Spice and Herb Frauds: Types, Incidence, and Detection: The State of the Art
Abstract
1. Introduction
1.1. Herbs and Spices Sensorial and Health-Related Properties
1.2. World Market of Herbs and Spices: Economic Relevance
1.3. Quality Protection of Herbs and Spices
2. Types of Frauds
- Adulteration and product tampering: Addition of a foreign or inferior-quality substance or element; by replacing a more valuable substance or element with less valuable or inert ingredients, so that they no longer match the implicit or explicit claims associated with the agri-food product. Adulterations can be carried out by the following actions: substitution, dilution, removal, unapproved/undeclared enhancement and concealment, and unapproved/undeclared treatment, process, or product. In the case of the addition of components, these could reduce the quality and alter the composition of the food itself, potentially causing health risks to consumers [22].
- Counterfeit: Intellectual Property Rights (IPR) infringement, including any aspects of the genuine agri-food product or packaging being replicated, for instance, the process of copying the brand name, packaging concept, or processing method for economic gain.
- Document forgery: The process of creating, adapting, altering, misrepresenting, or imitating documents such as certificates, passports, analytical test reports, declarations of compliance, and other identification, and administrative documents.
- Grey market activities: Production, theft, and diversion involving unauthorized sales channels for agri-food products (traceability issues).
- Misdescription/mislabelling/misbranding: Placing of explicit false claims or distorting the information on the label/packaging of expiry/production date, nutrition/health claims, geographical claims (excluding PGO, PDI, TSG), quality terms, and/or quantity (weight and volume).
- A different part of the same botanical plant, rather than the one declared, to the extent that this would mislead the customer.
- Technically avoidable amounts of parts from other botanical plants than the one declared.
- Ingredients, additives, dyes, or any other constituent not approved for use in herbs and spices.
- Ingredients, additives, dyes, or any other constituent approved for use in food but unlawfully not declared or indicated in a form which might mislead the customer.
- Herbs and spices that have had any valuable constituent omitted or removed which misleads the customer (e.g., spent and partially spent herbs and spices, de-oiled material, and defatted material).
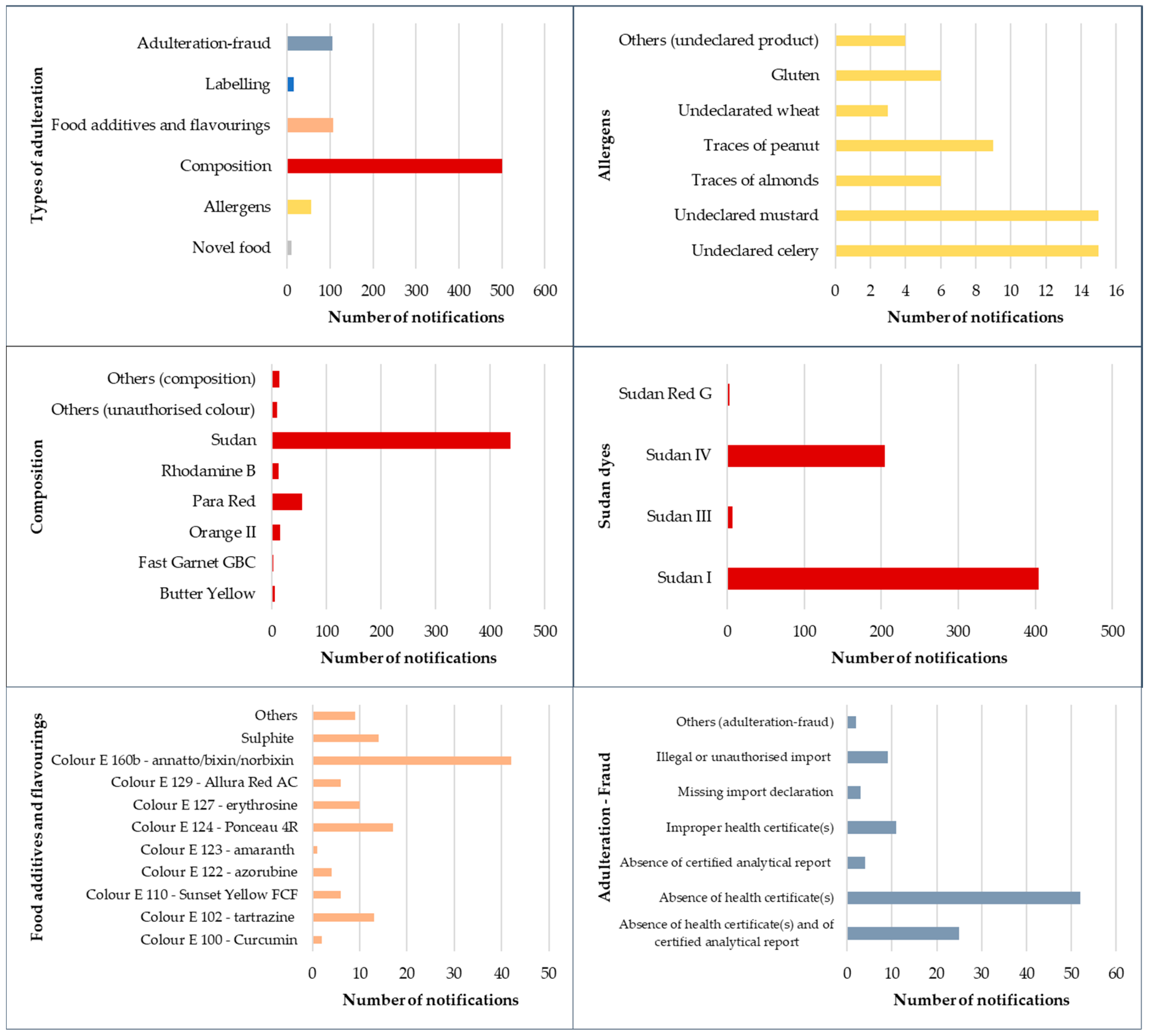
3. Fraud Incidence
4. Scientific Articles Related to Fraud and Adulteration
5. Methods for Detecting Spice and Herb Frauds and Adulterations
| Spices/Herbs | Fraud/Adulteration | Technique/Detection Method | Main Results | References |
|---|---|---|---|---|
| Paprika powder | Sudan I and Rhodamine B | HPLC-MS/MS | Detection of illegal synthetic dyes in Chinese paprika powder samples. Detection limits ranging from 0.013 ng/mL to 0.054 ng/mL, suggesting that the method is promising for accurate quantification of Sudan dyes at trace levels in foodstuffs. | [61] |
| Chili powder and paprika | Sudan I and II | HPLC-DAD | Detection of oil-soluble synthetic dyes in chilli products. The screening was based on the fingerprint differences of a normal unadulterated chilli sample with tested chilli samples. Limits of detection 0.40–2.41 mg/kg. The screening method was simple and had the possibility of finding the existence of the adulterated dyes which could not be identified using known standard analytes as control. | [62] |
| Chili powder | Sudan I–IV, Sudan Red 7B, Sudan Red G, Sudan Orange G, Para Red, and Methyl Red | UHPLC-DAD | Detection of the nine illegal dyes most frequently found in chilli-containing spices (the red dyes Sudan I–IV, Sudan Red 7B, Sudan Red G, Sudan Orange G, Para Red, and Methyl Re). Limits of detection showed lower values than required by European Union regulations and were in the range of 3.3–10.3 µg/L for standard solutions, and 5.6–235.6 µg kg−1 for chilli-containing spices. | [63] |
| Turmeric, curry, hot paprika, and sweet paprika | Synthetic dyes | HPLC-DAD | Very simple and fast detection of Sudan dyes (I, II, III and IV) in commercial spices up to a concentration of 5 mg/L. | [64] |
| Chili powder | Sudan | LC-UV/Vis | Simple detection of illegal dyes in foods such as Orange II, Sudan I–IV, Sudan Black B, Sudan Red 7B, Sudan Red G, Methanil Yellow, Dimethyl Yellow, Auramine O, Bixin, Fast Garnett GBC, Rhodamine B, Oil Orange SS, Orange G, Sudan Orange G, Naphthol Yellow, Acid Red 73, Toluidine Red, Sudan Red B, and Para Red in five matrices. The limits of detection, recovery and precision are considered adequate for a screening method. | [65] |
| Saffron | Geographical origin | HPLC-DAD | Differentiation of saffron spices produced at different sites on basis of crocin, safranal, picrocrocin and its derivatives and flavonoids. Statistical multivariate analysis of HPLC data offers the real possibility of differentiating PDO saffron from high-quality spices produced in close sites. | [66] |
| Saffron | Geographical origin | HPLC-DAD | Geographical discrimination of saffron samples from Iran and China on basis on picrocrocin and two types of crocin. The samples were well-separated according to their HPLC fingerprint data using PCA and orthogonal partial least squares discriminant analysis (OPLS-DA). | [67] |
| Saffron | Authenticity of saffron | HPLC-MS | Geographical discrimination of saffron samples on basis of glycerophospholipids and their oxidized lipids. The method allows for the distinguishing between PDO saffron and labelled Spanish saffron. | [68] |
| Paprika | Authenticate the geographical origin | HPLC-FLD | Phenolic acid and polyphenolic compounds were used as chemical markers to assess the classification of paprika from five European regions. The chromatographic fingerprints were also used to detect and quantitate two different paprika geographical-origin blend scenarios by partial least squares (PLS) regression. | [69] |
| Oregano | Olive leaves, myrtle leaves, cistus, hazelnut | LC-HRMS | Differentiation of oregano from olive leaves, myrtle leaves, cistus, and hazelnut by biomarker identification. | [37] |
| Oregano and sage | Olive leaves | GC-MS | Differentiation of ground oregano and sage from ground olive leaves on basis on two markers generated from the biophenol fraction. The detection limit was low, at 1%. | [70] |
| Bay leaves | Cinnamomum tamala, Litsea glaucescens, Pimenta racemosa, Syzygium polyanthum and Umbellularia californica leaves | GC-MS | Differentiation of bay leaf from its common surrogates (Cinnamomum tamala, Litsea glaucescens, Pimenta racemosa, Syzygium polyanthum and Umbellularia californica leaves). | [71] |
| Saffron | Turmeric and marigold | HS-GC-FID | Adulteration of saffron with two of the principal plant-derived adulterants: turmeric (Curcuma longa L.) and marigold (Calendula officinalis L.). The method, based on a combination of chemometrics with gas chromatography, may provide a rapid and low-cost screening method for the authentication of saffron. | [72] |
| Lemon balm | Nepeta cataria L. | CZE | Differentiation of the Melissa officinales L. from Nepeta cataria L. on basis on hidroxycinnamic acid contents for detection of commercial substitutions. | [73] |
| Vanilla | Artificial flavourings Adulterations | CZE) | Identification of natural vanilla by detection of p-hydroxybenzaldehyde, p-hydroxybenzoic acid, vanillin, and vanillic acid; identification of artificial flavourings by detection of ethyl vanillin; identification of adulterations by detection of coumarin in vanilla samples. Limits of detection from 2 to 5 μg/mL. | [74] |
| Smoked paprika | Adulteration with non-smoked paprika from non-authorized varieties | CZE | Detection of frauds in smoked paprika POD “Pimentón de La Vera” by mixing with non-authorized varieties. Methanol soluble proteins and hidrophilic and hidrophobic protein fractions allowed the detection limit of 5%. | [75,76] |
| Saffron | Curcuma species | RAPD-PCR | Four RAPD primers (OPA 02, OPA 04, OPA 07, and OPC 05) were used. RAPD banding pattern of two Curcuma species, namely Curcuma longa L. and Curcuma zedoaria (Christm.) Roscoe, and three market samples were tested to evaluate adulteration. Three market samples of turmeric powder were adulterated with Curcuma zeadoaria (Christm.) Roscoe. | [77] |
| Chili powder | Dried red beet powder, almond shell dust and powdered Ziziphus nummularia fruits | RAPD-PCR | Three selected RAPD primers (OPA-2, OPA-15 and OPA10) which produced adulterant-specific bands in simulated samples were used for analysing market samples of chilli powder. Out of the six market samples analysed, one sample showed an amplified Ziziphus nummularia-specific band, indicating the occurrence of adulteration in market samples. All the market samples tested were free from dried red beet pulp or almond dust adulteration. | [78] |
| Black pepper | Carica papaya | RAPD-PCR | Five decamer oligonucleotide primers (OPC-1, OPC-4, OPC-6, OPC-7 and OPC-8) discriminated Piper nigrum, as well as Carica papaya, by the presence and absence of unique bands. | [79] |
| Smoked paprika P.D.O. “Pimentón de la Vera” (autochthonous varieties of pepper: Jaranda, Jariza, and Bola) | Paprika elaborated from varieties of pepper foreign to the La Vera region, in central western Spain (varieties: Papri Queen, Papri King, Sonora, PS9794, and Papri Ace) | RAPD-PCR | RAPD-PCR with primers S13 and S22: two molecular markers of 641 and 704 bp, respectively, were obtained, which allowed all of the smoked paprika varieties to be differentiated from paprikas elaborated with the five foreign varieties. | [80] |
| Oregano | Plants lacking a clearly detectable essential-oil profile (Rubus sp., Cistus incanus L., Rhus coriaria L) | RAPD-PCR | Thirteen RAPD primers discriminated between oregano and its adulterants, allowing their detection in oregano samples with a limit of detection of 1%. | [81] |
| Oregano | Cistus incanus L., Rubus caesius L., and Rhus coriaria L. | SCAR-PCR | Detection limits at 1% for the adulteration of oregano. | [82] |
| Oregano | Olive leaves | SCAR-PCR | Detection limits at 1% for the adulteration of oregano. | [83] |
| Saffron | Curcuma species | SCAR-PCR | Two pairs of SCAR primers were designed from the RAPD markers ‘Cur 01’ and ‘Cur 02’, respectively. Six market samples of turmeric powder and four simulated standards besides the genuine samples were analysed using the specific SCAR markers. Both the SCAR markers detected the presence of Curcuma zedoaria/Curcuma malabarica adulteration in four market samples and inall the simulated standards prepared in different concentrations. The efficiency of the SCAR markers for detecting adulteration even at low concentrations (10 g adulterant/kg of turmeric powder) substantiates their applicability as a qualitative diagnostic tool for detecting plant-based adulterants in turmeric powder. | [84] |
| Chili powder | Dried red beet pulp and powdered Ziziphus mummularia fruits | SCAR-PCR | Red beet pulp-specific SCAR primer pair, B1, and Ziziphus nummularia-specific SCAR primer pair, Z1, were designed from the corresponding RAPD marker sequences to amplify SCAR markers of 320 bp and 389 bp, respectively. SCAR markers could detect the adulterants at a concentration as low as 10 g adulterant kg/blended sample. The Z. nummularia SCAR marker could detect the presence of Z. nummularia fruit adulteration in one of the commercial samples. All the market samples tested were free from red beet pulp adulteration. | [51] |
| Saffron | Safflower and Calendula | SCAR-PCR and DNA barcoding | SCAR markers SAFL4, SAFL40, SCCt131, and ScCO390 were useful for simple, accurate, specific, and sensitive detection of safflower/Calendula adulteration in saffron. Out of the three DNA barcodes (psbA-trnH, ITS2, and rbcLa) used, psbA-trnH was considered ideal for detection of adulterants in saffron as it gave different product sizes for saffron and safflower/Calendula. Detection limits of safflower (0.5%) and Calendula (3%) in saffron. | [85] |
| Saffron | Safflower, corn | SCAR and ITS multiplex PCR-based assay | Six pairs of SCAR primers were designed which were able to amplify reproducible saffron DNA with expected sizes and no amplification in corn and safflower DNA. In this study, a primer pair was also designed based on ITS sequences for specific amplification of safflower DNA. PCR reactions specifically amplified 613 bp of ITS region in safflower genome. The multiplex PCR assays were further established for the joint use of some SCAR and ITS markers efficiently. | [86] |
| “Florinis” Greek pepper | Florinis-type pepper and Karatzova peppers | ISSR | Differentiation of “Florinis Greek” pepper from its adulterants. The economic interest in ‘Florinis’ peppers has led to many adulteration events. In that aspect, genetic profiles of ‘Florinis’, a ‘Florinis’-type and ‘Karatzova’ peppers, were studied using inter-simple sequence repeat (ISSR) molecular markers and an automated fragment detection system. The molecular protocol established during this study may successfully discriminate the original ‘Florinis’ cultivar from the ‘Florinis’-type peppers and ‘Karatzova’ cultivar. | [87] |
| Oregano | Adulterants | SSR | Simple sequence repeat (SSR) markers were developed from expressed sequence tags (ESTs) of essential oil glands of oregano. Thirteen EST-SSR loci were evaluated using 20 individual plants of oregano and 19 plants of Origanum majorana. | [88] |
| Saffron | Safflower | SCAR-RAPD | Identification of the adulterant (safflower petals) in commercial saffron samples by amplification of two specific bands by SCAR primers designed from RAPD bands. Limit of detection: 1% of safflower. | [89] |
| Turmeric powder | Cassava, wheat, barley, rye starches | DNA barcoding | ITS was the ideal locus among the three testes (rbcL, ITS and matk) to discriminate the Curcuma species. Adulterants including Curcuma zedoaria (in one sample) and cassava starch, wheat, barley, and rye (in other two samples). | [90] |
| Cumin, garlic, fennel, cinnamon, pepper, bay leaves, clove | Wheat, sorghum, maize, soybean, rice species | DNA barcoding | A total of 22 species (16 types of spices and 6 adulterations) were collected for this study. ITS2 and psbA-trnH were used as barcoding loci. Only two types of natural spices (fennel and liquorice) were correctly labelled; the other 14 spices had different amounts of adulteration. | [91] |
| Sixteen types of culinary spices from Beijing Tong Ren Tang Group. Coriander, bay leaf, white pepper, and cumin | Triticum aestivum (wheat), Oryza sativa (rice) and Zea mays (maize) | DNA barcoding | Evaluation of five barcodes (ITS2, rbcL, trnL (UAA), trnL (P6 Loop), and psbA-trnH). Combination of two barcodes (ITS and psbA-trnH) gave a higher species’ resolution rate (95.5%). Thirty commercial products were evaluated, with 93.3% of the tested products being authentic and 6.7% indicating adulteration with rice. | [92] |
| Basil, oregano, paprika | Wind-pollinated plant species, wind-spread plant species | DNA metabarcoding | In this study, DNA metabarcoding was used for the identification and authentication of 62 products containing basil, oregano, and paprika, collected from different retailers and importers in Norway. Results showed varying degrees of discrepancy between the constituent species and those listed on the product labels, despite high product authenticity. | [93] |
| Saffron | Daucus carota, Carthamus tinctorius, Calendula officinalis, Dendranthema morifolium (Ramat.) Tzvel., Nelumbo nucifera, Hemerocallis fulva (L.) L., and Zea mays | Barcoding melting curve analysis method (Bar-MCA) | The universal chloroplast plant DNA barcoding region trnH-psbA was used to identify adulterants of saffron. Differences between the melting temperatures of saffron and its adulterants can be used to discriminate authentic and adulterated saffron. | [94] |
| Spices from Lamiaceae family | Adulterants | DNA barcoding | The barcode regions (rpoB, rbcL, matK and trnH-psbA) were tested. Results suggest that the non-coding trnH-psbA intergenic spacer was the most suitable marker for molecular spice identification, followed by matK. Both markers were almost invariably able to distinguish spice species from closest taxa, with the exclusion of samples belonging to the genus Oregano. | [95] |
| Saffron | Adulterants | Real-time PCR + HRM analysis and DNA mini-barcodes | ITS1 and matK region markers for Crocus genus detection. ITS2 locus for species-specific detection of Crocus sativus and Crocus cartwrightiamus | [96] |
| Turmeric powder | Sudan Red, starch, and Metanil Yellow | NIR spectroscopy | Controlled (PCA) and uncontrolled (PLS-DA and CMCA) pattern-recognition techniques for the detection and classification of Sudan Red, starch and Metanil Yellow fraud were applied to spectra. The overall precision of the SIMCA and PLS-DA classifiers were 82% and 92%, respectively | [97] |
| White pepper | Corn flour | 1. Portable NIR spectrometer 2. Hyperspectral imaging | 1. Recognition models by LDA, SVM, PLS-DA and SIMCA. The SIMCA model performed best in quality grading. For optimized PLS model on piperine concentration, prediction of unknown samples generated an R2p of 0.970, RMSEP of 0.111, and RPD value of 5.72. 2. The MCR-ALS was used to reduce the dimensionality of multivariate data. Minimum adulteration content of 1%. | [98] |
| Ginger powder | Chickpea powder | Hyperspectral imaging | Recognition models by convolutional neural networks (CNN). CNN was able to grade the images of ginger powder with 99.70% accuracy, compared to other classifiers. | [99] |
| Nutmeg | Seven adulterant materials: pericarp, two creamy spent, three brown spent, and one shell | Hyperspectral imaging | Data were pre-processed using standard normal variate (SNV) treatment. An artificial neural network (ANN) model showed the ability to detect adulteration at levels as low as 5%. | [100] |
| Cinnamon | Cinnamon cassia (10, 50, and 100%) | NIR spectroscopy | Average discrimination percentages of 99.25 and 100.00% for recognition models PLS-DA and probabilistic neural network (PNN), respectively. | [101] |
| Cinnamon | Cinnamon cassia | Hyperspectral imaging | PLS-DA and support vector machine (SVM) reached a similar performance to classify samples according to origin, with error = 3.3% and accuracy = 96.7%. | [102] |
| Turmeric | Starch | FT-NIR spectroscopy | Wavelength regions selected: 1400–1550 nm and 1900–2050 nm by variable importance in projection (VIP) method. PLSR model (R2 > 0.91). | [103] |
| Ginger | Corn starch, soybean flour, and wheat flour. | FT-NIR spectroscopy | Random forest (RF) and gradient boosting (GB) algorithms exhibited the highest accuracies (100%) in classification. PLSR models were built to further determine whether the adulterated levels of ginger adulteration with RPD values are greater than three for the three adulterants. | [104] |
| Saffron | Plant-derived adulterants | FT-NIR spectroscopy | PLS-DA on region 4000–600 cm−1 (99% correct classification of pure saffron and saffron adulterated at 5–20%). Synergy interval PLS (siPLS) with detection limits ranging from 1.0% to 3.1%. | [27] |
| Turmeric | Rice flour with tartrazine | Hyperspectral imaging | Functional relationship between the Bhattacharyya distance and the adulteration levels. Multivariate Gaussian. Model (R2 = 0.9816 and SSE = 1.1423). | [105] |
| Turmeric | Metanil Yellow, Sudan I | Raman spectroscopy | Self-modelling mixture analysis (SMA) was used to decompose the mixed spectral information. Linear correlation (R2 = 0.99). | [106] |
| Chili powder | Use of rhodamine B as a synthetic colourant | Indirect competitive ELISA | Immunoassay strategy was designed based on the heterologous strategy. Detection limit of indirect competitive ELISA was 0.002 μg/kg, showing a good sensitivity. | [107] |
| Chili | Sudan I, as a colorant | ELISA | Development of rapid ELISA method based on highly specific polyclonal antibodies. This ELISA method allows rapid, sensitive, and high-throughput screening of different food products for the presence of the illegal colorant. | [108] |
| Chili, curry, and mixes of soup and condiment | Detection of gluten-free product | ELISA kits | These authors detected levels exceeding the gluten threshold (20 ppm) in some of the condiment samples tested. | [50] |
| Cumin spices | Detection of traces of peanut protein | ELISA kits | A lack of sensitivity of the ELISA kit for traces of peanut protein in cumin spices because of false negative results. | [48] |
| Turmeric | Adulteration with other curcumin pigment | Light and scanning-electron microscopy | The turmeric powder can be identified by the presence of gelatinized starch granules, numerous oil cells, and parenchymatous cells in a microscopic view. The presence of calcium oxalate crystals in turmeric indicates adulteration with wild species. | [22] |
| Cumin, chilli, pepper and mustard powders | Adulterating substances (starch, plant straws, and monosodium glutamate) | Microscopic technique | Showed the efficiency of using microscopic technique to distinguish the micro-morphology characteristics of pure seasoning powders. | [109] |
| Black pepper powder | Adulteration with papaya seed powder | Microscopic technique | Meticulous microscopic examination of fatty oils, oil globules, starch granule, fibres and different features of parenchyma cells identified papaya seed powder in black pepper powder. | [110] |
| Fennel | Combined microscopy and GC-MS for the detection of adulteration of fennel seeds | Combined microscopy and GC-MS | Combined light microscopy coupled with fluorescence microscopy and GC-MS analysis allowed successful distinguishing of fennel seeds from two adulterants: dill (Anethum graveolens) and cumin (Cuminum cyminum). | [111] |
| Saffron | Adulterated with different percentages of dyes | Electronic nose or E-nose and a chemometric tool | The results of the analysis revealed that E-nose and a chemometric tool were able to differentiate authentic saffron samples from adulterated ones effectively, based on their aroma intensity. | [112] |
| Cumin | Adulterated with Moroccan coriander in different concentrations (5%, 20%, 50% and 70%) | E-nose and VE-tongue in combination with SPME-GC-MS | Compared the ability of E-nose and VE-tongue in combination with SPME-GC-MS to discriminate cumin samples from adulterated ones and those with different geographical origins; this was demonstrated. The results indicated that the VE-tongue has more potential (100% accuracy) for detection and discrimination than the other two methods. | [113] |
| Saffron | Safflower and corn-stigma adulteration in saffron | Electronic nose | The results revealed that the system can successfully recognise saffron adulteration with 100 % accuracy, and that it was able to successfully differentiate unadulterated saffron from adulterated saffron, with an adulteration level of more than 10%. | [114] |
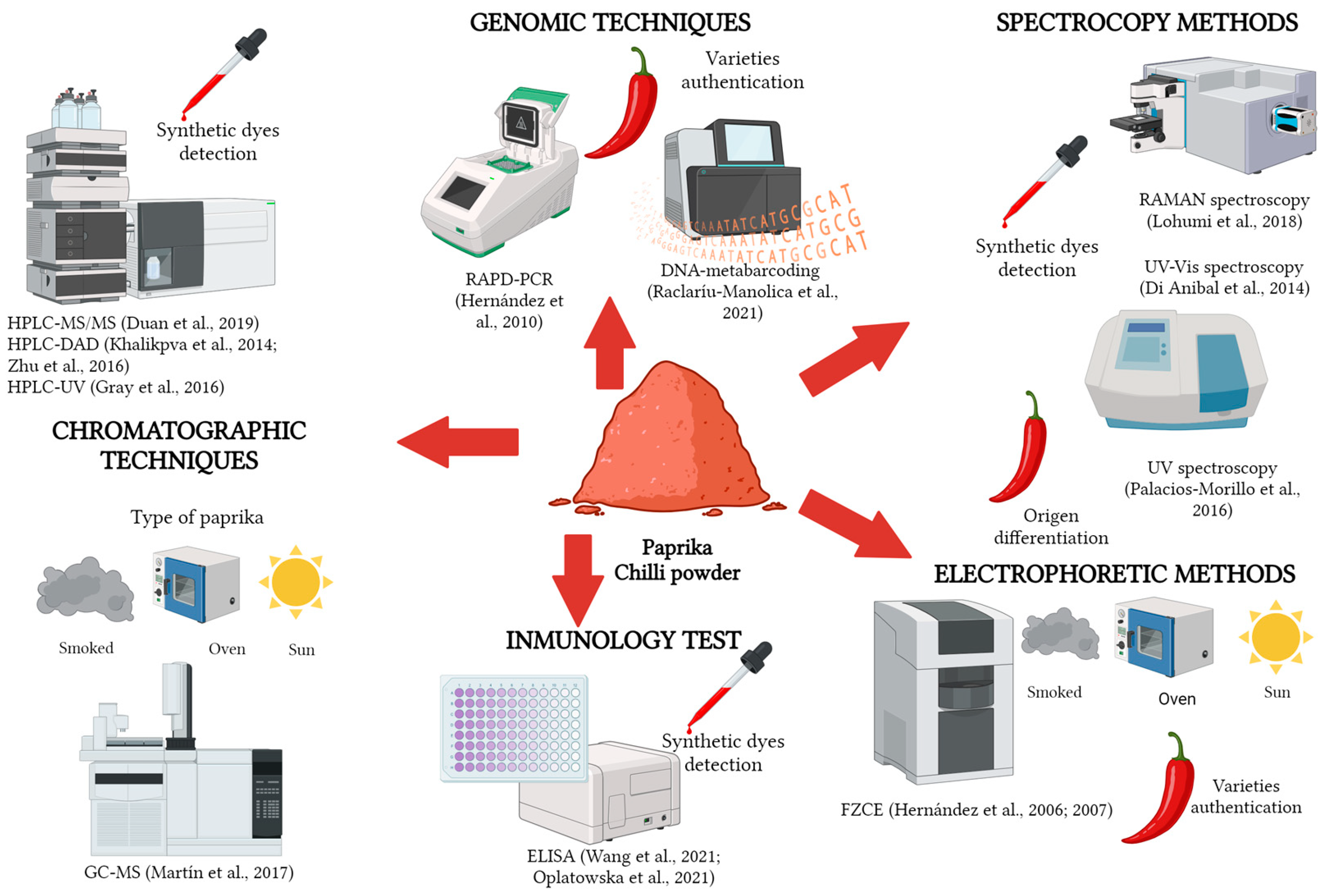
5.1. Chromatography Techniques
5.2. Electrophoretic Methods
5.3. Genomic Techniques
5.4. Spectroscopy and Image Analysis Methods
5.5. Other Techniques
5.5.1. Immunology Tests
5.5.2. Microscopy and Sensory Analysis Techniques
6. Adulteration Prevention Measurement for Spices and Herbs
7. Future Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ESA (European Association Spices). The ESA List of Culinary Herbs and Spices. 2018. Available online: https://www.esa-spices.org/download/esa-list-of-culinary-herbs-and-spices.pdf (accessed on 30 July 2023).
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive study on the antioxidant capacity and phenolic profiles of black seed and other spices and herbs: Effect of solvent and time of extraction. J. Food Meas. Charact. 2021, 15, 4561–4574. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Azeez, T.B.; Lunghar, J. Antiinflammatory effects of turmeric (Curcuma longa) and ginger (Zingiber officinale). In Inflammation and Natural Products; Gopi, S., Amalraj, A., Kunnumakkara, A., Thomas, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 127–146. [Google Scholar] [CrossRef]
- Willis, S.; Sunkara, R.; Hester, F.; Shackelford, L.; Walker, L.T.; Verghese, M. Chemopreventive and anti-inflammatory potential of select herbal teas and cinnamon in an in-vitro cell model. Food Nutr. Sci. 2019, 10, 1142–1156. [Google Scholar] [CrossRef][Green Version]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Talib, W.H.; AlHur, M.J.; Al Naimat, S.; Ahmad, R.E.; Al-Yasari, A.H.; Al-Dalaeen, A.; Thiab, S.; Mahmod, A.I. Anticancer effect of spices used in Mediterranean diet: Preventive and therapeutic potentials. Front. Nutr. 2022, 9, 905658. [Google Scholar] [CrossRef]
- Kaefer, C.M.; Milner, J.A. The role of herbs and spices in cancer prevention. J. Nutr. Biochem. 2008, 19, 347–361. [Google Scholar] [CrossRef]
- Choudhury, A.; Singh, P.A.; Bajwa, N.; Dash, S.; Bisht, P. Pharmacovigilance of herbal medicines: Concerns and future prospects. J. Ethnopharmacol. 2023, 3039, 116383. [Google Scholar] [CrossRef]
- Kalachaveedu, M.; Senthil, R.; Azhagiyamanavalan, S.; Ravi, R.; Meenakshisundaram, H.; Dharmarajan, A. Traditional medicine herbs as natural product matrices in cancer chemoprevention: A trans pharmacological perspective (scoping review). Phytother. Res. 2023, 37, 1539–1573. [Google Scholar] [CrossRef]
- Nanda, J.; Verma, N.; Mani, M. A Mechanistic Review on Phytomedicine and Natural Products in the Treatment of Diabetes. Curr. Diabetes Rev. 2023, 19, 44–54. [Google Scholar] [CrossRef]
- Rajawat, J.; Banerjee, M. A Review on Therapeutic Potential of Indian Herbal Plants to Counter Viral Infection and Disease Pathogenesis. Curr. Tradit. Med. 2023, 9, 136–144. [Google Scholar] [CrossRef]
- Usmani, K.; Jain, S.K.; Yadav, S. Mechanism of action of certain medicinal plants for the treatment of asthma. J. Ethnopharmacol. 2023, 317, 116828. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M.; Hickey, D.K.; Alonso-Gomez, M.; Wilkinson, M. Evaluation of antimicrobial activities of commercial herb and spice extracts against selected food-borne bacteria. J. Food Res. 2013, 2, 37. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT Statistical Database. Production. Crops and Livestock Products. Crops Primary. Download Data. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 April 2023).
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT Statistical Database. Trade. Crops and Livestock Products. Crops and Livestock Products. Download Data. 2021. Available online: https://www.fao.org/faostat/en/#data/TCL (accessed on 30 April 2023).
- Regulation (EU) No 1151/2012 of the European Parliament and of the Council of 21 November 2012 on Quality Schemes for Agricultural Products and Foodstuffs. 2012. Available online: https://eur-lex.europa.eu/eli/reg/2012/1151/oj (accessed on 30 July 2023).
- eAmbrosia, the EU Geografical Indication Register. Agricultural Products and Foodstuffs. 2023. Available online: https://ec.europa.eu/info/food-farming-fisheries/food-safety-and-quality/certification/quality-labels/geographical-indications-register/ (accessed on 2 May 2023).
- Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products. 2017. Available online: http://data.europa.eu/eli/reg/2017/625/oj (accessed on 30 July 2023).
- Winkler, B.; Maquet, A.; Reeves-Way, E.; Siegener, E.; Cassidy, T.; Valinhas De Oliveira, T.; Verluyten, J.; Jelic, M.; Muznik, A. Fighting Fraudulent and Deceptive Practices in the Agri-Food Chain; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Osman, A.G.; Raman, V.; Haider, S.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Overview of analytical tools for the identification of adulterants in commonly traded herbs and spices. J. AOAC Int. 2019, 102, 376–385. [Google Scholar] [CrossRef] [PubMed]
- ESA (European Association Spices). Adulteration Awareness Document. 2018. Available online: https://www.esa-spices.org/index-esa.html/publications-esa?amp (accessed on 30 July 2023).
- Muggeridge, M.; Clay, M. Quality Specifications for Herbs and Spices. Handbook of Herbs and Spices. 2001, 1. Available online: https://books.google.es/books?hl=es&lr=&id=cKjAgAAQBAJ&oi=fnd&pg=PA13&dq=Muggeridge+and+Clay,+2001&ots=BCebA6LK04&sig=-PMSY1T9RUdq5c-XlqjiZnIoZbg#v=onepage&q=Muggeridge%20and%20Clay%2C%202001&f=false (accessed on 30 July 2023).
- Galvin-King, P.; Haughey, S.A.; Elliott, C.T. Herb and spice fraud; the drivers, challenges and detection. Food Control 2018, 88, 85–97. [Google Scholar] [CrossRef]
- Reinholds, I.; Bartkevics, V.; Silvis, I.C.; van Ruth, S.M.; Esslinger, S. Analytical techniques combined with chemometrics for authentication and determination of contaminants in condiments: A review. J. Food Compos. Anal. 2015, 44, 56–72. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Polissiou, M.G. Assessing saffron (Crocus sativus L.) adulteration with plant-derived adulterants by diffuse reflectance infrared Fourier transform spectroscopy coupled with chemometrics. Talanta 2017, 162, 558–566. [Google Scholar] [CrossRef]
- Soffritti, G.; Busconi, M.; Sánchez, R.A.; Thiercelin, J.M.; Polissiou, M.; Roldán, M.; Fernández, J.A. Genetic and epigenetic approaches for the possible detection of adulteration and auto-adulteration in saffron (Crocus sativus L.) spice. Molecules 2016, 21, 343. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Lafeuille, J.L.; Ragupathy, S.; Newmaster, S. A Global Threat with Public Health and Economic Chapter 10. Spice and herb fraud. In Food Fraud; Academic Press: Cambridge, MA, USA, 2021; pp. 177–218. [Google Scholar] [CrossRef]
- Lakshmi, V.; Pradesh, A. Food adulteration. Int. J. Sci. Invent. Today 2012, 1, 106–113. Available online: http://www.ijsit.com/admin/ijsit_files/FOOD%20ADULTERATION_1.2.4.pdf (accessed on 30 July 2023).
- Ballin, N.Z.; Sørensen, A.T. Coumarin content in cinnamon containing food products on the Danish market. Food Control 2014, 38, 198–203. [Google Scholar] [CrossRef]
- Lee, S.; Lohumi, S.; Lim, H.S.; Gotoh, T.; Goto, T.; Cho, B.K.; Kim, M.S.; Lee, S.H. Development of a detection method for adulterated onion powder using Raman spectroscopy. J. Fac. Agric. Kyushu Univ. 2015, 60, 151–156. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, W.H.; Kim, M.S.; Mo, C.; Bae, H.; Cho, B.K. Detection of starch adulteration in onion powder by FT-NIR and FT-IR spectroscopy. J. Agric. Food Chem. 2014, 62, 9246–9251. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Lohumi, S.; Cho, B.K.; Kim, M.S.; Lee, S.H. Development of nondestructive detection method for adulterated powder products using Raman spectroscopy and partial least squares regression. J. Korean Soc. Nondestruct. Test. 2014, 34, 283–289. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Benes, E.; Bázár, G.; Kovács, Z.; Fodor, M. Agricultural potentials of molecular spectroscopy and avance for food authentication: An overview. Processes 2022, 10, 214. [Google Scholar] [CrossRef]
- Bononi, M.; Tateo, F. LC-ESI-MS/MS identification of oleuropein as marker of Olea europaea L., leaves used as a bulking agent in ground oregano and sage. Ital. J. Food Sci. 2011, 23, 245–251. Available online: https://air.unimi.it/handle/2434/164528 (accessed on 30 July 2023).
- Black, C.; Haughey, S.A.; Chevallier, O.P.; Galvin-King, P.; Elliott, C.T. A comprehensive strategy to detect the fraudulent adulteration of herbs: The oregano approach. Food Chem. 2016, 210, 551–557. [Google Scholar] [CrossRef]
- Gopu, C.L.; Aher, S.; Mehta, H.; Paradkar, A.R.; Mahadik, K.R. Simultaneous determination of cinnamaldehyde, eugenol and piperine by HPTLC densitometric method. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2008, 19, 116–121. [Google Scholar] [CrossRef]
- De Mey, E.; De Maere, H.; Dewulf, L.; Paelinck, H.; Sajewicz, M.; Fraeye, I.; Kowalska, T. Application of accelerated solvent extraction (ASE) and thin layer chromatography (TLC) to determination of piperine in commercial samples of pepper (Piper nigrum L.). J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2980–2988. [Google Scholar] [CrossRef]
- De Guzman, C.C.; Zara, R.R. Vanilla. In Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2012; Volume 1, pp. 547–589. [Google Scholar] [CrossRef]
- Lead Action News. Adulteration of Paprika in Hungary. 1995. Available online: http://www.lead.org.au/lanv3n3/lanv3n3-6.html (accessed on 30 July 2023).
- The Express Tribune. Crackdown: 3000 kg Adulterated Red Chilli Powder Seized. 2016. Available online: https://tribune.com.pk/story/1088806/crackdown-3000-kg-adulterated-red-chili-powderseized (accessed on 29 May 2023).
- Nallappan, K.; Dash, J.; Ray, S.; Pesala, B. Identification of adulterants in turmeric powder using terahertz spectroscopy. In Proceedings of the 2013 38th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Mainz, Germany, 1–6 September 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1–2. [Google Scholar] [CrossRef]
- Everstine, K.; Spink, J.; Kennedy, S. Economically motivated adulteration (EMA) of food: Common characteristics of EMA incidents. J. Food Prot. 2013, 76, 723–735. [Google Scholar] [CrossRef]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef]
- Tarantelli, T. Adulteration with Sudan dye has triggered several spice recalls. Food Safety Tech. 2017, 1–2. Available online: https://foodsafetytech.com/feature_article/adulteration-sudan-981 (accessed on 30 July 2023).
- Hagh-Nazari, S.; Keifi, N. Saffron and various fraud manners in its production and trades. Acta Hortic. 2006, 739, 411–416. [Google Scholar] [CrossRef]
- Garber, E.A.; Parker, C.H.; Handy, S.M.; Cho, C.Y.; Panda, R.; Samadpour, M.; Reynaud, D.H.; Ziobro, G.C. Presence of undeclared food allergens in cumin: The need for multiplex methods. J. Agric. Food Chem. 2016, 64, 1202–1211. [Google Scholar] [CrossRef]
- Surojanametakul, V.; Khaiprapai, P.; Jithan, P.; Varanyanond, W.; Shoji, M.; Ito, T.; Tamura, H. Investigation of undeclared food allergens in commercial Thai food products. Food Control 2012, 23, 107554. [Google Scholar] [CrossRef]
- Sharma, G.M.; Pereira, M.; Williams, K.M. Gluten detection in foods available in the United States–a market survey. Food Chem. 2015, 169, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, K.; Syamkumar, S.; Siju, S.; Sasikumar, B. SCAR markers for adulterant detection in ground chilli. Br. Food J. 2011, 113, 656–668. [Google Scholar] [CrossRef]
- Agres, T. The Cumin Scandal: Accidental or Fraudulent. Food Qual. Saf. 2015. Available online: https://www.foodqualityandsafety.com/article/the-cumin-scandal-accidental-or-fraudulent/ (accessed on 3 May 2023).
- Śmiechowska, M.; Newerli-Guz, J.; Skotnicka, M. Spices and Seasoning Mixes in European Union—Innovations and Ensuring Safety. Foods 2021, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Galaxy Scientific. Fast Detection of Paprika Adulteration Using FT-NIR Spectroscopy. 2016. Available online: https://www.azom.com/article.aspx?ArticleID=13251# (accessed on 30 July 2023).
- ASTA. Spice Adulteration—White Paper. 2011. Available online: http://docshare04.docshare.tips/files/6810/68105104.pdf (accessed on 30 July 2023).
- Galvin-King, P.; Haughey, S.A.; Elliott, C.T. The detection of substitution adulteration of paprika with spent paprika by the application of molecular spectroscopy tools. Foods 2020, 9, 944. [Google Scholar] [CrossRef]
- Scarano, D.; Rao, R. DNA markers for food products authentication. Diversity 2014, 6, 579–596. [Google Scholar] [CrossRef]
- RASFF (Rapid Alert System Feed and Food)-Window. European Commission. 2023. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 2 May 2023).
- Maquet, A.; Lievens, A.; Paracchini, V.; Kaklamanos, G.; De La Calle Guntinas, M.B.; Garlant, L.; Papoci, S.; Pietretti, D.; Ždiniaková, T.; Breidbach, A.; et al. Results of an EU Wide Coordinated Control Plan to Establish the Prevalence of Fraudulent Practices in the Marketing of Herbs and Spices; Joint Research Centre (JRC) Technical Report; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- Web of Science Core Collection. 2023. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 15 May 2023).
- Duan, H.L.; Mou, Z.L.; Wang, J.; Ma, S.Y.; Zhan, H.Y.; Zhang, Z.Q. Magnetically modified porous β-cyclodextrin polymers for dispersive solid-phase extraction high-performance liquid chromatography analysis of Sudan dyes. Food Anal. Methods 2019, 12, 1429–1438. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Y.; Zhou, C.; Zhao, B.; Yun, W.; Huang, S.; Tao, P.; Tu, D.; Chen, S. A screening method of oil-soluble synthetic dyes in chilli products based on multi-wavelength chromatographic fingerprints comparison. Food Chem. 2016, 192, 441–451. [Google Scholar] [CrossRef]
- Khalikova, M.A.; Šatínský, D.; Šmidrkalová, T.; Solich, P. On-line SPE–UHPLC method using fused core columns for extraction and separation of nine illegal dyes in chilli-containing spices. Talanta 2014, 130, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Di Anibal, C.V.; Odena, M.; Ruisánchez, I.; Callao, M.P. Determining the adulteration of spices with Sudan I-II-II-IV dyes by UV–visible spectroscopy and multivariate classification techniques. Talanta 2009, 79, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.M.; Walker, M.J.; Burn, M.J.S.; Mazur, M.; Niedzwiedzka, K.; Liszka, K.; Burns, D.T. Illegal dyes in food and spices—A 2006 LGC LC-UV/visible method reviewed and updated for 19 dyes. J. Assoc. Public Anal. 2016, 44, 18–39. [Google Scholar]
- D’Archivio, A.A.; Giannitto, A.; Maggi, M.A.; Ruggieri, F. Geographical classification of Italian saffron (Crocus sativus L.) based on chemical constituents determined by high-performance liquid-chromatography and by using linear discriminant analysis. Food Chem. 2016, 212, 110–116. [Google Scholar] [CrossRef]
- Liu, J.; Chen, N.; Yang, J.; Yang, B.; Ouyang, Z.; Wu, C.; Yuan, Y.; Wang, W.; Chen, M. An integrated approach combining HPLC, GC/MS, NIRS, and chemometrics for the geographical discrimination and commercial categorization of saffron. Food Chem. 2018, 253, 284–292. [Google Scholar] [CrossRef]
- Rubert, J.; Lacina, O.; Zachariasova, M.; Hajslova, J. Saffron authentication based on liquid chromatography high resolution tandem mass spectrometry and multivariate data analysis. Food Chem. 2016, 204, 201–209. [Google Scholar] [CrossRef]
- Campmajó, G.; Rodríguez-Javier, L.R.; Saurina, J.; Núñez, O. Assessment of paprika geographical origin fraud by high-performance liquid chromatography with fluorescence detection (HPLC-FLD) fingerprinting. Food Chem. 2021, 352, 129397. [Google Scholar] [CrossRef]
- Bononi, M.; Fiordaliso, I.; Tateo, F. Rapid GC/MS test for identification of Olea europaea L. leaves in ground oregano. Ital. J. Food Sci. 2010, 22, 479–483. [Google Scholar]
- Raman, V.; Bussmann, R.W.; Khan, I.A. Which bay leaf is in your spice rack?–A quality control study. Planta Med. 2017, 83, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Morozzi, P.; Zappi, A.; Gottardi, F.; Locatelli, M.; Melucci, D. A quick and efficient non-targeted screening test for saffron authentication: Application of chemometrics to gas-chromatographic data. Molecules 2019, 24, 2602. [Google Scholar] [CrossRef] [PubMed]
- Acosta, G.; Arce, S.; Martínez, L.D.; Llabot, J.; Gomez, M.R. Monitoring of phenolic compounds for the quality control of Melissa officinalis products by capillary electrophoresis. Phytochem. Anal. 2012, 23, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Martín, A.; Aranda, E.; Bartolomé, T.; Córdoba, M.G. Detection of smoked paprika “Pimentón de La Vera” adulteration by free zone capillary electrophoresis (FZCE). J. Agric. Food Chem. 2006, 54, 4141–4147. [Google Scholar] [CrossRef]
- Hernández, A.; Martín, A.; Aranda, E.; Bartolomé, T.; Córdoba, M.G. Application of temperature-induced phase partition of proteins for the detection of smoked paprika adulteration by free zone capillary electrophoresis (FZCE). Food Chem. 2007, 105, 1219–1227. [Google Scholar] [CrossRef]
- Sasikumar, B.; Syamkumar, S.; Remya, R.; John Zachariah, T. PCR based detection of adulteration in the market samples of turmeric powder. Food Biotechnol. 2007, 18, 299–306. [Google Scholar] [CrossRef]
- Dhanya, K.; Syamkumar, S.; Jaleel, K.; Sasikumar, B. Random amplified polymorphic DNA technique for detection of plant based adulterants in chilli powder (Capsicum annuum). J. Spices Aromat. Crops. 2008, 17, 75–81. Available online: https://core.ac.uk/download/pdf/236023706.pdf (accessed on 30 July 2023).
- Khan, S.; Mirza, K.J.; Anwar, F.; Abdin, M.Z. Development of RAPD markers for authentication of Piper nigrum (L.). Environ. We Int. J. Sci. Tech. 2010, 5, 47–56. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=cf547f60308d0e35e3ec91d6af0d0d0a576946b7 (accessed on 30 July 2023).
- Hernández, A.; Aranda, E.; Martín, A.; Benito, M.J.; Bartolomé, T.; Córdoba, M.G. Efficiency of DNA typing methods for detection of smoked paprika “Pimentón de La Vera” adulteration used in the elaboration of dry-cured Iberian pork sausages. J. Agric. Food Chem. 2010, 58, 11688–11694. [Google Scholar] [CrossRef]
- Marieschi, M.; Torelli, A.; Poli, F.; Sacchetti, G.; Bruni, R. RAPD-based method for the quality control of Mediterranean oregano and its contribution to pharmacognostic techniques. J. Agric. Food Chem. 2009, 57, 1835–1840. [Google Scholar] [CrossRef]
- Marieschi, M.; Torelli, A.; Poli, F.; Bianchi, A.; Bruni, R. Quality control of commercial Mediterranean oregano: Development of SCAR markers for the detection of the adulterants Cistus incanus L., Rubus caesius L. and Rhus coriaria L. Food Control 2010, 21, 998–1003. [Google Scholar] [CrossRef]
- Marieschi, M.; Torelli, A.; Bianchi, A.; Bruni, R. Development of a SCAR marker for the identification of Olea europaea L.: A newly detected adulterant in commercial Mediterranean oregano. Food Chem. 2011, 126, 705–709. [Google Scholar] [CrossRef]
- Dhanya, K.; Syamkumar, S.; Siju, S.; Sasikumar, B. Sequence characterized amplified region markers: A reliable tool for adulterant detection in turmeric powder. Food Res. Int. 2011, 44, 2889–2895. [Google Scholar] [CrossRef]
- Bansal, S.; Thakur, S.; Mangal, M.; Mangal, A.K.; Gupta, R.K. Identification of suitable locus for specific detection of biological adulterants of saffron. Food Anal. Methods 2019, 12, 2509–2517. [Google Scholar] [CrossRef]
- Babaei, S.; Talebi, M.; Bahar, M. Developing a SCAR and ITS reliable multiplex PCR-based assay for safflower adulterant detection in saffron samples. Food Control 2014, 35, 323–328. [Google Scholar] [CrossRef]
- Mougiou, N.; Trikka, F.; Michailidou, S.; Pantoura, M.; Argiriou, A. Molecular and biochemical characterization of the Greek pepper (Capsicum annuum) cultivars “Florinis” and “Karatzova”. Pol. J. Food Nutr. Sci. 2021, 71, 109477. [Google Scholar] [CrossRef]
- Novak, J.; Lukas, B.; Bolzer, K.; Grausgruber-Gröger, S.; Degenhardt, J. Identification and characterization of simple sequence repeat markers from a glandular Origanum vulgare expressed sequence tag. Mol. Ecol. Resour. 2008, 8, 599–601. [Google Scholar] [CrossRef]
- Javanmardi, N.; Bagheri, A.; Moshtaghi, N.; Sharifi, A.; Hemati Kakhki, A. Identification of Safflower as a fraud in commercial Saffron using RAPD/SCAR. J. Cell Mol. Res. 2011, 3, 31–37. Available online: https://jcmr.um.ac.ir/article_26196_d4ff2b0bcf8b3b6573f992f3b0206ad1.pdf (accessed on 30 July 2023).
- Parvathy, V.A.; Swetha, V.P.; Sheeja, T.E.; Sasikumar, B. Detection of plant-based adulterants in turmeric powder using DNA barcoding. Pharm. Biol. 2015, 53, 1774–1779. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Y.; Sun, W.; Wu, L.; Xiong, C.; Zhu, Z.; Zhao, H.; Zhang, B.; Wang, C.; Liu, X. An efficient DNA barcoding based method for the authentication and adulteration detection of the powdered natural spices. Food Control 2019, 106, 106745. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Xing, R.R.; Liu, K.H.; Ge, Y.Q.; Chen, Y. Species identification of culinary spices with two-locus DNA barcoding. Food Control 2023, 150, 109742. [Google Scholar] [CrossRef]
- Raclariu-Manolică, A.C.; Anmarkrud, J.A.; Kierczak, M.; Rafati, N.; Thorbek, B.L.G.; Schrøder-Nielsen, A.; De Boer, H.J. DNA metabarcoding for quality control of basil, oregano, and paprika. Front. Plant Sci. 2021, 12, 665618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Cao, L.; Yuan, Y.; Chen, M.; Jin, Y.; Huang, L. Barcoding melting curve analysis for rapid, sensitive, and discriminating authentication of saffron (Crocus sativus L.) from its adulterants. Biomed Res. Int. 2014, 2014, 10. [Google Scholar] [CrossRef]
- De Mattia, F.; Bruni, I.; Galimberti, A.; Cattaneo, F.; Casiraghi, M.; Labra, M. A comparative study of different DNA barcoding markers for the identification of some members of Lamiacaea. Food Res. Int. 2011, 44, 693–702. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Meira, L.; Oliveira, M.B.P.; Mafra, I. Exploiting DNA mini-barcodes as molecular markers to authenticate saffron (Crocus sativus L.). Food Control 2016, 65, 21–31. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Bayati, M.R.; Emadi, B. An evaluation of IR spectroscopy for authentication of adulterated turmeric powder using pattern recognition. Food Chem. 2021, 364, 130406. [Google Scholar] [CrossRef]
- Chen, R.; Mei, J.; Du, G.; Shi, Y.; Huang, Y. Convenient detection of white pepper adulteration by portable NIRS and spectral imaging with chemometrics. Microchem. J. 2020, 182, 107925. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Abbaspour-Gilandeh, Y.; Heidarbeigi, K.; Momeny, M. Detection of fraud in ginger powder using an automatic sorting system based on image processing technique and deep learning. Comput. Biol. Med. 2021, 136, 104764. [Google Scholar] [CrossRef]
- Guide to Protecting and Defending Food and Drink from Deliberate Attack. 2017. Available online: https://www.food.gov.uk/sites/default/files/pas962017.pdf (accessed on 26 February 2019).
- Kiani, S.; van Ruth, S.M.; van Raamsdonk, L.W.; Minaei, S. Hyperspectral imaging as a novel system for the authentication of spices: A nutmeg case study. LWT Food Sci. Technol. 2019, 104, 61–69. [Google Scholar] [CrossRef]
- Cantarelli, M.A.; Moldes, C.A.; Marchevsky, E.J.; Azcarate, S.M.; Camiña, J.M. Low-cost analytic method for the identification of Cinnamon adulteration. Microchem. J. 2020, 159, 105513. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Brasil, Y.L.; Lima, A.F.; Pretel, H.A.; Godoy, H.T.; Barbin, D.; Siche, R. Rapid and non-destructive cinnamon authentication by NIR-hyperspectral imaging and classification chemometrics tools. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 289, 122226. [Google Scholar] [CrossRef]
- Kar, S.; Tudu, B.; Jana, A.; Bandyopadhyay, R. FT-NIR spectroscopy coupled with multivariate analysis for detection of starch adulteration in turmeric powder. Food Addit. Contam Part A 2021, 36, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.X.; Guo, S.; Zhang, X.; Yan, H.; Zhang, Z.Y.; Chen, X. Rapid detection of adulteration in powder of ginger (Zingiber officinale Roscoe) by FT-NIR spectroscopy combined with chemometrics. Food Chem. 2022, 15, 100450. [Google Scholar] [CrossRef] [PubMed]
- Bandara, W.G.C.; Prabhath, G.W.K.; Dissanayake, D.W.S.C.B.; Herath, V.R.; Godaliyadda, G.M.R.I.; Ekanayake, M.P.B. Validation of multispectral imaging for the detection of selected adulterants in turmeric samples. J. Food Eng. 2020, 266, 109700. [Google Scholar] [CrossRef]
- Dhakal, S.; Schmidt, W.F.; Kim, M.; Tang, X.; Peng, Y.; Chao, K. Detection of additives and chemical contaminants in turmeric powder using FT-IR spectroscopy. Foods 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, X.; Zhong, P.; Li, Z.; Tang, Q.; Huang, X.; Zherdev, A.V.; Dzantiev, B.B.; Eremin, S.A.; Xiao, Z.; et al. Heterologous immunoassay strategy for enhancing detection sensitivity of banned dye rhodamine B in fraudulent food. Chem. Biol. Technol. Agric. 2021, 8, 17. [Google Scholar] [CrossRef]
- Oplatowska, M.; Stevenson, P.J.; Schulz, C.; Hartig, L.; Elliott, C.T. Development of a simple gel permeation clean-up procedure coupled to a rapid disequilibrium enzyme-linked immunosorbent assay (ELISA) for the detection of Sudan I dye in spices and sauces. Anal. Bioanal. Chem. 2011, 401, 1411–1422. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, M. Study on the microscopic identification of the adulterated plant origin powdered seasonings. Discourse J. Agric. Food Sci. 2014, 2, 264–269. [Google Scholar] [CrossRef]
- Vadivel, V.; Ravichandran, N.; Rajalakshmi, P.; Brindha, P.; Gopal, A.; Kumaravelu, C. Microscopic, phytochemical, HPTLC, GC–MS and NIRS methods to differentiate herbal adulterants: Pepper and papaya seeds. J. Herbal Med. 2018, 11, 36–45. [Google Scholar] [CrossRef]
- Ma, X.; Mao, W.; Zhou, P.; Li, P.; Li, H. Distinguishing Foeniculum vulgare fruit from two adulterants by combination of microscopy and GC-MS analysis. Microsc. Res. Tech. 2015, 78, 633–641. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Integration of computer vision and electronic nose as non-destructive systems for saffron adulteration detection. Comput. Electron. Agric. 2017, 141, 46–53. [Google Scholar] [CrossRef]
- Tahri, K.; Tiebe, C.; El Bari, N.; Hübert, T.; Bouchikhi, B. Geographical classification and adulteration detection of cumin by using electronic sensing coupled to multivariate analysis. Procedia Technol. 2017, 27, 240–241. [Google Scholar] [CrossRef]
- Heidarbeigi, K.; Mohtasebi, S.S.; Foroughirad, A.; Ghasemi-Varnamkhasti, M.; Rafiee, S.; Rezaei, K. Detection of adulteration in saffron samples using electronic nose. Int. J. Food Prop. 2015, 18, 1391–1401. [Google Scholar] [CrossRef]
- Martín, A.; Hernández, A.; Aranda, E.; Casquete, R.; Velázquez, R.; Bartolomé, T.; Córdoba, M.G. Impact of volatile composition on the sensorial attributes of dried paprikas. Food Res. Int. 2017, 100, 691–697. [Google Scholar] [CrossRef]
- Di Anibal, C.; Rodriguez, M.S.; Albertengo, L. UV-visible spectroscopy and multivariate classification as a screening tool to identify adulteration of culinary spices with Sudan I and blends of Sudan I+IV dyes. Food Anal. Methods 2014, 7, 1090–1096. [Google Scholar] [CrossRef]
- Palacios-Morillo, A.; Jurado, J.M.; Alcázar, A.; Pablos, F. Differentiation of Spanish paprika from Protected Designation of Origin based on color measurements and pattern recognition. Food Control 2016, 62, 243–249. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, H.; Kim, M.S.; Qin, J.; Kandpal, L.M.; Bae, H.; Rahman, A.; Cho, B.K. Calibration and testing of a Raman hyperspectral imaging system to reveal powdered food adulteration. PLoS ONE 2018, 13, e0195253. [Google Scholar] [CrossRef]
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896. [Google Scholar] [CrossRef]
- Cserháti, T.; Forgács, E.; Morais, M.H.; Mota, T.; Ramos, A. Separation and quantitation of colour pigments of chili powder (Capsicum frutescens) by high-performance liquid chromatography–diode array detection. J. Chromatogr. A 2000, 896, 69–73. [Google Scholar] [CrossRef]
- Ellis, D.I.; Muhamadali, H.; Haughey, S.A.; Elliott, C.T.; Goodacre, R. Point-and-shoot: Rapid quantitative detection methods for on-site food fraud analysis—Moving out of the laboratory and into the food supply chain. Anal. Methods 2015, 7, 9401–9414. [Google Scholar] [CrossRef]
- Wadood, S.A.; Boli, G.; Xiaowen, Z.; Hussain, I.; Yimin, W. Recent development in the application of analytical techniques for the traceability and authenticity of food of plant origin. Microchem. J. 2020, 152, 104295. [Google Scholar] [CrossRef]
- Kamal, M.; Karoui, R. Analytical methods coupled with chemometric tools for determining the authenticity and detecting the adulteration of dairy products: A review. Trends Food Sci. Technol. 2015, 46, 27–48. [Google Scholar] [CrossRef]
- Przybylska, A.; Gackowski, M.; Koba, M. Application of capillary electrophoresis to the analysis of bioactive compounds in herbal raw materials. Molecules 2021, 26, 2135. [Google Scholar] [CrossRef] [PubMed]
- Maher, H.M.; Al-Zoman, N.Z.; Al-Shehri, M.M.; Al-Showiman, H.; Al-Taweel, A.M.; Fawzy, G.A.; Perveen, S. Determination of luteolin and apigenin in herbs by capillary electrophoresis with diode array detection. Instrum. Sci. Technol. 2015, 43, 611–625. [Google Scholar] [CrossRef]
- Głowacki, R.; Furmaniak, P.; Kubalczyk, P.; Borowczyk, K. Determination of total apigenin in herbs by micellar electrokinetic chromatography with UV detection. J. Anal. Methods Chem. 2016, 2016, 3827832. [Google Scholar] [CrossRef] [PubMed]
- Anubala, S.; Sekar, R.; Nagaiah, K. Determination of curcuminoids and their degradation products in turmeric (Curcuma longa) rhizome herbal products by non-aqueous capillary electrophoresis with photodiode array detection. Food Anal. Methods 2016, 9, 2567–2578. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Hashemi, P.; Günaydın, K.; Erim, F.B. The sensitive capillary electrophoretic-LIF method for simultaneous determination of curcuminoids in turmeric by enhancing fluorescence intensities of molecules upon inclusion into (2-hydroxypropyl)-β-cyclodextrin. Electrophoresis 2015, 36, 2516–2521. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Quan, F.; Chen, P.; Qian, J.; Zhou, L.; Pu, Q. Sensitive analysis of curcuminoids via micellar electrokinetic chromatography with laser-induced native fluorescence detection and mixed micelles-induced fluorescence synergism. J. Chromatogr. A 2018, 1564, 207–213. [Google Scholar] [CrossRef]
- Adımcılar, V.; Kalaycıoğlu, Z.; Aydoğdu, N.; Dirmenci, T.; Kahraman, A.; Erim, F.B. Rosmarinic and carnosic acid contents and correlated antioxidant and antidiabetic activities of 14 Salvia species from Anatolia. J. Pharm. Biomed. Anal. 2019, 175, 112763. [Google Scholar] [CrossRef]
- Rabanes, H.R.; Guidote, A.M., Jr.; Quirino, J.P. Micellar electrokinetic chromatography of the constituents in Philippine lagundi (Vitex negundo) herbal products. Microchem. J. 2014, 112, 153–158. [Google Scholar] [CrossRef]
- Hashemi, P.; Erim, F.B. Analysis of vitamin B2 in saffron stigmas (Crocus sativus L.) by capillary electrophoresis coupled with laser-induced fluorescence detector. Food Anal. Methods 2016, 9, 2395–2399. [Google Scholar] [CrossRef]
- Uncu, A.T.; Uncu, A.O.; Frary, A.; Doganlar, S. Authentication of botanical origin in herbal teas by plastid noncoding DNA length polymorphisms. J. Agric. Food Chem. 2015, 63, 5920–5929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trindade, H. Molecular biology of aromatic plants and spices. A review. Flavour Fragr. J. 2010, 25, 272–281. [Google Scholar] [CrossRef]
- Mei, Z.; Khan, M.A.; Fu, J. Genetic authentication of Eclipta prostrate (Asteraceae) from Penthorum chinense (Penthoraceae) by Sequence Characterized Amplified Region (SCAR) markers. Rev. Biol. Trop. 2020, 68, 180–188. [Google Scholar] [CrossRef]
- Vera, D.N.; Ruisánchez, I.; Callao, M.P. Establishing time stability for multivariate qualitative methods Case study: Sudan I and IV adulteration in food spices. Food Control 2018, 92, 341–347. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Staikidou, C.; Kyriakoudi, A.; Tsimidou, M.Z. A stepwise approach for the detection of carminic acid in saffron with regard to religious food certification. Food Chem. 2018, 267, 410–419. [Google Scholar] [CrossRef]
- D’Archivio, D.N.; Maggi, M.A. Geographical identification of saffron (Crocus sativus L.) by linear discriminant analysis applied to the UV–visible spectra of aqueous extracts. Food Chem. 2017, 219, 408–413. [Google Scholar] [CrossRef]
- Negi, A.; Pare, A.; Meenatchi, R. Emerging techniques for adulterant authentication in spices and spice products. Food Control 2021, 127, 108113. [Google Scholar] [CrossRef]
- Kucharska-Ambrożej, K.; Karpinska, J. The application of spectroscopic techniques in combination with chemometrics for detection adulteration of some herbs and spices. Microchem. J. 2020, 153, 104278. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Cruz-Tirado, J.P.; Barbin, D.F. Nontargeted analytical methods as a powerful tool for the authentication of spices and herbs: A review. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 670–689. [Google Scholar] [CrossRef]
- De Lima, A.B.S.; Batista, A.S.; De Jesus, J.C.; De Jesus Silva, J.; De Araújo, A.C.M.; Santos, L.S. Fast quantitative detection of black pepper and cumin adulterations by near-infrared spectroscopy and multivariate modelling. Food Control 2020, 107, 106802. [Google Scholar] [CrossRef]
- Lixourgioti, P.; Goggin, K.A.; Zhao, X.; Murphy, D.J.; van Ruth, S.; Koidis, A. Authentication of cinnamon spice samples using FT-IR spectroscopy and chemometric classification. LWT Food Sci. Technol. 2022, 154, 112760. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; de França, R.L.; Tumbajulca, M.; Barraza-Jáuregui, G.; Barbin, D.F.; Siche, R. Detection of cumin powder adulteration with allergenic nutshells using FT-IR and portable NIRS coupled with chemometrics. J. Food Compos. Anal. 2023, 116, 105044. [Google Scholar] [CrossRef]
- Massaro, A.; Bragolusi, M.; Tata, A.; Zacometti, C.; Lefevre, S.; Frégière-Salomon, A.; Lafeuille, J.L.; Sammarco, G.; Candalino, I.F.; Suman, M. Non-targeted authentication of black pepper using a local web platform: Development, validation and post-analytical challenges of a combined NIR spectroscopy and LASSO method. Food Control 2023, 145, 109477. [Google Scholar] [CrossRef]
- Modupalli, N.; Naik, M.; Sunil, C.K.; Natarajan, V. Emerging non-destructive methods for quality and safety monitoring of spices. Trends Food Sci. Technol. 2021, 108, 133–147. [Google Scholar] [CrossRef]
- Chao, K.; Dhakal, S.; Schmidt, W.F.; Qin, J.; Kim, M.; Peng, Y.; Huang, Q. Raman and IR spectroscopic modality for authentication of turmeric powder. Food Chem. 2020, 320, 126567. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Y.; Sun, X.; Wei, X.; Lin, Z.; Zhang, X.; Shi, J.; Battino, M.; Gong, Y.; Shi, B.; et al. SERS determination of hydroxy-α-sanshool in spicy hotpot seasoning: The strategy to restrain the interference of capsaicin and its mechanism. Food Chem. 2023, 413, 135644. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Yu, S.; Fu, H.; He, S.; Yang, B.; Nan, T.; Yuan, Y.; Huang, L. Prediction of chemical indicators for quality of Zanthoxylum spices from multi-regions using hyperspectral imaging combined with chemometrics. Front. Sustain. Food Syst. 2022, 6, 1036892. [Google Scholar] [CrossRef]
- Orrillo, I.; Cruz-Tirado, J.P.; Cardenas, A.; Oruna, M.; Carnero, A.; Barbin, D.F.; Siche, R. Hyperspectral imaging as a powerful tool for identification of papaya seeds in black pepper. Food Control 2019, 101, 45–52. [Google Scholar] [CrossRef]
- Florián-Huamán, J.; Cruz-Tirado, J.P.; Barbin, D.F.; Siche, R. Detection of nutshells in cumin powder using NIR hyperspectral imaging and chemometrics tools. J. Food Compos. Anal. 2022, 108, 104407. [Google Scholar] [CrossRef]
- Hashemi-Nasab, F.S.; Talebian, S.; Parastar, H. Multiple adulterants detection in turmeric powder using Vis-SWNIR hyperspectral imaging followed by multivariate curve resolution and classification techniques. Microchem. J. 2023, 185, 108–203. [Google Scholar] [CrossRef]
- Serrano, R.; Da Silva, G.; Silva, O. Application of light and scanning electron microscopy in the identification of herbal medicines. Microscopy 2010, 3, 182–190. [Google Scholar]
- De Vito, S.; Salvato, M.; Massera, E.; Miglietta, M.; Fattoruso, G.; Di Francia, G. Advanced pattern recognition techniques for fast and reliable e-nose response analysis in NDTs scenarios. Sensors 2015, 319, 235–240. [Google Scholar] [CrossRef]
- Morling, A.; McNaughton, R. A 2016 Baseline Food Crime Annual Strategic Assessment. 2016. Available online: https://www.food.gov.uk/sites/default/files/fsafood-crime-assessment-2016.pdf (accessed on 30 July 2023).
- Van Rijswijk, W.; Frewer, L.J. Consumer needs and requirements for food and ingredient traceability information. Int. J. Consum. Stud. 2012, 36, 282–290. [Google Scholar] [CrossRef]
- Charlebois, S.; Schwab, A.; Henn, R.; Huck, C.W. Food fraud: An exploratory study for measuring consumer perception towards mislabeled food products and influence on self-authentication intentions. Trends Food Sci. Technol. 2016, 50, 211–218. [Google Scholar] [CrossRef]
- Barrere, V.; Everstine, K.; Théolier, J.; Godefroy, S. Food fraud vulnerability assessment: Towards a global consensus on procedures to manage and mitigate food fraud. Trends Food Sci. Technol. 2020, 100, 131–137. [Google Scholar] [CrossRef]
- Soon, J.M.; Krzyzaniak, S.C.; Shuttlewood, Z.; Smith, M.; Jack, L. Food fraud vulnerability assessment tools used in food industry. Food Control 2019, 101, 225–232. [Google Scholar] [CrossRef]
- Spink, J.; Moyer, D.C.; Speier-Pero, C. Introducing the food fraud initial screening model (FFIS). Food Control 2016, 69, 306–314. [Google Scholar] [CrossRef]
- SSAFE. SSAFE Food Fraud Vulnerability Assessment Tool. 2018. Available online: https://www.ssafe-food.org/tools/food-fraud-vulnerability-assessment-tool (accessed on 30 July 2023).
- Silvis, I.C.J.; Van Ruth, S.M.; Van Der Fels-Klerx, H.J.; Luning, P.A. Assessment of food fraud vulnerability in the spices chain: An explorative study. Food Control 2017, 81, 80–87. [Google Scholar] [CrossRef]

| Herbs and Spices | Area Mill. ha | Production Mill. t | Imports USD Mill. | Main Producers |
|---|---|---|---|---|
| Anise, badian, coriander, cumin, caraway, fennel, and juniper berries | 2.30 | 2.70 | 1398.58 | India, Türkiye, Mexico, Russia, Syria |
| Chillies and peppers, dry (Capsicum spp.) | 1.62 | 4.84 | 2773.00 | India, Bangladesh, Thailand, China, Ethiopia |
| Cinnamon and cinnamon-tree flowers | 0.30 | 0.23 | 1048.17 | China, Indonesia, Vietnam, Sri Lanka, Madagascar |
| Cloves (whole stems) | 0.67 | 0.19 | 451.52 | Indonesia, Madagascar, Tanzania, Comoros, Sri Lanka |
| Ginger | 0.45 | 4.90 | 1506.24 | India, Nigeria, China, Indonesia, Nepal |
| Mustard seed | 0.63 | 0.53 | 316.37 | Nepal, Russia, Canada, Myanmar, Ukraine |
| Nutmeg, mace, cardamoms | 0.47 | 0.15 | 1452.57 | India, Indonesia, Guatemala, Nepal, Sri Lanka |
| Other stimulant, spicy and aromatic crops | 1.41 | 3.15 | 2236.67 | India, Ethiopia, Türkiye, Bangladesh, Yemen |
| Pepper (Piper spp.) | 0.68 | 0.79 | 2025.28 | Vietnam, Brazil, Indonesia, Burkina Faso, India |
| Peppermint, spearmint | 0.00 | 0.04 | 3.67 | Morocco, Argentina, Mexico, Japan, Georgia |
| Sesame seed | 12.51 | 6.35 | 3570.79 | Sudan, India, Tanzania, Myanmar, China |
| Vanilla | 0.09 | 0.01 | 901.44 | Madagascar, Indonesia, Mexico, Papua New Guinea, China |
| Continent | Production (Mill. t) | Exports (Mill. t) | Imports (Mill. t) | Exports (USD Mill.) | Imports (USD Mill.) |
|---|---|---|---|---|---|
| Africa | 6.27 | 1.40 | 0.35 | 2631.21 | 733.56 |
| America | 0.90 | 0.72 | 0.89 | 2028.27 | 3085.37 |
| Asia | 16.39 | 4.09 | 4.66 | 9720.66 | 9316.54 |
| Europe | 0.30 | 0.76 | 1.28 | 2563.04 | 4369.02 |
| Oceania | 0.02 | 0.01 | 0.04 | 69.47 | 179.81 |
| World | 23.87 | 6.97 | 7.22 | 17,012.65 | 17,684.30 |
| Type and Country Zone | Product Type | Name | Country |
|---|---|---|---|
| Protected Designation of Origin (PDO) European Union | Saffron | Krokos Kozanis | Greece |
| Azafrán de la Mancha | Spain | ||
| Zafferano dell’Aquila; Zafferano di San Gimignano; Zafferano di Sardegna | Italy | ||
| Paprika | Pimentón de La Vera; Pimentón de Murcia; Pimentón de Mallorca | Spain | |
| Piment d’Espelette | France | ||
| Szegedi paprika; Kalocsai fűszerpaprika-őrlemény | Hungary | ||
| Žitavská paprika | Slovakia | ||
| Cumin | Český kmín | Czechia | |
| Protected Geographical Indication (PGI) European Union | Thyme | Thym de Provence | France |
| Vanilla | Vanille de l’île de La Réunion | France | |
| Protected Geographical Indication (PGI) non-EU countries | Pepper | Poivre de Penja | Cameroon |
| Pimienta | Poivre de Kampot | Cambodia | |
| Ginger | Luoping Xiao Huang Jiang | China | |
| Cinnamon | Ceylon Cinnamon | Sri Lanka |
| Spices and Herbs | Product | Adulteration (Novel Food Ingredient) | Date | Notifying Country | Distribution | Origin |
| Oregano | Oregano | Novel food olive leaf | 2020 | Germany | Netherlands, France, Austria, Switzerland, Algeria, Germany | Türkiye |
| Spices | Spice mix | Angelica sinensis | 2020 | Finland | Hong Kong, Finland, Netherlands | Hong Kong |
| Spices and Herbs | Product | Adulteration (Undeclared Product or Other Botanical Plants or Allergens) | Date | Notifying Country | Distribution | Origin |
| Basil | Basil | Celery | 2022 | Cyprus | Cyprus | Greece |
| Coriander | Coriander | Mustard | 2021 | Netherlands | Netherlands | Ukraine |
| Coriander | Mustard | 2022 | Spain | Portugal, Sweden, Belgium, Bulgaria, Andorra, Spain, Chile, France, Colombia, Panama, Costa Rica, Italy, Honduras, Lithuania, Mexico | Spain | |
| Cumin | Cumin (ground) | Gluten | 2020 | European Commission | Spain, Andorra | Spain |
| Cumin (ground) | Mustard | 2021 | Spain | Spain | France | |
| Cumin (ground) | Sesame | 2022 | Spain | Dominican Republic, Portugal, Bulgaria, Switzerland, United States, Germany, Andorra, Spain, France, United Kingdom, Guinea, Mexico | Spain, India | |
| Curry | Curry (Madras curry) | Gluten | 2021 | Spain | Spain, Portugal | Spain |
| Curry (powder) | Traces of peanut | European Commission | Northern Ireland | India | ||
| Spearmint | Spearmint (crushed) | Celery | 2022 | Cyprus | Cyprus | Egypt |
| Spices | Spice mix | Mustard | 2021 | Spain | Spain, France, Portugal | Spain |
| Spice mix | Traces of gluten, mustard and lupin | Netherlands | Netherlands, Aruba, Lithuania | Netherlands | ||
| Spices and Herbs | Product | Adulteration (Composition: Illegal Dyes) | Date | Notifying Country | Distribution | Origin |
| Cumin | Cumin | Auramine O and cis-Bixin | 2022 | Lithuania | Spain, Portugal, Greece, Czech Republic, Lithuania | India |
| Curry | Curry | Sudan I | 2021 | Austria | Austria, Hungary | Türkiye |
| Curry | Rhodamine B | Austria | Netherlands, France, Austria, Germany | Türkiye | ||
| Curry | Sudan I | Netherlands | Netherlands, Belgium | India | ||
| Curry (powder) | Sudan I and Sudan IV | Belgium | Distribution restricted to notifying country | Türkiye | ||
| Curry (powder) | Orange II | 2022 | Belgium | Belgium | Cameroon | |
| Pepper | Chilli pepper (powder) | Orange II | 2020 | Belgium | Belgium | Cameroon |
| Pepper (dried) | Orange II | Belgium | Germany | Cameroon | ||
| Pepper (grind dried) | Orange II | Belgium | United Kingdom | Nigeria | ||
| Cayenne pepper (powder) | Orange II and Sudan I | Belgium | United Kingdom | Nigeria | ||
| Chilli pepper (powder) | Orange II | 2021 | Belgium | Germany | Ghana | |
| Chilli pepper (powder) | Orange II | Belgium | Netherlands, Belgium | Nigeria | ||
| Chilli pepper (powder) | Orange II | Belgium | Germany | Togo | ||
| Chilli pepper (powder) | Orange II and Sudan I | Belgium | France | Togo | ||
| Chilli pepper (powder) | Sudan I, Sudan IV and Rhodamine B | 2022 | Belgium | Product not (yet) placed on the market | Bangladesh | |
| Chilli crushed with seed | Sudan I, Sudan III and Sudan IV | Germany | Germany, Italy, Luxembourg | Unknown | ||
| Pepper (crushed) | Sudan I and Sudan IV | Switzerland | Distribution restricted to notifying country | China | ||
| Sumac | Sumac (ground) | Sudan I and Sudan Orange G | 2022 | Latvia | Latvia, Russia, Poland, Croatia | Türkiye |
| Sumac (ground) | Sudan IV | Switzerland | Switzerland | Türkiye | ||
| Sumac (ground) | Sudan IV | Switzerland | Distribution restricted to notifying country | Türkiye | ||
| Sumac | Sudan IV | Germany | Denmark, Poland, France, Sweden, Slovenia, Austria, Germany | Türkiye | ||
| Spices and herbs | Spices | Sudan I | 2020 | Latvia | Latvia | Uzbekistan |
| Spices and herbs | Sudan I | 2021 | Latvia | Uzbekistan | Russia | |
| Spices | Sudan IV | 2022 | Belgium | France, Belgium, Germany | France | |
| Spices (couscous spice mix) | Sudan I and Sudan IV | Switzerland | Latvia, Malta, Netherlands, Portugal, Romania, Austria, Belgium, Switzerland, Germany, Denmark, Spain, France, Italy, Lithuania | Lebanon | ||
| Spices (spice preparation) | Sudan IV | Switzerland | Switzerland | Türkiye | ||
| Spices | Sudan II, Sudan III and Sudan IV | Latvia | Latvia | Russia | ||
| Herbs (Italian product “granelli d’erbe”) | Aloe-emodin and emodin | France | France | Italy | ||
| Turmeric | Turmeric | Residue of lead and Sudan I | 2022 | Belgium | Product not (yet) placed on the market | Bangladesh |
| Spices and Herbs | Product | Adulteration (Undeclared or Unauthorised Food Additives and Flavourings) | Date | Notifying Country | Distribution | Origin |
| Cinnamon | Cinnamon | Sulphite | 2021 | Belgium | Northern Ireland, Austria, Belgium, Switzerland, Czech Republic, Germany, Denmark, Spain, Finland, France, Greece, Croatia, Hungary, Ireland, Iceland, Italy, Lithuania, Luxembourg, Latvia, Netherlands, Norway, Poland, Portugal, Romania, Sweden, Slovenia, Slovakia | United Kingdom |
| Cinnamon | Sulphite | Belgium | Denmark, Netherlands, France, Sweden, Belgium, Italy, Germany | Sri Lanka | ||
| Cumin | Cumin (powder) | Colour E104—Quinoline yellow | 2022 | Spain | Spain | India |
| Cumin | Colour E 160b—annatto/bixin/norbixin | 2022 | Lithuania | Spain, Portugal, Greece, Czech Republic, Lithuania | India | |
| Herbs | Dried lily bulbs | E220—Sulphur dioxide | 2021 | Denmark | Denmark, Spain, Netherlands, France, Austria, Hungary, Germany | China |
| Spices | Tandoori masala | Colour E 102—tartrazine and colour E 129—Allura Red | 2022 | Denmark | Denmark | Spain |
| Sumac | Spice preparation | Colour E 122—azorubine and Colour E 124—Ponceau 4R | 2022 | Switzerland | Switzerland | Türkiye |
| Spices and Hrbs | Product | Adulteration (Labelling Absent) | Date | Notifying Country | Distribution | Origin |
| Ginger | Ginger (powder) | Missing allergen labelling | 2022 | Germany | Denmark, Latvia, Estonia, Finland, Poland, Germany | India |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez, R.; Rodríguez, A.; Hernández, A.; Casquete, R.; Benito, M.J.; Martín, A. Spice and Herb Frauds: Types, Incidence, and Detection: The State of the Art. Foods 2023, 12, 3373. https://doi.org/10.3390/foods12183373
Velázquez R, Rodríguez A, Hernández A, Casquete R, Benito MJ, Martín A. Spice and Herb Frauds: Types, Incidence, and Detection: The State of the Art. Foods. 2023; 12(18):3373. https://doi.org/10.3390/foods12183373
Chicago/Turabian StyleVelázquez, Rocío, Alicia Rodríguez, Alejandro Hernández, Rocío Casquete, María J. Benito, and Alberto Martín. 2023. "Spice and Herb Frauds: Types, Incidence, and Detection: The State of the Art" Foods 12, no. 18: 3373. https://doi.org/10.3390/foods12183373
APA StyleVelázquez, R., Rodríguez, A., Hernández, A., Casquete, R., Benito, M. J., & Martín, A. (2023). Spice and Herb Frauds: Types, Incidence, and Detection: The State of the Art. Foods, 12(18), 3373. https://doi.org/10.3390/foods12183373






