Non-Destructive Classification of Organic and Conventional Hens’ Eggs Using Near-Infrared Hyperspectral Imaging
Abstract
1. Introduction
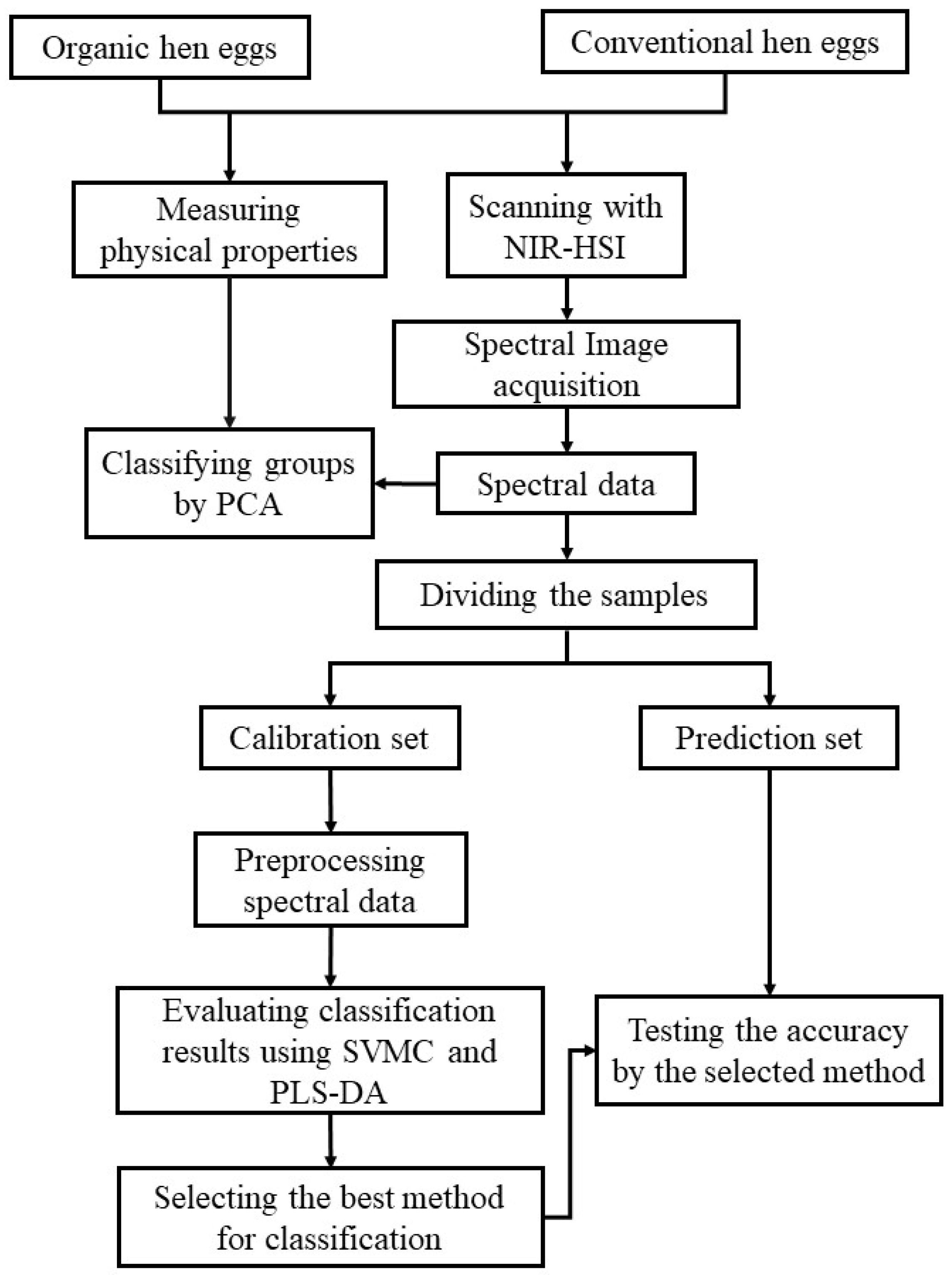
2. Materials and Methods
2.1. Hens’ Egg Samples
2.2. NIR-HSI
2.3. Physical Properties Measured Using Non-Destructive Methods
2.3.1. Density Determination
2.3.2. Shell Color Measurement
2.4. Physiochemical Properties Measured Using Destructive Methods
2.5. Data Analysis
3. Results and Discussion
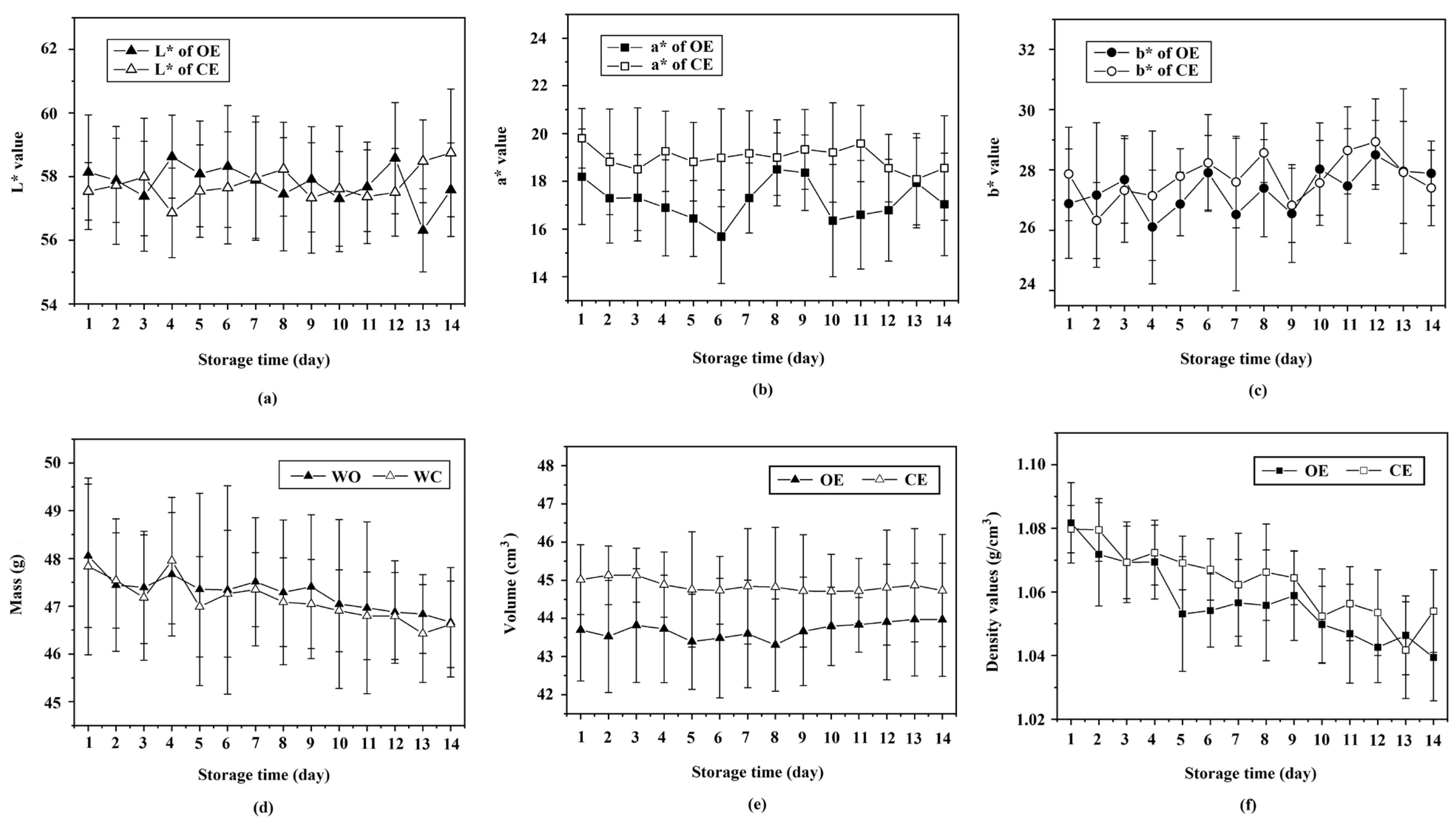
3.1. Determining Physical Properties via Non-Destructive Methods
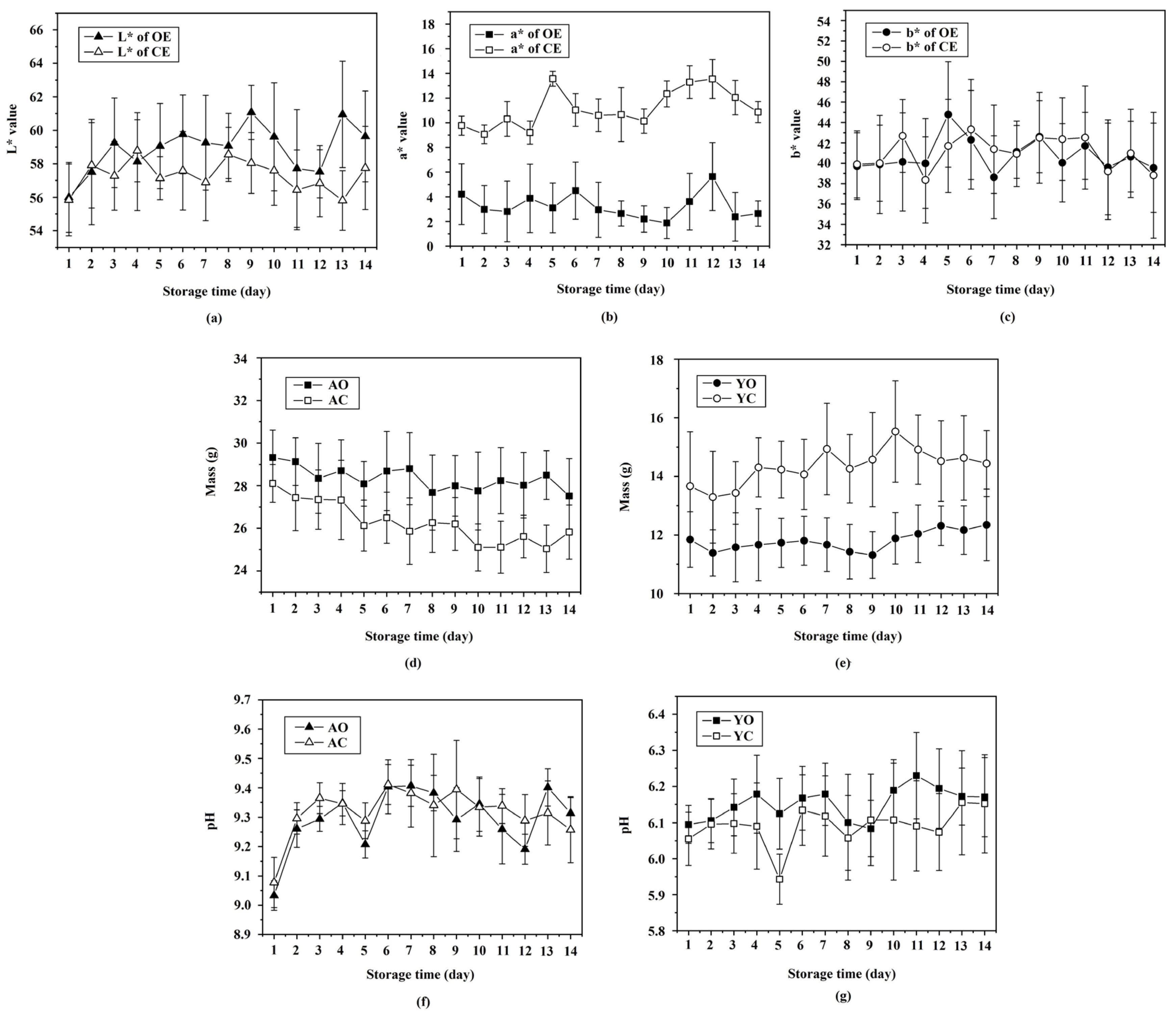
3.2. Determining Physiochemical Properties via Destructive Methods
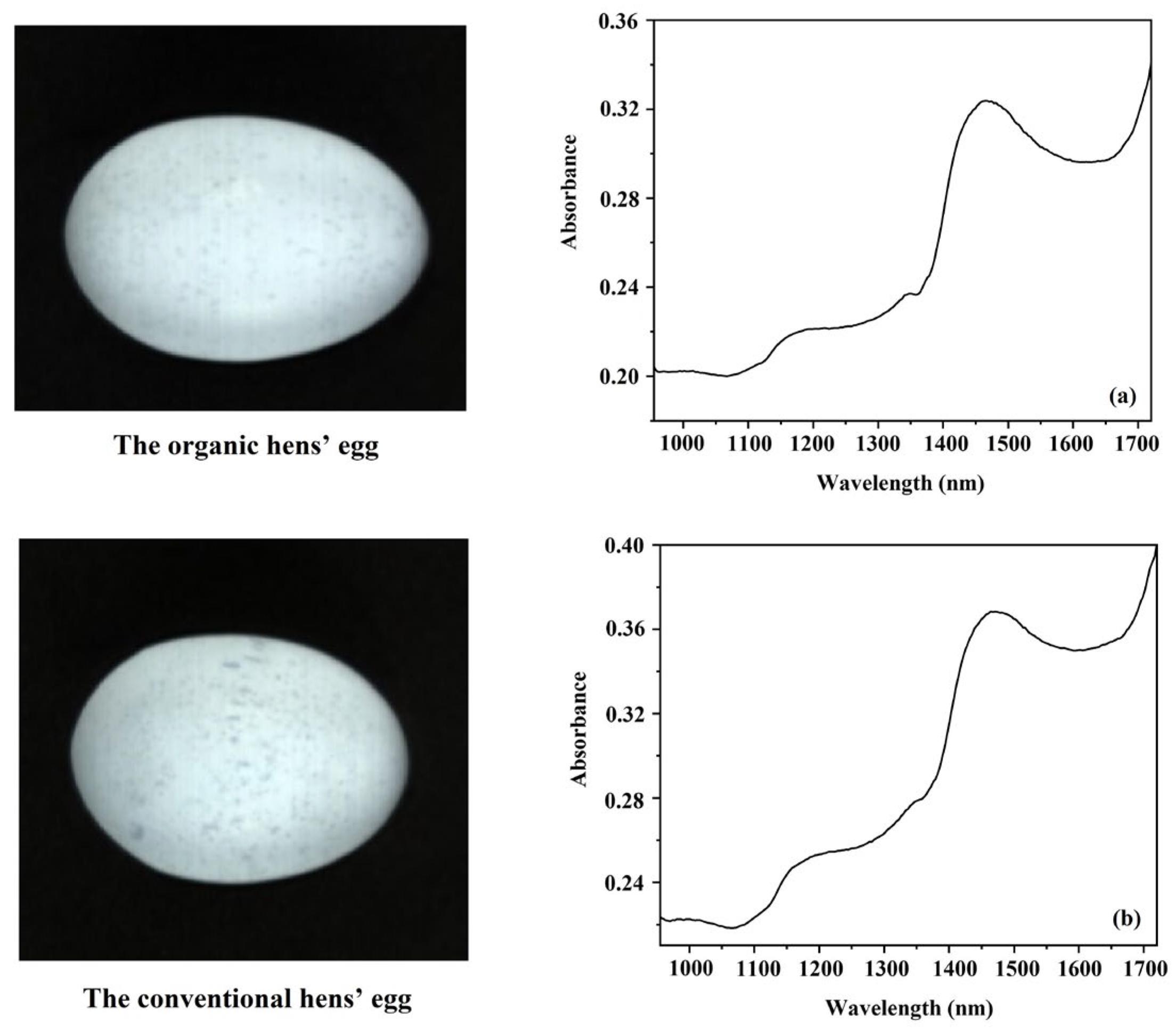
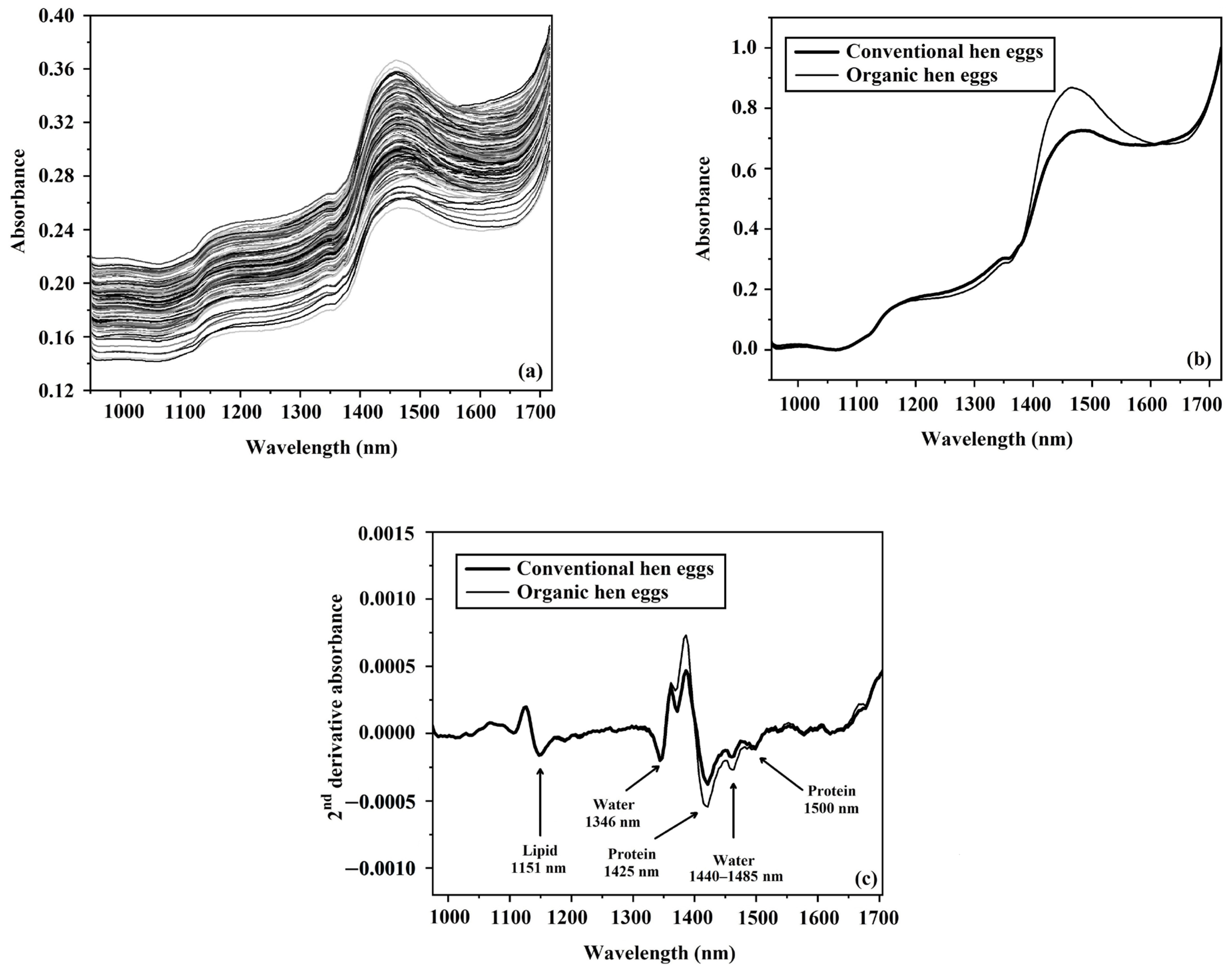
3.3. Absorbance Spectra
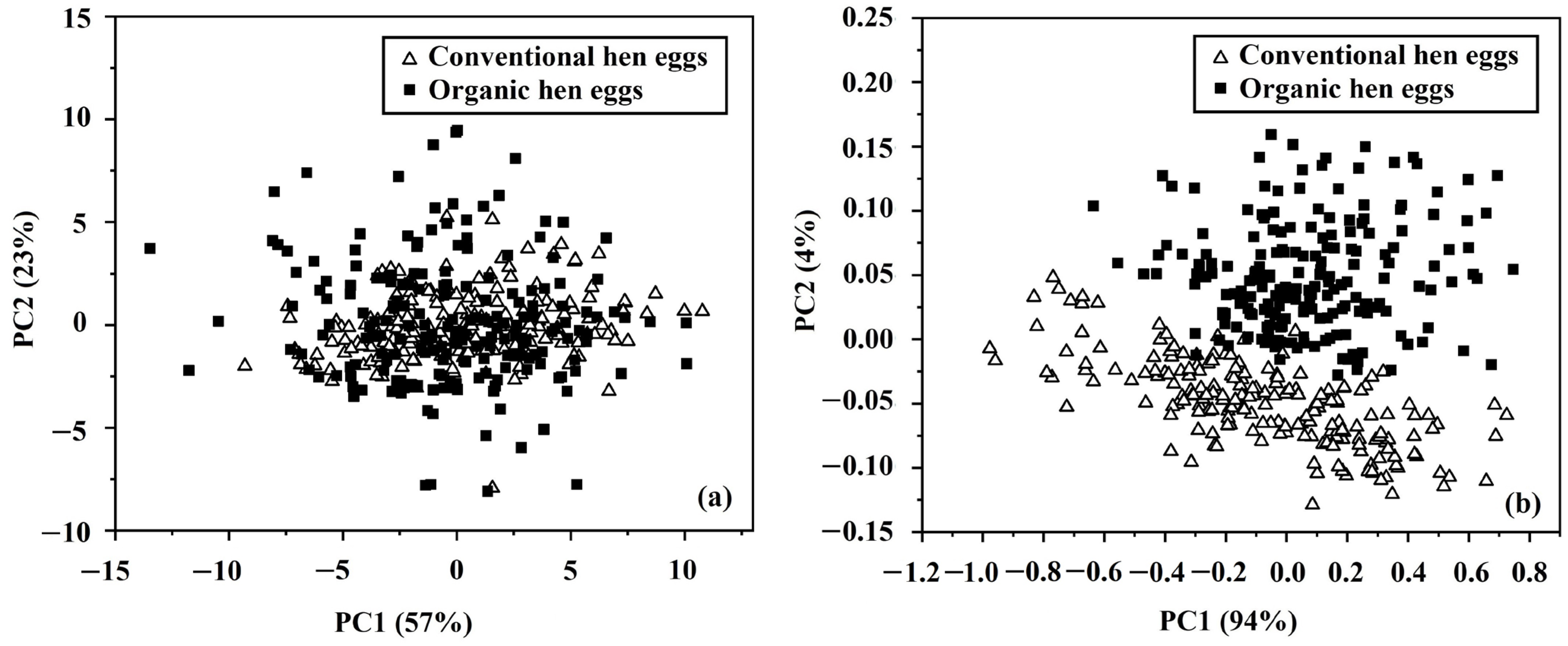
3.4. Discriminant Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campmajo, G.; Cayero, I.; Saurina, J.; Nunez, O. Classification of hen eggs by HPLC-UV fingerprinting and chemometric methods. Foods 2019, 8, 310. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 14 November 2022).
- McDougal, T. Global Egg Production Continues to Rise. Available online: https://www.poultryworld.net/Eggs/Articles/2020/6/Global-egg-production-continues-to-rise-604164E/ (accessed on 29 June 2022).
- Karoui, R.; Ketelaere, B.D.; Kemps, B.; Bamelis, F.; Mertens, K.; Baerdemaeker, J.D. Chapter 15: Eggs and Egg Products. In Infrared Spectroscopy for Food Quality Analysis and Control; Sun, D.W., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 399–414. [Google Scholar] [CrossRef]
- Wang, X.; Son, M.; Meram, C.; Wu, J. Mechanism and potential of egg consumption and egg bioactive components on Type-2 diabetes. Nutrients 2019, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Kassis, N.; Drake, S.R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Development of nutraceutical egg products with omega-rich oils. LWT-Food Sci. Technol. 2010, 43, 777–783. [Google Scholar] [CrossRef]
- Blair, R. Nutrition and Feeding of Organic Poultry, 2nd ed.; CAB International: Oxford, UK, 2008; p. 278. [Google Scholar]
- Hidalgo, A.; Rossi, M.; Clerici, F.; Ratti, S. A market study on the quality characteristics of eggs from different housing systems. Food Chem. 2008, 106, 1031–1038. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Cooperatives. Organic Agriculture. Part 2: Organic Livestock (TAS 9000 Part 2–2011). Available online: https://www.acfs.go.th/standard/download/eng/Organic_Livestock.pdf (accessed on 14 November 2022).
- Ministry of Agriculture and Cooperatives. Good Agricultural Practices for Layer Farm (TAS 6909-2010). Available online: https://www.acfs.go.th/standard/download/eng/GAP_LayerFarm.pdf (accessed on 14 November 2022).
- Hafez, T.A.; El-Ghayaty, H.A.; Megahed, A.A. A comparison between organic and conventionally produced eggs. Assiut Vet. Med. J. 2013, 59, 55–59. [Google Scholar] [CrossRef]
- Ma, X.; Chen, L.; Yin, L.; Li, Y.; Yang, Z.; Li, G.; Shan, H. Risk analysis of 24 residual antibiotics in poultry eggs in Shandong, China (2018–2020). Vet. Sci. 2022, 9, 126. [Google Scholar] [CrossRef]
- Kamali, A.; Mirlohi, M.; Etebari, M.; Sepahi, S. Occurrence of tetracycline residue in table eggs and genotoxic effects of raw and heated contaminated egg yolks on hepatic cells. Iran. J. Public Health 2020, 49, 1355–1363. [Google Scholar] [CrossRef]
- Ebied, N.A.; Abdou, M.S.; Abass, M.E. Microbial quality and residues of some antibiotics in conventional and organic table eggs at Retail Stores in Kafrelsheikh Governorate. Alex. J. Vet. Sci. 2021, 69, 98–112. [Google Scholar] [CrossRef]
- Florkiewicz, A.F.; Deren, K.; Florkiewicz, A.; Topolska, K.; Juszczak, L.; Cieslik, E. The quality of eggs (organic and nutraceutical vs. conventional) and their technological properties. Poult. Sci. 2017, 96, 2480–2490. [Google Scholar] [CrossRef]
- Banaszewska, D.; Biesiada-Drzazga, B.; Marciniuk, M.; Hrnčár, C.; Arpášová, H.; Kaim-Mirowski, S. Comparison of the quality of cage and organic eggs available in retail and their content of selected macroelements. Acta Sci. Pol. Technol. Aliment. 2020, 19, 159–167. [Google Scholar] [CrossRef]
- Minelli, G.; Sirri, F.; Folegatti, E.; Meluzzi, A.; Franchini, A. Egg quality traits of laying hens reared in organic and conventional systems. Ital. J. Anim. Sci. 2007, 6, 728–730. [Google Scholar] [CrossRef]
- Heflin, L.E.; Malheiros, R.; Anderson, K.E.; Johnson, L.K.; Raatz, S.K. Mineral content of eggs differs with hen strain, age, and rearing environment. Poult. Sci. 2018, 97, 1605–1613. [Google Scholar] [CrossRef]
- BBC News. Germany Investigates Organic Egg ‘Fraud’. Available online: https://www.bbc.com/news/world-europe-21573158 (accessed on 24 November 2021).
- Ruth, S.; Alewijn, M.; Rogers, K.; Newton-Smith, E.; Tena, N.; Bollen, M.; Koot, A. Authentication of organic and conventional eggs by carotenoid profiling. Food Chem. 2011, 126, 1299–1305. [Google Scholar] [CrossRef]
- Tres, A.; O’Neill, R.; Van Ruth, S.M. Fingerprinting of fatty acid composition for the verification of the identity of organic eggs. Lipid Technol. 2011, 23, 40–42. [Google Scholar] [CrossRef]
- He, H.; Sun, D. Hyperspectral imaging technology for rapid detection of various microbial contaminants in agricultural and food products. Trends Food Sci. Technol. 2015, 46, 99–109. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part I: Fundamentals. Innov. Food Sci. Emerg. Technol. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- Chen, S.Y.; Hsu, S.H.; Ko, C.Y.; Hsu, K.H. Real-time defect and freshness inspection on chicken eggs using hyperspectral imaging. Food Control 2023, 150, 109716. [Google Scholar] [CrossRef]
- Yao, K.; Sun, J.; Chen, C.; Xu, M.; Zhou, X.; Cao, Y.; Tian, Y. Non-destructive detection of egg qualities based on hyperspectral imaging. J. Food Eng. 2022, 325, 111024. [Google Scholar] [CrossRef]
- Suktanarak, S.; Teerachaichayut, S. Non-destructive quality assessment of hens’ eggs using hyperspectral images. J. Food Eng. 2017, 215, 97–103. [Google Scholar] [CrossRef]
- Yao, K.; Sun, J.; Cheng, J.; Xu, M.; Chen, C.; Zhou, X.; Dai, C. Development of Simplified Models for Non-Destructive Hyperspectral Imaging Monitoring of S-ovalbumin Content in Eggs during Storage. Foods 2022, 11, 2024. [Google Scholar] [CrossRef]
- Xu, J.; Riccioli, C.; Sun, D. Comparison of hyperspectral imaging and computer vision for automatic differentiation of organically and conventionally farmed salmon. J. Food Eng. 2017, 196, 170–182. [Google Scholar] [CrossRef]
- Teerachaichayut, S.; Ho, H.T. Non-destructive prediction of total soluble solids, titratable acidity and maturity index of limes by near infrared hyperspectral imaging. Postharvest Biol. Technol. 2017, 133, 20–25. [Google Scholar] [CrossRef]
- Lei, T.; Lin, X.; Sun, D. Rapid classification of commercial Cheddar cheeses from different brands using PLSDA, LDA and SPA-LDA models built by hyperspectral data. J. Food Meas. Charact. 2019, 13, 3119–3129. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Pierna, J.A.F.; Rogez, H.; Barbin, D.F.; Baeten, V. Authentication of Cocoa (Theobroma cacao) bean hybrids by NIR-hyperspectral imaging and chemometrics. Food Control 2020, 118, 107445. [Google Scholar] [CrossRef]
- Rios-Reina, R.; Callejon, R.M.; Amigo, J.M. Feasibility of a rapid and non-destructive methodology for the study and discrimination of pine nuts using near-infrared hyperspectral analysis and chemometrics. Food Control 2021, 130, 108365. [Google Scholar] [CrossRef]
- Khamsopha, D.; Sahachairungrueng, W.; Teerachaichayut, S. Utilizing near infrared hyperspectral imaging for quantitatively predicting adulteration in tapioca starch. Food Control 2021, 123, 107781. [Google Scholar] [CrossRef]
- Sricharoonratana, M.; Thompson, A.K.; Teerachaichayut, S. Use of near infrared hyperspectral imaging as a nondestructive method of determining and classifying shelf life of cakes. LWT-Food Sci. Technol. 2021, 136, 110369. [Google Scholar] [CrossRef]
- Sahachairungrueng, W.; Teerachaichayut, S. Nondestructive quality assessment of longans using near infrared hyperspectral imaging. Agric. Eng. Int. CIGR J. 2022, 24, 217–227. [Google Scholar] [CrossRef]
- Sahachairungrueng, W.; Meechan, C.; Veerachat, N.; Thompson, A.K.; Teerachaichayut, S. Assessing the Levels of Robusta and Arabica in Roasted Ground Coffee Using NIR Hyperspectral Imaging and FTIR Spectroscopy. Foods 2022, 11, 3122. [Google Scholar] [CrossRef]
- Tantinantrakun, A.; Sukwanit, S.; Thompson, A.K.; Teerachaichayut, S. Nondestructive evaluation of SW-NIRS and NIR-HSI for predicting the maturity index of intact pineapples. Postharvest Biol. Technol. 2023, 195, 112141. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Cooperatives. Hen Egg (TAS 6702–2010). Available online: https://www.acfs.go.th/standard/download/eng/hen%20egg_ENG.pdf (accessed on 14 November 2022).
- Mohsenin, N.N. Chapter 3: Physical characteristics. In Physical Properties of Plant and Animal Materials: Structure, Physical Characteristics and Mechanical Properties, 1st ed.; Gordon and Breach Science Publishers: New York, NY, USA, 1970; pp. 66–76. [Google Scholar]
- Kleinebecker, T.; Klaus, V.H.; Holzel, N. Reducing sample quantity and maintaining high prediction quality of grassland biomass properties with near Infrared Reflectance Spectroscopy. J. Near Infrared Spectrosc. 2011, 19, 495–505. [Google Scholar] [CrossRef]
- Ribeiro, J.P.O.; Medeiros, A.D.; Caliari, I.P.; Trancoso, A.C.R.; Miranda, R.M.; Freitas, F.C.L.; Silva, L.J.; Dias, D.C.F. FT-NIR and linear discriminant analysis to classify chickpea seeds produced with harvest aid chemicals. Food Chem. 2021, 342, 128324. [Google Scholar] [CrossRef]
- Teye, E.; Uhomoibhi, J.O. Nondestructive Authentication of Cocoa Bean Cultivars by FT-NIR Spectroscopy and Multivariate Techniques. Focus Sci. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Musicant, D.R.; Kumar, V.; Ozgur, A. Optimizing F-measure with support vector machines. In Proceedings of the Sixteenth International Florida Artificial Intelligence Research Society Conference, St. Augustine, FL, USA, 12–14 May 2003. [Google Scholar]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Rashid, N.A.; Nasaruddin, N.; Kassim, K.; Hazwani, A.; Rahim, H.A. Comparison Analysis: Large Data Classification Using PLS-DA and Decision Trees. Math. Methods Stat. 2019, 8, 100–105. [Google Scholar] [CrossRef]
- Brasil, Y.L.; Cruz-Tirado, J.P.; Barbin, D.F. Fast online estimation of quail eggs freshness using portable NIR spectrometer and machine learning. Food Control 2022, 131, 108418. [Google Scholar] [CrossRef]
- Sokolowicz, Z.; Dykiel, M.; Augustynska-Prejsnar, A.; Krawczyk, J. The effect of storage duration on some quality traits and composition of eggs from different housing systems. Ann. Anim. Sci. 2022, 22, 459–475. [Google Scholar] [CrossRef]
- Eke, M.O.; Olaitan, N.I.; Ochefu, J.H. Effect of storage conditions on the quality attributes of shell (Table) eggs. Niger. Food J. 2013, 31, 18–24. [Google Scholar] [CrossRef]
- Brodacki, A.; Batkowska, J.; Drabik, K.; Chabroszewska, P.; Luczkiewicz, P. Selected quality traits of table eggs depending on storage time and temperature. Br. Food J. 2019, 121, 2016–2026. [Google Scholar] [CrossRef]
- Lordelo, M.; Fernandes, E.; Bessa, R.J.B.; Alves, S.P. Quality of egg different laying hen production systems, from indigenous breeds and specialty eggs. Poult. Sci. 2016, 96, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Lee, K.T.; Lee, W.I.; Han, Y.K. Effects of storage temperature and time on the quality of eggs from laying hens at peak production. Asian-australas. J. Anim. Sci. 2011, 24, 279–284. [Google Scholar] [CrossRef]
- Zotte, A.D.; Cullere, M.; Pellattiero, E.; Sartori, A.; Marangon, A.; Bondesan, V. Is the farming method (Cage, barn, organic) a relevant factor for marketed egg quality traits. Livest. Sci. 2021, 246, 104453. [Google Scholar] [CrossRef]
- Lee, M.H.; Cho, E.J.; Choi, E.S.; Sohn, S.H. The effect of storage time and temperature on egg quality in commercial eggs. Korean J. Clin. Lab. Sci. 2016, 43, 31–38. [Google Scholar]
- Workman, J.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy, 1st ed.; Taylor and Francis Group LLC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 546–562. [Google Scholar]
- Picouet, P.A.; Gou, P.; Hyypio, R.; Castellari, M. Implementation of NIR technology for at-line rapid detection of sunflower oil adulterated with mineral oil. J. Food Eng. 2018, 230, 18–27. [Google Scholar] [CrossRef]
- Sato, T. Nondestructive measurements of lipid content and fatty acid composition in Rapeseeds (Brassica napus L.) by Near Infrared Spectroscopy. Plant Prod. Sci. 2008, 11, 146–150. [Google Scholar] [CrossRef]
- Muncan, J.; Tsenkova, R. Aquaphotomics approach for monitoring different steps of purification process in water treatment systems. Talanta 2019, 206, 120253. [Google Scholar] [CrossRef]
- Chen, J.; Ren, X.; Zhang, Q.; Diao, X.; Shen, Q. Determination of protein, total carbohydrates and crude fat contents of foxtail millet using effective wavelengths in NIR spectroscopy. J. Cereal Sci. 2013, 58, 241–247. [Google Scholar] [CrossRef]
- Hsu, C.W.; Chang, C.C.; Lin, C.J. A Practical Guide to Support Vector Classification. Available online: https://www.csie.ntu.edu.tw/~cjlin/papers/guide/guide.pdf (accessed on 20 January 2023).
- Lindstrom, S.W.; Geladi, P.; Jonsson, O.; Pettersson, F. The importance of balanced data sets for partial least squares discriminant analysis: Classification problems using hyperspectral imaging data. J. Near Infrared Spectrosc. 2011, 19, 233–241. [Google Scholar] [CrossRef]

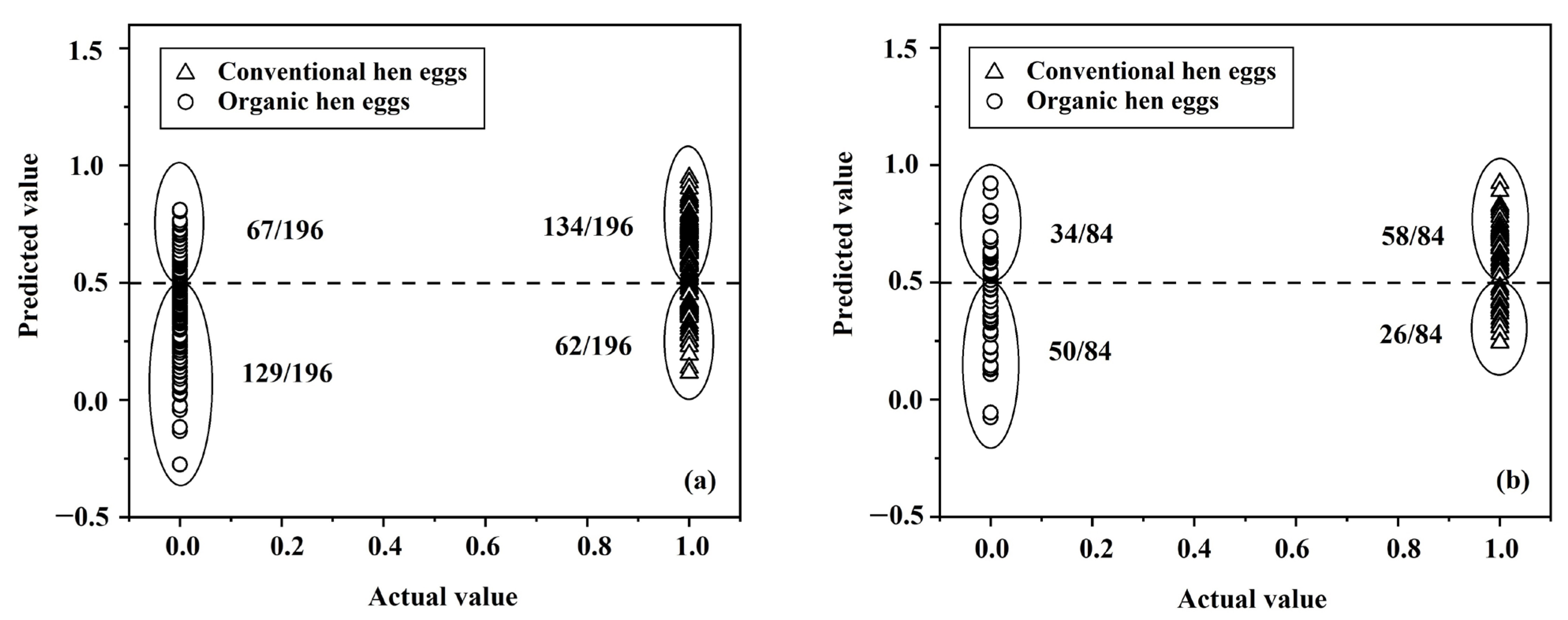
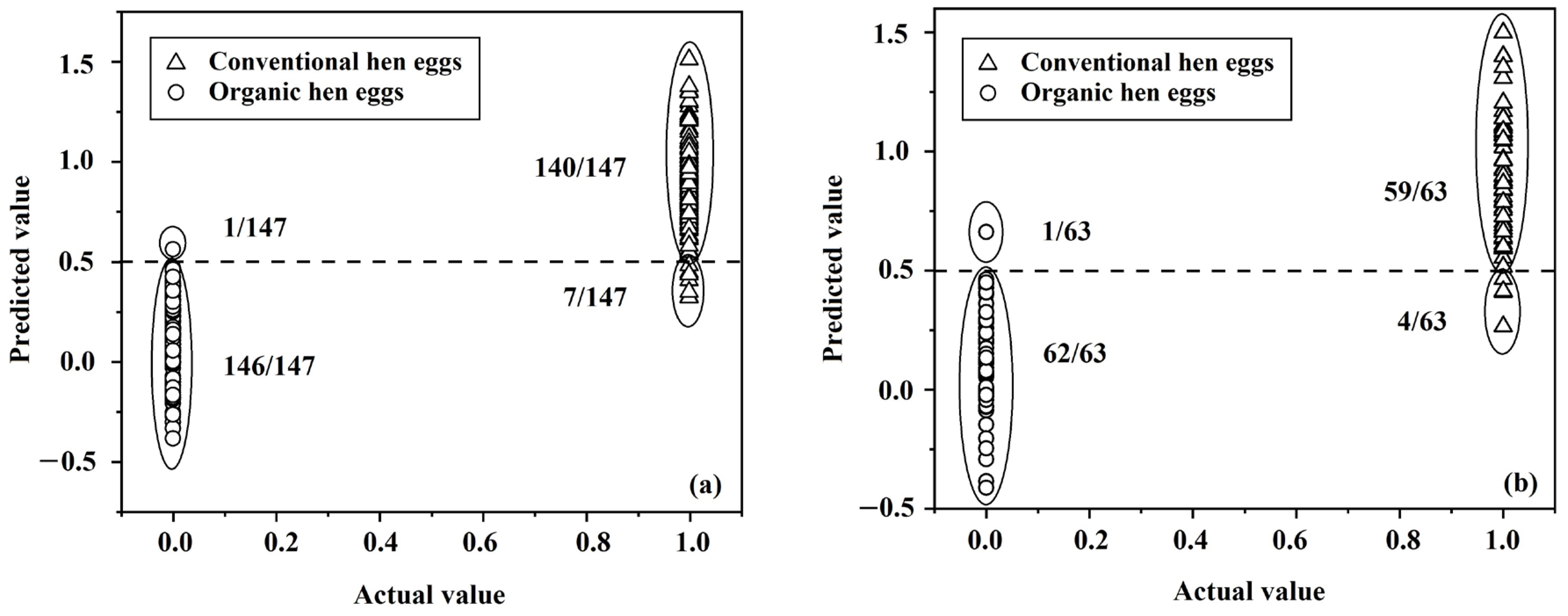
| Model | DATA SET | Conventional Hen Eggs | Organic Hen Eggs | % Accuracy | % Specificity | % Sensitivity | % Error Rate | ||
|---|---|---|---|---|---|---|---|---|---|
| True | False | True | False | ||||||
| (TN) | (FN) | (TP) | (FP) | ||||||
| LDA | Calibration | 134/196 | 62/196 | 129/196 | 67/196 | 67.09 | 67.54 | 66.67 | 32.91 |
| Prediction | 58/84 | 26/84 | 50/84 | 34/84 | 64.29 | 65.79 | 63.04 | 35.71 | |
| Model | Pre-Treatment | Conventional Hen Eggs | Organic Hen Eggs | % Accuracy | % Specificity | % Sensitivity | % Error Rate | ||
|---|---|---|---|---|---|---|---|---|---|
| True | False | True | False | ||||||
| (TN) | (FN) | (TP) | (FP) | ||||||
| SVMC | 1 1st derivative + 2 SNV | 143/147 | 4/147 | 142/147 | 5/147 | 96.94 | 96.62 | 97.26 | 3.06 |
| PLS-DA | 1st derivative + 3 MSC | 140/147 | 7/147 | 146/147 | 1/147 | 97.28 | 99.29 | 95.42 | 2.72 |
| Model | Pre-Treatment | Conventional Hen Eggs | Organic Hen Eggs | % Accuracy | % Specificity | % Sensitivity | %Error Rate | ||
|---|---|---|---|---|---|---|---|---|---|
| True | False | True | False | ||||||
| (TN) | (FN) | (TP) | (FP) | ||||||
| PLS-DA | 1 1st derivative 2 MSC | 59/63 | 4/63 | 62/63 | 1/63 | 96.03 | 98.33 | 93.94 | 3.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahachairungrueng, W.; Thompson, A.K.; Terdwongworakul, A.; Teerachaichayut, S. Non-Destructive Classification of Organic and Conventional Hens’ Eggs Using Near-Infrared Hyperspectral Imaging. Foods 2023, 12, 2519. https://doi.org/10.3390/foods12132519
Sahachairungrueng W, Thompson AK, Terdwongworakul A, Teerachaichayut S. Non-Destructive Classification of Organic and Conventional Hens’ Eggs Using Near-Infrared Hyperspectral Imaging. Foods. 2023; 12(13):2519. https://doi.org/10.3390/foods12132519
Chicago/Turabian StyleSahachairungrueng, Woranitta, Anthony Keith Thompson, Anupun Terdwongworakul, and Sontisuk Teerachaichayut. 2023. "Non-Destructive Classification of Organic and Conventional Hens’ Eggs Using Near-Infrared Hyperspectral Imaging" Foods 12, no. 13: 2519. https://doi.org/10.3390/foods12132519
APA StyleSahachairungrueng, W., Thompson, A. K., Terdwongworakul, A., & Teerachaichayut, S. (2023). Non-Destructive Classification of Organic and Conventional Hens’ Eggs Using Near-Infrared Hyperspectral Imaging. Foods, 12(13), 2519. https://doi.org/10.3390/foods12132519




