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Amin, K.; Yu, H.; Yang, H.; Guo, Z.; Liu, J. Advances in aptamers, and application of mycotoxins detection: A review. Food Res. Int. 2023, 170, 113022. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, W.; Wen, Q.; Xu, D.; Ren, J.L.; Lin, Q.L. Aptamer-engineered nanomaterials to aid in mycotoxin determination. Food Control 2022, 135, 108661. [Google Scholar] [CrossRef]
- Gamal, M.H.; Taha, M.; Jesus, S.-G.; Sarah, A.-A.; Okon, J.E.; Mosaad, A.A.-W.; Elsayed, E.H. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2022, 144, 109350. [Google Scholar] [CrossRef]
- Lv, X.Y.; Xu, X.Y.; Miao, T.; Zang, X.F.; Geng, C.; Li, Y.P.; Cui, B.; Fang, Y.S. A ratiometric electrochemiluminescent/electrochemical strategy based on novel designed BPYHBF nanorod and Fc-MOF with tungsten for ultrasensitive AFB1 detection. Sens. Actuator B Chem. 2022, 352, 131026. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Zhao, R.Y.; Qu, Z.X.; He, B.S. FeMOF-based nanostructured platforms for T-2 toxin detection in beer by a “fence-type” aptasensing principle. Anal. Bioanal. Chem. 2022, 414, 7999–8008. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, H.L.; Wei, M.; Ren, W.J.; Zhang, Y.R.; Jiang, L.Y.; Wei, T.; He, B.S. A DNAzyme-assisted triple-amplified electrochemical aptasensor for ultra-sensitive detection of T-2 toxin. Sens. Actuator B Chem. 2021, 328, 129063. [Google Scholar] [CrossRef]
- Shukla, S.; Haldorai, Y.; Khan, I.; Kang, S.M.; Kwak, C.H.; Gandhi, S.; Bajpai, V.K.; Huh, Y.S.; Han, Y.K. Bioreceptor-free, sensitive and rapid electrochemical detection of patulin fungal toxin, using a reduced graphene oxide@SnO2 nanocomposite. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110916. [Google Scholar] [CrossRef]
- Hamami, M.; Mars, A.; Raouafi, N. Biosensor based on antifouling PEG/Gold nanoparticles composite for sensitive detection of aflatoxin M1 in milk. Microchem. J. 2021, 165, 106102. [Google Scholar] [CrossRef]
- An, X.S.; Shi, X.J.; Zhang, H.; Yao, Y.; Wang, G.X.; Yang, Q.Q.; Xia, L.M.; Sun, X. An electrochemical immunosensor based on a combined amplification strategy with the GO-CS/CeO2-CS nanocomposite for the detection of aflatoxin M-1. New J. Chem. 2020, 44, 1362–1370. [Google Scholar] [CrossRef]
- Zhang, C.; Du, C.C.; Liu, W.; Guo, T.; Zhou, Y.; Zhou, H.Y.; Zhang, Y.H.; Liu, X.Z.; Ma, L. A high sensitivity electrochemical immunosensor based on monoclonal antibody coupled flower- nano-ZnO for detection of tenuazonic acid. Agriculture 2022, 12, 204. [Google Scholar] [CrossRef]
- Chen, Z.X.; Yang, M.; Li, Z.Y.; Liao, W.C.; Chen, B.Q.; Yang, T.; Hu, R.; Yang, Y.H.; Meng, S. Highly sensitive and convenient aptasensor based on Au NPs@Ce-TpBpy COF for quantitative determination of zearalenone. RSC Adv. 2022, 12, 17312–17320. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhu, X.W.; Zhang, L.; Song, Q.; Xu, H.Y.; Liu, X.Q. Electrochemical sensor based on CuO nanoparticles-modified graphene oxide (CuO@GO) nanocomposites for determination of zearalenone mycotoxins in food samples. Int. J. Electrochem. Sci. 2021, 16, 210435. [Google Scholar] [CrossRef]
- Jahangiri-Dehaghani, F.; Zare, H.R.; Shekari, Z.; Benvidi, A. Development of an electrochemical aptasensor based on Au nanoparticles decorated on metal-organic framework nanosheets and p-biphenol electroactive label for the measurement of aflatoxin B1 in a rice flour sample. Anal. Bioanal. Chem. 2022, 414, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.L.; Wu, G.; Ding, Z.Y.; Xie, J. Construction of a nanoscale metal-organic framework aptasensor for fluorescence ratiometric sensing of AFB1 in real samples. Food Chem. 2023, 416, 135805. [Google Scholar] [CrossRef]
- Zhao, X.D.; Wang, Y.; Li, J.Z.; Huo, B.Y.; Qin, Y.K.; Zhang, J.Y.; Chen, M.M.; Peng, Y.; Bai, J.L.; Li, S.; et al. A fluorescence aptasensor based on controlled zirconium-based MOFs for the highly sensitive detection of T-2 toxin. Spectrochim. Acta Pt. A Mol. Biomol. Spectrosc. 2021, 259, 119893. [Google Scholar] [CrossRef]
- Yiting, F.; Huanhuan, Y.; Jiaxin, L.; Khalid, A.; Bo, L.; Wendan, J.; Sainan, W.; Hongling, F.; Hansong, Y.; Zhijun, G. Single-walled carbon nanohorn-based fluorescence energy resonance transfer aptasensor platform for the detection of aflatoxin B1. Foods 2023, 12, 2880. [Google Scholar] [CrossRef]
- Xuan, Z.; Wu, Y.; Liu, H.; Li, L.; Ye, J.; Wang, S. Copper oxide nanoparticle-based immunosensor for zearalenone analysis by combining automated sample pre-processing and high-throughput terminal detection. Sensors 2021, 21, 6538. [Google Scholar] [CrossRef]
- Wang, C.Q.; Huang, X.Y.; Tian, X.Y.; Zhang, X.R.; Yu, S.S.; Chang, X.H.; Ren, Y.; Qian, J. A multiplexed FRET aptasensor for the simultaneous detection of mycotoxins with magnetically controlled graphene oxide/Fe3O4 as a single energy acceptor. Analyst 2019, 144, 6004–6010. [Google Scholar] [CrossRef]
- Pang, H.; Li, H.; Zhang, W.; Mao, J.; Zhang, L.X.; Zhang, Z.W.; Zhang, Q.; Wang, D.; Jiang, J.; Li, P.W. Fullerenol quantum dots-based highly sensitive fluorescence aptasensor for patulin in apple juice. Toxins 2022, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.L.; Ma, J.J.; Cheng, Y.Q.; Wang, H.G.; Sun, J.H.; Yan, Y.X. Quantum dot nanobead-based fluorescent immunochromatographic assay for simultaneous quantitative detection of fumonisin B-1, dexyonivalenol, and zearalenone in grains. Food Control 2020, 117, 107331. [Google Scholar] [CrossRef]
- Wang, F.X.; Li, Z.P.; Jia, H.P.; Lu, R.H.; Zhang, S.B.; Pan, C.P.; Zhang, Z.Q. An ultralow concentration of Al-MOFs for turn-on fluorescence detection of aflatoxin B-1 in tea samples. Food Chem. 2022, 383, 132389. [Google Scholar] [CrossRef]
- Li, Z.S.; Xu, X.H.; Fu, Y.C.; Guo, Y.N.; Zhang, Q.; Zhang, Q.Y.; Yang, H.; Li, Y.B. A water-stable luminescent metal-organic framework for effective detection of aflatoxin B1 in walnut and almond beverages. RSC Adv. 2019, 9, 620–625. [Google Scholar] [CrossRef]
- Guo, X.D.; Wen, F.; Qiao, Q.Q.; Zheng, N.; Saive, M.; Fauconnier, M.L.; Wang, J.Q. A novel graphene oxide-based aptasensor for amplified fluorescent detection of aflatoxin M-1 in milk powder. Sensors 2019, 19, 3840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Wang, Y.L.; Nan, M.N.; Li, Y.C.; Yun, J.M.; Wang, Y.; Bi, Y. Novel colorimetric aptasensor based on unmodified gold nanoparticle and ssDNA for rapid and sensitive detection of T-2 toxin. Food Chem. 2021, 348, 129128. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.P.; Zhang, G.H.; Chen, W.; Wu, S.X.; Yang, H.L.; Zhou, Y. Multicolor immunosensor for detection of zearalenone based on etching Au NBPs mediated by HRP. J. Food Compos. Anal. 2023, 115, 105014. [Google Scholar] [CrossRef]
- Lv, X.Q.; Foda, M.F.; He, J.L.; Zhou, J.J.; Cai, J. Robust and facile label-free colorimetric aptasensor for ochratoxin A detection using aptamer-enhanced oxidase-like activity of MnO2 nanoflowers. Food Chem. 2023, 401, 134144. [Google Scholar] [CrossRef]
- Althaga, I.I.; Ahmed, S.A.; El-Said, W.A. Colorimetric aflatoxins immunoassay by using silica nanoparticles decorated with gold nanoparticles. Spectrochim. Acta Pt. A Mol. Biomol. Spectrosc. 2021, 246, 118999. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, H.J.; Qu, X.F.; Wang, W.L.; Yang, C.; Peng, C.F.; Zhang, Y. A smartphone based photothermal-colorimetric immunochromatographic sensor for ultrasensitive and ultra-wide concentration range detection of deoxynivalenol. Microchem. J. 2023, 190, 108675. [Google Scholar] [CrossRef]
- Khansili, N.; Krishna, P.M. Sensitive metal oxide-clay nanocomposite colorimetric sensor development for aflatoxin detection in foods: Corn and almond. ACS Omega 2021, 6, 14911–14925. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.Y.; Li, Z.Y.; Hu, R.; Yang, Y.H.; Yang, T. A visual peroxidase mimicking aptasensor based on Pt nanoparticles-loaded on iron metal organic gel for fumonisin B1 analysis in corn meal. Biosens. Bioelectron. 2022, 209, 114241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.R.; Li, L.B.; Zhou, Z.; Yang, X.D.; Hao, N.; Guo, Y.S.; Wang, K. A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef]
- Huang, S.Y.; Lai, W.Q.; Liu, B.Q.; Xu, M.D.; Zhuang, J.Y.; Tang, D.P.; Lin, Y.X. Colorimetric and photothermal dual-mode immunoassay of aflatoxin B-1 based on peroxidase-like activity of Pt supported on nitrogen-doped carbon. Spectrochim. Acta Pt. A Mol. Biomol. Spectrosc. 2023, 284, 121782. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Lv, Y.; Qi, S.; Zhang, Y.; Wang, Z.P. Sensitive colorimetric aptasensor based on stimuli-responsive metal-organic framework nano-container and trivalent DNAzyme for zearalenone determination in food samples. Food Chem. 2022, 371, 131145. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.H.; Byun, J.Y.; Kim, J.H.; Kim, M.G. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Lerdsri, J.; Thunkhamrak, C.; Jakmunee, J. Development of a colorimetric aptasensor for aflatoxin B1 detection based on silver nanoparticle aggregation induced by positively charged perylene diimide. Food Control 2021, 130, 108323. [Google Scholar] [CrossRef]
- Guo, R.; Ji, Y.; Chen, J.N.; Ye, J.; Ni, B.X.; Li, L.; Yang, Y.T. Multicolor visual detection of deoxynivalenol in grain based on magnetic immunoassay and enzymatic etching of plasmonic gold nanobipyramids. Toxins 2023, 15, 351. [Google Scholar] [CrossRef]
- Lu, W.Y.; Tian, Y.; Teng, W.P.; Qiu, X.C.; Li, M. Plasmonic colorimetric immunosensor based on Poly-HRP and AuNS etching for tri-modal readout of small molecule. Talanta 2023, 265, 124883. [Google Scholar] [CrossRef]
- Song, L.C.; Li, J.L.; Li, H.; Chang, Y.W.; Dai, S.J.; Xu, R.M.; Dou, M.H.; Li, Q.J.; Lv, G.P.; Zheng, T.S. Highly sensitive SERS detection for Aflatoxin B1 and Ochratoxin A based on aptamer-functionalized photonic crystal microsphere array. Sens. Actuator B Chem. 2022, 364, 131778. [Google Scholar] [CrossRef]
- He, P.H.; Hassan, M.M.; Yang, W.J.; Shi, Z.X.; Zhou, X.Y.; Xu, Y.; Ouyang, Q.; Chen, Q.S. Rapid and stable detection of three main mycotoxins in rice using SERS optimized AgNPs@K30 coupled multivariate calibration. Food Chem. 2023, 398, 133883. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, C.G.; Li, J.X.; Wang, W.Q.; Yu, Q.; Wang, C.W.; Wang, S.Q. Graphene oxide-based three-dimensional Au nanofilm with high-density and controllable hotspots: A powerful film-type SERS tag for immunochromatographic analysis of multiple mycotoxins in complex samples. Chem. Eng. J. 2022, 448, 137760. [Google Scholar] [CrossRef]
- Chen, R.P.; Wang, H.; Sun, C.Q.; Zhao, Y.G.; He, Y.; Nisar, M.S.; Wei, W.S.; Kang, H.Q.; Xie, X.L.; Du, C.M.; et al. Au@SiO2 SERS nanotags based lateral flow immunoassay for simultaneous detection of aflatoxin B1 and ochratoxin A. Talanta 2023, 258, 124401. [Google Scholar] [CrossRef]
- Xie, X.Q.; Pan, M.F.; Hong, L.P.; Liu, K.X.; Yang, J.Y.; Wang, S.; Song, Y.; Wang, S. Carbon dots-embedded fluorescent molecularly imprinted photonic crystals hydrogel strip for accurate and selective detection of rutin in sophora japonica products. Sens. Actuator B Chem. 2022, 368, 132196. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.; Radovic, M.; Rizzotto, F.; Vizzini, P.; Jaric, S.; Pavlovic, Z.; Radonic, V.; Nikolic, M.V.; Vidic, J. Advances in nanomaterials-based electrochemical biosensors for foodborne pathogen detection. Nanomaterials 2021, 11, 2700. [Google Scholar] [CrossRef]
- Lin, X.F.; Yu, W.Y.; Tong, X.Y.; Li, C.X.; Duan, N.; Wang, Z.P.; Wu, S.J. Application of nanomaterials for coping with mycotoxin contamination in food safety: From detection to control. Crit. Rev. Anal. Chem. 2022, 34, 2076063. [Google Scholar] [CrossRef] [PubMed]
- Bahadir, E.B.; Sezginturk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.N.; Peng, D.P.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.X.; Shabbir, M.A.; Cheng, G.Y.; Yuan, Z.H. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Song, S.; Park, S.; Joo, C. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens. Bioelectron. 2020, 152, 112015. [Google Scholar] [CrossRef]
- Soh, J.H.; Chan, H.-M.; Ying, J.Y. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today 2020, 30, 100831. [Google Scholar] [CrossRef]
- Su, Z.X.; Dou, W.C.; Liu, X.J.; Ping, J.F.; Li, D.Y.; Ying, Y.B.; Xie, L.J. Nano-labeled materials as detection tags for signal amplification in immunochromatographic assay. TrAC Trends Anal. Chem. 2022, 154, 116673. [Google Scholar] [CrossRef]
- Bartosh, A.V.; Urusov, A.E.; Petrakova, A.V.; Kuang, H.; Zherdev, A.V.; Dzantiev, B.B. Highly sensitive lateral flow test with indirect labelling for zearalenone in baby food. Food Agric. Immunol. 2020, 31, 653–666. [Google Scholar] [CrossRef]
- Tang, X.Q.; Li, P.W.; Zhang, Q.; Zhang, Z.W.; Zhang, W.; Jiang, J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal. Chem. 2017, 89, 11520–11528. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, W.Y.; Mao, Y.H.; Qiu, X.C.; Du, D.L. An enhanced immunochromatography assay based on gold growth on the surface of E. coli carrier for the simultaneous detection of mycotoxins. Talanta 2023, 251, 123798. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.H.; Zhang, Y.L.; Al-Hartomy, O.; Wageh, S.; Al-Sehemi, A.G.; Hao, Y.B.; Gao, L.F.; Wang, H.; Zhang, H. Colloidal quantum dots: Synthesis, composition, structure, and emerging optoelectronic applications. Laser Photonics Rev. 2022, 50, 202200551. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, H.; Wu, S.; Lv, J.; Mei, L.; Zhou, H.; Lin, X.; Han, X. A quantum dot fluorescent microsphere based immunochromatographic strip for detection of brucellosis. BMC Vet. Res. 2021, 17, 48. [Google Scholar] [CrossRef]
- Linghu, X.; Qiu, J.P.; Wang, S.S.; Lu, Y. Fluorescence immunoassay based on magnetic separation and ZnCdSe/ZnS quantum dots as a signal marker for intelligent detection of sesame allergen in foods. Talanta 2023, 256, 124323. [Google Scholar] [CrossRef]
- Lu, Y.N.; Shan, Y.K.; Huang, H.C.; Zhu, L.; Li, B.J.; Wang, S.Y.; Liu, F. Quantum dot microsphere-based immunochromatography test strip enabled sensitive and quantitative on-site detections for multiple mycotoxins in grains. Food Chem. 2022, 376, 131868. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers chemistry: Chemical modifications and conjugation strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef]
- Acquah, C.; Agyei, D.; Obeng, E.M.; Pan, S.R.; Tan, K.X.; Danquah, M.K. Aptamers: An emerging class of bioaffinity ligands in bioactive peptide applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.Y.; Peng, J.; Shan, S.; Liu, D.F.; Huang, Y.N.; Lai, W.H. Green enzyme-linked immunosorbent assay based on the single-stranded binding protein-assisted aptamer for the detection of mycotoxin. Anal. Chem. 2020, 92, 8422–8426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Zhang, C.; Wang, J.L.; Knopp, D. Recent progress in rapid determination of mycotoxins based on emerging biorecognition molecules: A review. Toxins 2022, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Jia, B.Y.; Sheng, P.; Liao, X.F.; Shi, L.C.; Fang, L.; Zhou, L.D.; Kong, W.J. Aptasensors for mycotoxins in foods: Recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2032–2073. [Google Scholar] [CrossRef]
- Sun, S.M.; Xie, Y.L. An enhanced enzyme-linked aptamer assay for the detection of zearalenone based on gold nanoparticles. Anal. Methods 2021, 13, 1255–1260. [Google Scholar] [CrossRef]
- Niazi, S.; Khan, I.M.; Yu, Y.; Pasha, I.; Shoaib, M.; Mohsin, A.; Mushtaq, B.S.; Akhtar, W.; Wang, Z.P. A “turnon” aptasensor for simultaneous and time-resolved fluorometric determination of zearalenone, trichothecenes A and aflatoxin B-1 using WS2 as a quencher. Microchim. Acta 2019, 186, 575. [Google Scholar] [CrossRef]
- Mousivand, M.; Javan-Nikkhah, M.; Anfossi, L.; Di Nardo, F.; Salina, M.; Bagherzadeh, K. High performance aptasensing platform development through in silico aptamer engineering for aflatoxin B1 monitoring. Food Control 2023, 145, 109418. [Google Scholar] [CrossRef]
- Chen, X.J.; Gao, D.; Sun, F.X.; Li, Z.Z.; Wang, Y.; Qiu, C.X.; He, K.F.; Wang, J. Nanomaterial-based aptamer biosensors for ochratoxin A detection: A review. Anal. Bioanal. Chem. 2022, 414, 2953–2969. [Google Scholar] [CrossRef]
- Al-Hawary, S.I.S.; Sapaev, I.B.; Althomali, R.H.; Saleh, E.A.M.; Qadir, K.; Romero-Parra, R.M.; Ouda, G.I.; Hussien, B.M.; Ramadan, M.F. Recent progress in screening of mycotoxins in foods and other commodities using MXenes-based nanomaterials. Crit. Rev. Anal. Chem. 2023, 17, 2222412. [Google Scholar] [CrossRef]
- Kaur, A.; Pandey, K.; Kaur, R.; Vashishat, N.; Kaur, M. Nanocomposites of carbon quantum dots and graphene quantum dots: Environmental applications as sensors. Chemosensors 2022, 10, 367. [Google Scholar] [CrossRef]
- Agarwal, K.; Rai, H.; Mondal, S. Quantum dots: An overview of synthesis, properties, and applications. Mater. Res. Express 2023, 10, 062001. [Google Scholar] [CrossRef]
- Sun, P.; Xing, Z.P.; Li, Z.Z.; Zhou, W. Recent advances in quantum dots photocatalysts. Chem. Eng. J. 2023, 458, 141399. [Google Scholar] [CrossRef]
- Singh, A.K.; Sri, S.; Garimella, L.B.V.S.; Dhiman, T.K.; Sen, S.; Solanki, P.R. Graphene quantum dot-based optical sensing platform for aflatoxin B1 detection via the resonance energy transfer phenomenon. ACS Appl. Bio. Mater. 2022, 5, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.Y.; Zhang, D.; Zhang, L.X.; Ouyang, H.; Fu, Z.F. Alkaline hydrolysis behavior of metal-organic frameworks NH2-MIL-53(Al) employed for sensitive immunoassay via releasing fluorescent molecules. ACS Appl. Mater. Interfaces 2019, 11, 35597–35603. [Google Scholar] [CrossRef] [PubMed]
- Rowland, C.E.; Brown, C.W.; Medintz, I.L.; Delehanty, J.B. Intracellular FRET-based probes: A review. Methods Appl. Fluoresc. 2015, 3, 042006. [Google Scholar] [CrossRef]
- Fang, C.; Huang, Y.; Zhao, Y. Review of FRET biosensing and its application in biomolecular detection. Am. J. Transl. Res. 2023, 15, 694–709. [Google Scholar]
- Oh, H.-K.; Joung, H.-A.; Jung, M.; Lee, H.; Kim, M.-G. Rapid and simple detection of ochratoxin A using fluorescence resonance energy transfer on lateral flow mmunoassay (FRET-LFI). Toxins 2019, 11, 292. [Google Scholar] [CrossRef]
- Tang, Z.W.; Liu, X.; Su, B.C.; Chen, Q.; Cao, H.M.; Yun, Y.H.; Xu, Y.; Hammock, B.D. Ultrasensitive and rapid detection of ochratoxin A in agro-products by a nanobody-mediated FRET-based immunosensor. J. Hazard. Mater. 2020, 387, 121678. [Google Scholar] [CrossRef]
- Zhao, X.D.; Wang, Y.; Li, J.Z.; Huo, B.Y.; Huang, H.; Bai, J.L.; Peng, Y.; Li, S.; Han, D.P.; Ren, S.Y.; et al. A fluorescence aptasensor for the sensitive detection of T-2 toxin based on FRET by adjusting the surface electric potentials of UCNPs and MIL-101. Anal. Chim. Acta 2021, 1160, 338450. [Google Scholar] [CrossRef]
- Dai, S.L.; Wu, S.J.; Duan, N.; Wang, Z.P. A luminescence resonance energy transfer based aptasensor for the mycotoxin ochratoxin A using upconversion nanoparticles and gold nanorods. Microchim. Acta 2016, 183, 1909–1916. [Google Scholar] [CrossRef]
- Qin, G.X.; Zhou, Q.F.; Li, H.L.; Yan, F.Y.; He, J.; Wei, Y.N.; Wang, H.J.; Chen, Y.X.; Lao, S.B.; Yang, Y.X.; et al. A sensitive WS2 nanosheet sensing platform based on chemiluminescence resonance energy transfer for the detection of ochratoxin A. Aust. J. Chem. 2022, 75, 362–368. [Google Scholar] [CrossRef]
- Mortezaei, M.; Dadmehr, M.; Korouzhdehi, B.; Hakimi, M.; Ramshini, H. Colorimetric and label free detection of gelatinase positive bacteria and gelatinase activity based on aggregation and dissolution of gold nanoparticles. J. Microbiol. Methods 2021, 191, 106349. [Google Scholar] [CrossRef] [PubMed]
- Nandhakumar, M.; Thangaian, D.T.; Kasi, N. Topical progress of gold nanoparticles towards diverse: Metal ion sensing through optical spectrometry and electrochemical techniques-A short review. J. Mater. Res. Technol. 2023, 22, 1185–1209. [Google Scholar] [CrossRef]
- Wu, L.X.; Wang, M.; Wei, D.Z. Advances in gold nanoparticles for mycotoxin analysis. Analyst 2021, 146, 1793–1806. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, L.Z.; Huang, T.Z.; Liu, X.; Chen, Q.; Jin, G.Y.; Cao, H.M. Gold nanoparticle-based colorimetric aptasensor for rapid detection of multiple mycotoxins in rice. Anal. Methods 2021, 13, 5749–5755. [Google Scholar] [CrossRef]
- He, Z.Y.; Chen, Q.Y.; Ding, S.S.; Wang, G.Q.; Takarada, T.; Maeda, M. Suppressed DNA base pair stacking assembly of gold nanoparticles in an alcoholic solvent for enhanced ochratoxin A detection in Baijiu. Analyst 2023, 148, 1291–1299. [Google Scholar] [CrossRef]
- Dadmehr, M.; Shahi, S.C.; Malekkiani, M.; Korouzhdehi, B.; Tavassoli, A. A stem-loop like aptasensor for sensitive detection of aflatoxin based on graphene oxide/AuNPs nanocomposite platform. Food Chem. 2023, 402, 134212. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; Yu, W.D.; Wang, Y.W.; Song, P.; Xu, Q.; Ming, D.M.; Yang, Y.Q. A switchable and signal-amplified aptasensor based on metal organic frameworks as the quencher for turn-on detection of T-2 mycotoxin. Anal. Bioanal. Chem. 2021, 413, 6595–6603. [Google Scholar] [CrossRef]
- Ong, J.Y.; Pike, A.; Tan, L.L. Recent advances in conventional methods and electrochemical aptasensors for mycotoxin detection. Foods 2021, 10, 1437. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.M.; Tang, X.Q.; Zhang, Z.W.; Li, P.W. Recent advances in electrochemical sensors for mycotoxin detection in food. Electroanalysis 2023, 35, 202100223. [Google Scholar] [CrossRef]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly imprinted polymer-based electrochemical sensors for food contaminants determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Lu, L.; Hu, X.Q.; Zhu, Z.W.; Li, D.; Tian, S.Y.; Chen, Z.X. Review-electrochemical sensors and biosensors modified with binary nanocomposite for food safety. J. Electrochem. Soc. 2019, 167, 037512. [Google Scholar] [CrossRef]
- Ramya, M.; Kumar, P.S.; Rangasamy, G.; Shankar, V.U.; Rajesh, G.; Nirmala, K.; Saravanan, A.; Krishnapandi, A. A recent advancement on the applications of nanomaterials in electrochemical sensors and biosensors. Chemosphere 2022, 308, 136416. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yang, M.; Jiang, M.; Huang, X.J.; Liu, W.Q.; Xie, P.H. Carbon-based nanomaterials—A promising electrochemical sensor toward persistent toxic substance. TrAC Trends Anal. Chem. 2019, 119, 115624. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Gorab, M.G.; Hashemi, S.M.; Javanshir, S.; Cohan, R.A.; et al. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Mu, Z.D.; Ma, L.Y.; Wang, J.; Zhou, J.; Yuan, Y.H.; Bai, L.J. A target-induced amperometic aptasensor for sensitive zearalenone detection by CS@AB-MWCNTs nanocomposite as enhancers. Food Chem. 2021, 340, 128128. [Google Scholar] [CrossRef]
- Lai, H.H.; Ming, P.T.; Wu, M.Q.; Wang, S.M.; Sun, D.P.; Zhai, H.Y. An electrochemical aptasensor based on P-Ce-MOF@MWCNTs as signal amplification strategy for highly sensitive detection of zearalenone. Food Chem. 2023, 423, 136331. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Zhao, L.; Nguyen, E.P.; Arola, H.; Nevanen, T.K.; Adam, V.; Zitka, O.; Merkoci, A. Inkjet-printed electrochemically reduced graphene oxide microelectrode as a platform for HT-2 mycotoxin immunoenzymatic biosensing. Biosens. Bioelectron. 2020, 156, 112109. [Google Scholar] [CrossRef]
- Ong, C.C.; Sangu, S.S.; Illias, N.M.; Gopinath, S.C.B.; Saheed, M.S.M. Iron nanoflorets on 3D-graphene-nickel: A ‘Dandelion’ nanostructure for selective deoxynivalenol detection. Biosens. Bioelectron. 2020, 154, 112088. [Google Scholar] [CrossRef]
- Bagdziunas, G. Theoretical design of molecularly imprinted polymers based on polyaniline and polypyrrole for detection of tryptophan. Mol. Syst. Des. Eng. 2020, 5, 1504–1512. [Google Scholar] [CrossRef]
- Kazemi, F.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. Biosensing applications of polyaniline (PANI)-based nanocomposites: A review. Polym. Rev. 2021, 61, 553–597. [Google Scholar] [CrossRef]
- Singh, A.K.; Lakshmi, G.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A simple detection platform based on molecularly imprinted polymer for AFB1 and FuB1 mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Costa, D.; Domingues, R.P.; Apreutesei, M.; Pedrosa, P.; Martin, N.; Correlo, V.M.; Reis, R.L.; Alves, E.; Barradas, N.P.; et al. Optimization of nanocomposite Au/TiO2 thin films towards LSPR optical-sensing. Appl. Surf. Sci. 2018, 438, 74–83. [Google Scholar] [CrossRef]
- Pellas, V.; Hu, D.V.; Mazouzi, Y.; Mimoun, Y.; Blanchard, J.; Guibert, C.; Salmain, M.; Boujday, S. Gold nanorods for LSPR biosensing: Synthesis, coating by silica, and bioanalytical applications. Biosensors 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Chen, C.; Yan, H. A portable optical fiber tip facet LSPR aptasensor for detection of fumonisin B1. IEEE Sens. J. 2022, 22, 17838–17844. [Google Scholar] [CrossRef]
- Lerdsri, J.; Soongsong, J.; Laolue, P.; Jakmunee, J. Reliable colorimetric aptasensor exploiting 72-Mers ssDNA and gold nanoprobes for highly sensitive detection of aflatoxin M1 in milk. J. Food Compos. Anal. 2021, 102, 103992. [Google Scholar] [CrossRef]
- Zhokhov, A.A.; Masalov, V.M.; Sukhinina, N.S.; Matveev, D.V.; Dolganov, P.V.; Dolganov, V.K.; Emelchenko, G.A. Photonic crystal microspheres. Opt. Mater. 2015, 49, 208–212. [Google Scholar] [CrossRef]
- Fathi, F.; Monirinasab, H.; Ranjbary, F.; Nejati-Koshki, K. Inverse opal photonic crystals: Recent advances in fabrication methods and biological applications. J. Drug Deliv. Sci. Technol. 2022, 72, 103377. [Google Scholar] [CrossRef]
- Dai, S.J.; Li, Q.J.; Li, W.; Zhang, Y.D.; Dou, M.H.; Xu, R.M.; Wang, T.T.; Lu, X.Y.; Wang, F.Y.; Li, J.L. Advances in functional photonic crystal materials for the analysis of chemical hazards in food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4900–4920. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Shen, P.; Liu, R.; Li, Y.W.; Xu, J.J.; Zheng, Q.; Zhang, Y.; Li, J.L.; Zheng, T.S. Aptamer fluorescence signal recovery screening for multiplex mycotoxins in cereal samples based on photonic crystal microsphere suspension array. Sens. Actuator B Chem. 2017, 248, 351–358. [Google Scholar] [CrossRef]
- Jiao, S.S.; Liu, J.; Sun, J.L.; Chang, Y.W.; Wang, S.W.; Dai, S.J.; Xu, R.M.; Dou, M.H.; Li, Q.J.; Wang, J.; et al. A highly sensitive and reproducible multiplex mycotoxin SERS array based on AuNPs-loaded inverse opal silica photonic crystal microsphere. Sens. Actuator B Chem. 2022, 355, 131245. [Google Scholar] [CrossRef]
| The United States | Total amount of AFB in food: <20 μg/kg; DON: <1000pg/kg, ZEN: <100 pg/kg; Milk and dairy products: AFM1 ≤ 0.5 μg/kg. |
| European Union | Agricultural products: Total amount of AFs: <4 μg/kg, AFB1: <2 μg/kg, OTA: <3 μg/kg, DON: <1000 μg/kg, ZEN: <50 μg/kg; Infant foods: Total amount of AFB: <2 μg/kg, AFB1 <0.1 μg/kg, AFM1: <0.025 μg/kg, OTA: <0.5 μg/kg, DON: <150 μg/kg, ZEN: <20 μg/kg |
| China | Corn, peanuts, and their products: AFB1: < 20 μg/kg, OTA: <5 μg/kg, DON: <1000 μg/kg, ZEN < 60 μg/kg; Other grains, beans, and fermented foods: AFB1: <5 μg/kg; Infant foods: AFB1: 5 μg/kg, AFM1: < 0.5μg/kg; Fresh milk and dairy products: AFM1: < 0.5μg/kg; Rice and vegetable oils (except corn oil and peanut oil): AFB1: <10 μg/kg. |
| Japan | Peanuts and their products: AFB1: <10 μg/kg; Wheat: DON: <1100 μg/kg; Apple juice: Patulin: <50 μg/kg. |
| Materials/Methods | Mycotoxins | Substrates | Properties of Materials | Results | Ref. |
|---|---|---|---|---|---|
| SPE | |||||
| PDA-IL-NFsM SPE coupled with UPLC-MS/MS | AFB1, AFB2, AFG1, AFG2, ST, FB1, FB2, OTA, ZEN, HT-2, T-2, DON, 3-AcDON, NIV, 15-AcDON | Corn, wheat | Various interception mechanisms with the target through hydrogen bonding, π-π interaction, and electrostatic or hydrophobic interaction; good simultaneous adsorption performance; significantly reducing the matrix effect | Linear range: 1.0–2000 μg/kg; LOD: 0.04–4.21 μg/kg; LOQ: 0.13–14.03 μg/kg; Recovery: 80.79–112.37 % (RSD: 2.91–14.82 %, n = 4) | [33] |
| Fe3O4@COF Magnetic SPE coupled with UHPLC-MS/MS | AFB1, OTA, ZEN, TEN, ALT, ALS, AME, AOH, TEA | Fruits | Abundant aromatic rings and carbonyl groups in Fe3O4@COF structure; through the strong π-π interaction and hydrogen bond between mycotoxin and Fe to realize effective enrichment of target mycotoxin | Linear range: 0.05–200 μg/kg; LOD: 0.01–0.50 μg/kg; LOQ: 0.10–1.00 μg/kg; Recovery: 74.25–111.75 % (RSD: 2.08–9.01 %, n = 5) | [34] |
| PDA@Fe3O4-MWCNTs Magnetic SPE coupled with HPLC-FLD | AFB1, AFB2, AFG1, AFG2, OTA, OTB | Edible vegetable oils | Good water solubility and dispersibility; largely eliminating the influence of matrix effect | Linear range: 1–100 μg/L; LOD: 0.2–0.5 μg/kg; LOQ: 0.6–1.5 μg/kg; Recovery: 70.15–89.25 % (RSD: ≤ 6.4 %, n = 6) | [35] |
| rGO/AuNPs SPE coupled with UHPLC-MS/MS | AFB1, AFM1, OTA, ZEA, α-ZOL, β-ZOL, ZAN, α-ZAL, β-ZAL | Milk | Good adsorbability; adding AuNPs increases the distance between graphene layers and minimizes agglomeration | Linear range: 0.02–200 ng/mL; LOD: 0.01–0.07 ng/mL; LOQ: 0.02–0.18 ng/mL; Recovery: 70.1–111.1 % (RSD: 2.0–11.1 %, n = 5) | [36] |
| MIL-101(Cr)@Fe3O4 Magnetic SPE coupled with UHPLC-MS/MS | AFB1, AFB2, AFG1, AFG2, OTA, OTB, T-2, HT-2, DAS | Maize, wheat, watermelon, and melon | Magnetic separation and adsorption capabilities involving polar or nonpolar forces, hydrogen bonding forces, and π-π conjugation with mycotoxin-rich functional groups | Linear range: 0.2–100 ng/mL LOD: 0.02–0.06 μg/kg; LOQ: 0.08–0.2 μg/kg Recovery: 83.5–108.5 % (RSD: 1.6–10.4 %, n = 5) | [37] |
| Fe3O4@SiO2-NH2 Magnetic SPE coupled with ELISA | AFB1 | Pixian douban | Rapid separation and enrichment under the external magnetic field; strong chemical stability, storage stability, and specificity combined with aptamer | Linear range: 0.5–2.0 ng/mL; LOD: 0.17 ng/mL; LOQ: 0.48 ng/mL; Recovery: 80.19–113.92 % (RSD: 2.30–7.28 %, n = 3) | [38] |
| HAS SPE coupled with HPLC-PHRED-FLD | AFB1 | Vegetable oils | Outstanding adsorption properties due to the large number of functional group hydrogen bonding, hydrophobicity, and π-π interactions; minimizing the pretreatment time and the amounts of organic solvents | Linear range: 0.10–50 μg/kg; LOD: 0.03–0.09 μg/kg; LOQ: 0.1–0.3 μg/kg; Recovery: 66.9–118.4 % (RSD: ≤ 7.2 %, n = 6) | [39] |
| UIO-66-NH2@MIPs SPE coupled with HPLC | AFB1, AFB2, AFG1, AFG2 | Wheat, rice, corn, soybean | Uniform and stable; the unique pore structure effectively improving the selective adsorption capacity; excellent affinity and selectivity | Linear range: 0.20–45 μg/kg; LOD: 0.06–0.13 μg/kg; LOQ: 0.24–0.45 μg/kg; Recovery: 74.3–98.6 % (RSD: 1.0–5.9 %, n = 6) | [40] |
| MWCNT-COOH + C18 SPE coupled with UPLC-MS/ MS | 21 mycotoxins (AFs, OTA, OTB, ZEN, T-2, ZEN et al.) | Corn, wheat | Significantly reducing the matrix effect; high-throughput screening of various targets; greatly improving the detection efficiency | LOQ: 0.5–25 μg/L; Recovery: 75.6–110.3 % (RSD: 0.3–10.7 %, n = 5) | [41] |
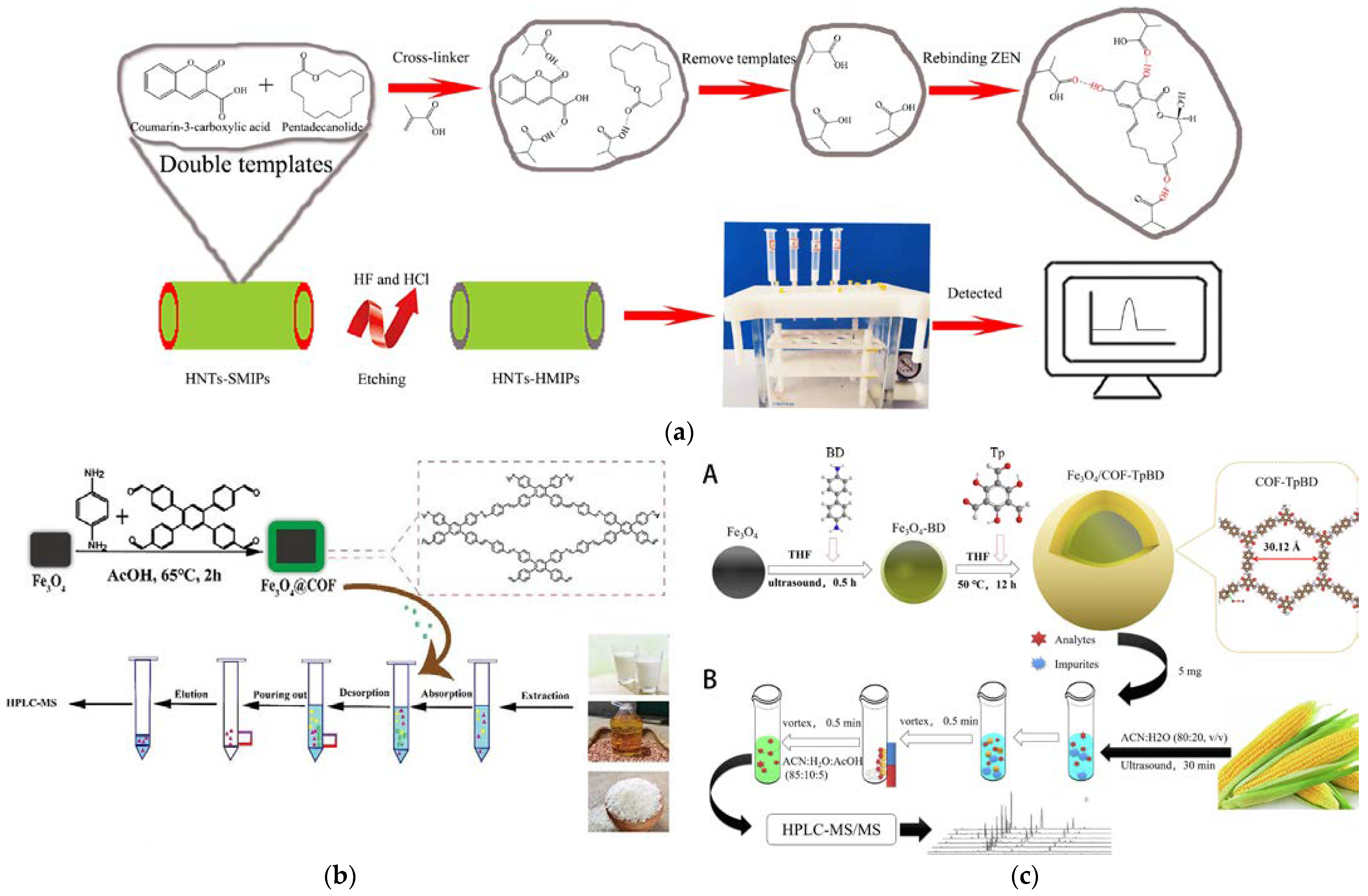
| HNTs-HMIPs SPE coupled with HPLC-FD (Figure 1a) | ZEN | Rice corn, red beans, oats, wheat | Hollow imprinted polymer; excellent adsorption due to the loose and porous characteristics | LOD: 0.5 μg/kg; LOQ: 4.17 μg/kg (Oat), 1.8 μg/kg (Wheat); Recovery: 77.13–102.4 % (RSD: ≤ 5.59 %, n = 6) | [42] |
| SPME | |||||
| AuNPs SPME coupled with UHPLC-MS/MS | PAT | Apple juice, fresh apple, apple baby food, orange juice | Capillary monolithic column directly modified by AuNPs; high specificity and high affinity | Linear range: 8.11–8.11 × 103 pmol/L; LOD: 2.17 pmol/L; Recovery: 85.4–106 % (RSD: 4.1–7.3 %, n = 5) | [43] |
| MAA-co-DVB SPME coupled with HPLC | AFB1, ZEN, STEH | Rice | High-strength micro/nanostructure containing a large number of acrylic groups forming hydrogen bonds with groups in the target structure; effectively overcoming the matrix effect | Linear range: 0.01–1.0 mg/kg; LOD: 0.689–2.030 μg/kg; LOQ: 5.36–14.4 μg/kg Recovery: 86.0–102.8 % (RSD: ≤ 4.8 %, n = 4) | [44] |
| Fe3O4@SiO2@Cu/Ni-NH2BDC Dispersive SPME coupled with HPLC-FLD | AFB1, AFB2, AFG1, AFG2 | River water, well water, rice | Chemical bonds formed between three components making the adsorbent more stable and magnetic; rapid separation | Linear range: 0.11–79.2 ng/mL; LOD: 0.01–0.04 ng/mL; LOQ: 0.04–0.15 ng/mL; Recovery: 92.0–97.8 % (RSD: 4.1–7.6 %) | [45] |
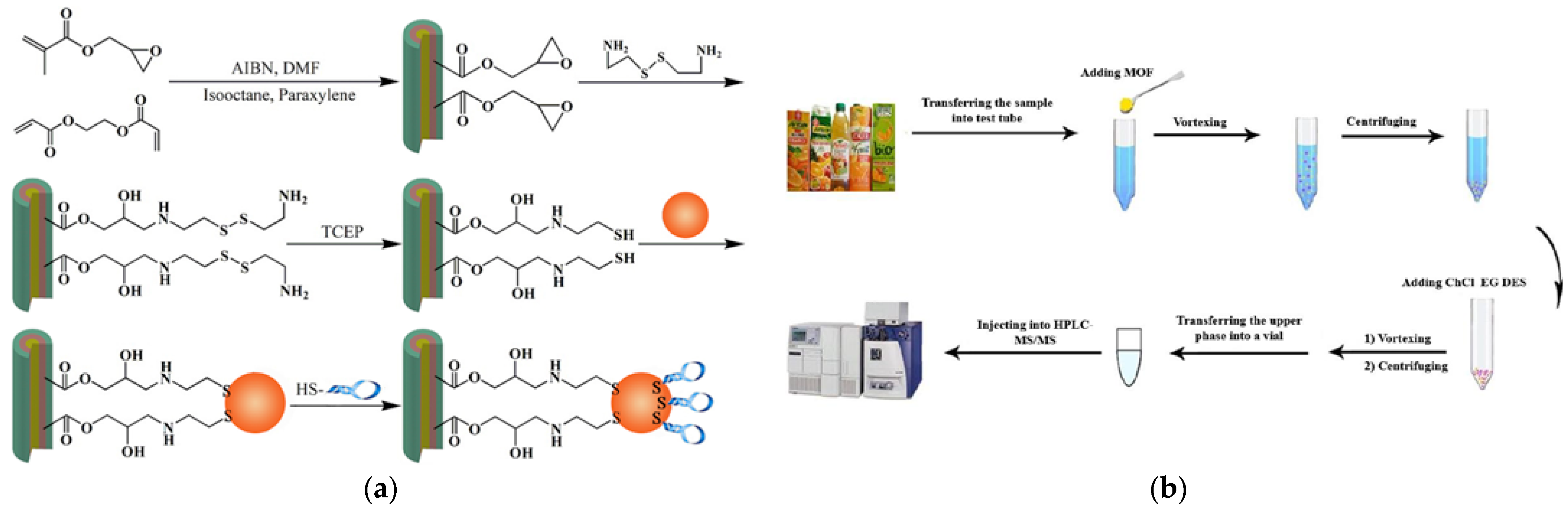
| MOF+VB3 Dispersive SPME coupled with HPLC-FLD | PAT, OTA, AFB1, AFB2, AFG1, AFG2 | Fruit juices, milk | Green organic linker; high surface area, high adsorption capacity, and excellent porosity to form a new green adsorbent | Linear range: 42.8–1 × 106 ng/L; LOD: 11.3–48.2 ng/L; LOQ: 42.8–161.6 ng/L; Recovery: 64.0–87.0 % (RSD: ≤5 %, n = 3) | [46,47] |
| Materials | Mycotoxins | Substrates | Action/Merits | Results | Ref. |
|---|---|---|---|---|---|
| Electrochemical Methods | |||||
| MWCNTs/Fc-MOF | AFB1 | Walnut | Sensing substrate with large specific surface area for Ab incubation; stable internal reference electrochemical signals; improving the sensitivity and reliability by self-correction | Linear range: 10 fg/mL–100 ng/mL; LOD: 5.39 fg/mL; Recovery: 88.1–106 % (RSD: 1.2 %, n = 3) | [106] |
| AuNPs/FeMOF@GO | T-2 | Bear | Larger specific surface area for aptamer loading; improving the electron transfer capacity | Linear range: 0.5–5.0 × 106 pg/mL; LOD: 0.19 pg/mL; Recovery: 92.5–97.8 % (RSD: 3.2–5.5 %, n = 3) | [107] |
| AuNPs/MnO2@GO | T-2 | Milk | Enhance the electrochemical active surface area for interface sensing and amplify the signal | Linear range: 2 fg/mL–20 ng/mL; LOD: 0.107 fg/mL; Recovery: 96.5–103.4 % (RSD: 3.8–4.3 %, n = 3) | [108] |
| rGO/SnO2 | PTA | Apple juice | High surface area and excellent electrocatalytic performance | Linear range: 50–600 nM; LOD: 0.6635 nM; Recovery: 74.33–99.26 % (CV: 0.944–2.95 %, n = 3) | [109] |
| PEG/AuNPs | AFM1 | Milk | Hydrated layer combining the hydrophilicity of PEG and high surface area of AuNPs; inhibiting protein corrosion and enhancing capacitance signal | Linear range: 20–300 pg/L; LOD: 7.14 pg/mL; Recovery: 101.6–105.5 % (RSD: <3%, n = 3) | [110] |
| GO-CS/CeO2-CS | AFM1 | Milk | Strong conductivity, large specific surface area, and good redox performance; accelerating the electron transfer and amplifying the electrochemical signal | Linear range: 0.01–1 mg/L; LOD: 0.009 mg/L; Recovery: 96.15–104.25 % (RSD: 2.7–4.2 %, n = 3) | [111] |
| ZnO NFs | TEA | Tomato, orange | Highly specific surface area and excellent conductivity; a carrier for efficient immobilization of monoclonal Abs and Ab bioconjugates | Linear range: 5 × 10−5 –5 × 10−1 μg/mL; LOD: 1.14 × 10−5 μg/mL; Recovery: 95.71–120.3 % (RSD: 4.15–8.67 %, n = 3) | [112] |
| AuNPs@Ce-TpBpy COF | ZEN | Cornmeal | Adjustable pore size; high specific surface area; porous structure | Linear range: 1 pg/mL–10.0 ng/mL; LOD: 0.389 pg/mL; Recovery: 93.0–104.7 % (RSD: 1.26–5.54 %, n = 3) | [113] |
| CuO@GO | ZEN | Milk | Large electrochemical active surface area; high active electron transfer site and high conductivity | Linear range: 10–150 ng/mL; LOD: 0.012ng/mL; Recovery: 84.4–97.0 % (RSD: 1.01–1.34 %, n = 4) | [114] |
| AuNPs/Ni-MOF | AFB1 | Rice flour | Larger specific surface area for improving electron transfer capacity and larger specific surface area, and amplifying electrochemical signals of the electrode | Linear range: 0.005–150.0 ng/mL; LOD: 0.001 ng/mL; Recovery: 98.7–101.3 % (RSD: 6.1–7.8 %, n = 3) | [115] |
| Fluorescence Methods | |||||
| NMOFs | AFB1 | Maize | Low complexity; low interferences; high specificity and stability | Linear range: 0–3.33 ng/mL; LOD: 0.08 ng/mL; Recovery: 89.11–102.40 % (RSD: 3.17–5.39 %, n = 3) | [116] |
| Zr-MOFs | T-2 | Milk, beer | Rich functional groups, easy to combine with auxiliary materials | Linear range: 0.5–100 ng/mL; LOD: 0.239 ng/mL; Recovery: 89.86–111.51 % (RSD: 2.0–2.9 %, n = 3) | [117] |
| SWCNH | AFB1 | Soybean oil | Remarkable specificity and stability | Linear range: 10–100 ng/mL; LOD: 4.1 ng/mL; Recovery: 85.9–102.3 % (RSD: 3.59–5.46 %, n = 3) | [118] |
| CuO NPs | ZEN | Wheat, maize | High stability and biocompatibility; signal source and carrier for signal amplification; automatic sample pretreatment; high-throughput terminal detection | Linear range: 16.0–1600.0 μg/kg; LOD: 0.33 μg/kg; Recovery: 99.2–104.9 % (RSD: 0.7–5.1 %, n = 3) | [119] |
| GO/Fe3O4 | AFB1, FB1 | Peanut | Double fluorescence emission peaks quenching at the same time; effectively removing by magnetic separation to eliminate background interference | Linear range: 10 pg/mL–300 ng/mL; LOD: 6.7 pg/mL; Recovery: 92.0–97.0 % (RSD: 4.2–6.4 %, n = 3) | [120] |
| FOQD | PAT | Apple juice | Excellent fluorescence quenching ability; remarkable dispersibility in water | Linear range: 0.02–1 ng/mL; LOD: 0.01 ng/mL; Recovery: 95.0–103.0 % (RSD: 3.1–5.3 %, n = 3) | [121] |
| QDNBs | FB1, DON, ZEN | Wheat, maize | Good biocompatibility; the carrier for immobilization of Ab molecules | Linear range: 0.295–69.867 ng/mL; LOD: 0.87 ng/mL; Recovery: 78.61–122.31 % (CV: 1.10–14.36 %, n = 3) | [122] |
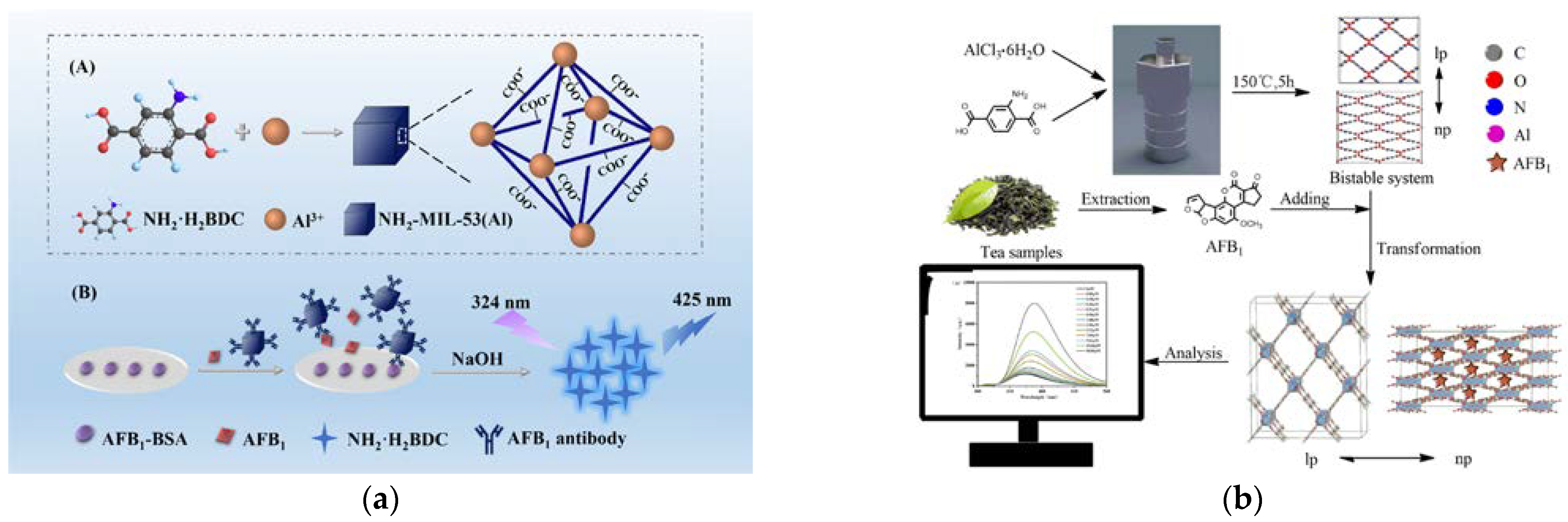
| Al-MOFs | AFB1 | Tea | Good stability from respiratory effect; multiple forces for target toxin recognition | Linear range: 0.05–9.61 μM; LOD: 11.67 ppb; Recovery: 78.86–115.29 % (RSD: 0.83–7.72 %, n = 3) | [123] |
| Zr-CAU-24 | AFB1 | Walnut, almond beverages | Strong metal–ligand bond strength; high water stability; high sensitivity | Linear range: 0.075–25 μM; LOD: 19.97 ppb; Recovery: 91–108 % | [124] |
| GO | AFM1 | Milk power | Protecting DNA aptamer from nuclease cleavage; target circulating signal amplification; high sensitivity | Linear range: 0.2–10 μg/kg; LOD: 0.05 μg/kg; Recovery: 98–126 % (SD: 1.48–6.3 μg/kg, n = 3) | [125] |
| Colorimetric Methods | |||||
| AuNPs | T-2 | Wheat, maize | Acting as signal source; high sensitivity and portable | Linear range: 0.1–5000 ng/mL; LOD: 57.8 pg/mL; Recovery: 90.9–108.4 % (RSD: 0.7–7.21 %, n = 3) | [126] |
| AuNBPs | ZEN | Cornmeal | Highly sensitive signal source | Linear range: 0.02–0.80 ng/mL; LOD: 0.011 ng/mL; Recovery: 89.5–107.0 % (CV: 4.39–6.7 %, n = 3) | [127] |
| MnO2 | OTA | Wheat flour, red wine | Excellent oxidase-like activity; using DNA regulate catalytic activity; high affinity for chromogenic substrates | Linear range: 0.05–33.35 ng/mL; LOD: 0.069 ng/mL; Recovery: 85.0–101.6 % (RSD: 1.33–6.83 %, n = 3) | [128] |
| Au NPs@m-SiNPs | AFs | Cornflakes, peanuts, butter, pecan nuts | Large surface area, high thermal stability, and chemical stability; acting as a fixed platform for AuNPs and direct immobilization of Abs | Linear range: 1–75 ng/mL; LOD: 0.16 ng/mL | [129] |
| BP-Au | DON | Maize, oat, millet | Remarkable color and photothermal conversion efficiency | Linear range: 0.1–8 ng/mL; LOD: 0.1 ng/mL; Recovery: 95.38–114.81 % (CV: 1.00–16.12 %, n = 3) | [130] |
| ZnOBt | AFs | Corn, almond | Color changes indirectly with targets through the oxidation–reduction reaction | Linear range: 0.5–20 ppb; LOD: 2.74 ppb; Recovery: 83.2–96.4 %; (RSD: 4.1–9.6 %, n = 5) | [131] |
| Pt NPs/Fe-MOG | FB1 | Corn | Excellent peroxidase simulation activity and high affinity for substrates | Linear range: 0.01–2000.0 ng/mL; LOD: 2.7 pg/mL; Recovery: 98.7–101.9 % (RSD: 3.0–4.7 %, n = 3) | [132] |
| FeO/GO, FeO@Au | AFB1, OTA | Peanut | Large specific surface area; strong interactive affinity; high peroxidase activity; double-target detection | Linear range: 0.5–250 ng/mL; LOD: 0.15 ng/mL; Recovery: 87.3–102.5 % (RSD: 4.7–8.4 %, n = 3) | [133] |
| Pt-CN | AFB1 | Peanut | Outstanding peroxidase simulation activity | Linear range: 1.0 pg/mL–10 ng/mL; LOD: 0.22 pg/mL; Recovery: 85.71–105.3 % (RSD: 2.23–8.33 %, n = 3) | [134] |
| UiO-66-NH2 | ZEN | Wheat, maize | Ultrahigh loading capacity; excellent affinity with specific aptamer; excellent catalytic performance | Linear range: 0.01–100 ng/mL; LOD: 0.36 pg/mL; Recovery: 94.6–108.7 % (RSD: 1.0–8.7 %, n = 6) | [135] |
| Other Strategies (LSPR and SERS) | |||||
| Aptamer-GNR LSPR | OTA | Grape juice | Carrier for the aptamer specifically recognized the target; increasing local refractive index to lead to the shift of extinction peak | Linear range: 10 pM–100 nM; LOD: 12.0 pM; Recovery: 85.5–116.9 % | [136] |
| PCPD-AgNPs LSPR | AFB1 | Peanut | Generating colorimetric signals based on LSPR changes of aggregated AgNPs for quantitative detection | Linear range: 0.2–6.0 ng/mL; LOD: 0.09 ng/mL; Recovery: 84.0–91.2 % (RSD: 0.6–1.8 %, n = 3) | [137] |
| Au NBPs LSPR | DON | Wheat, maize | Self-assembly into photonic crystals; enhancing the signal by coupling emission | Linear range: 0–2000 ng/mL; LOD: 57.93 ng/mL; Recovery: 93.7–107.5 % (RSD: 4.06–11.8 %, n = 4) | [138] |
| AuNPS LSPR | ZEN | Corn, wheat | Signal source; forming a stronger blue shift of LSPR peak, resulting in color change | Linear range: 0.04–2.96 ng/mL; LOD: 0.10 ng/mL (Naked eye), 0.07 ng/mL (Smartphone), 0.04 ng/mL (UV-spectra); Recovery: 76–112.6 % (RSD: 3.6–12.7 %, n = 3) | [139] |
| AUNPs-3D SPCM SERS | AFB1, OTA | Lily, Job’s tears seed, lotus seed | Great enhancement effect on SERS signal; good structural uniformity; accurate focusing position; overcoming the problem of SERS analysis and quantification | Linear range: 0.001–10 ng/mL; LOD: 0.034 pg/mL; Recovery: 80.23–116.20 % (CV: 6.3–7.16 %, n = 3) | [140] |
| AgNPs@K30 SERS | AFB1, OTA, OTB | Rice | High roughness surface; anisotropic SERS substrate for capturing target toxin to produce the signal | Linear range: 0.5–500 µg/kg; LOD: 1.133 µg/kg; Recovery: 85.57–107.1 % (RSD: 5.84–8.71 %, n = 5) | [141] |
| GO@Au-Au SERS | FB1, AFB1, ZEN | Corn, peanut | Larger reaction interface; excellent stability and dispersibility; greatly improved the SERS activity and colorimetric signal | Linear range: 0.00046–10 ng/mL; LOD: 0.529 pg/mL; Recovery: 90.03–113.75 % (RSD: 2.79–13.48 %, n = 3) | [142] |
| Au@SiO2 SERS | AFB1, OTA | Corn, rice, wheat | Improved plasmon resonance activity; chemical properties and biocompatibility; good dispersibility as SERS substrate | Linear range: 250 fg/mL–25 ng/mL; LOD: 0.24 pg/mL; Recovery: 87.0–108.0 % (RSD: 2.4–6.3 %, n = 4) | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Li, H.; Yang, J.; Wen, X.; Wang, S.; Pan, M. Nanoscale Materials Applying for the Detection of Mycotoxins in Foods. Foods 2023, 12, 3448. https://doi.org/10.3390/foods12183448
Hu X, Li H, Yang J, Wen X, Wang S, Pan M. Nanoscale Materials Applying for the Detection of Mycotoxins in Foods. Foods. 2023; 12(18):3448. https://doi.org/10.3390/foods12183448
Chicago/Turabian StyleHu, Xiaochun, Huilin Li, Jingying Yang, Xintao Wen, Shuo Wang, and Mingfei Pan. 2023. "Nanoscale Materials Applying for the Detection of Mycotoxins in Foods" Foods 12, no. 18: 3448. https://doi.org/10.3390/foods12183448
APA StyleHu, X., Li, H., Yang, J., Wen, X., Wang, S., & Pan, M. (2023). Nanoscale Materials Applying for the Detection of Mycotoxins in Foods. Foods, 12(18), 3448. https://doi.org/10.3390/foods12183448






