3.1. VOC Profile of Stored Potatoes
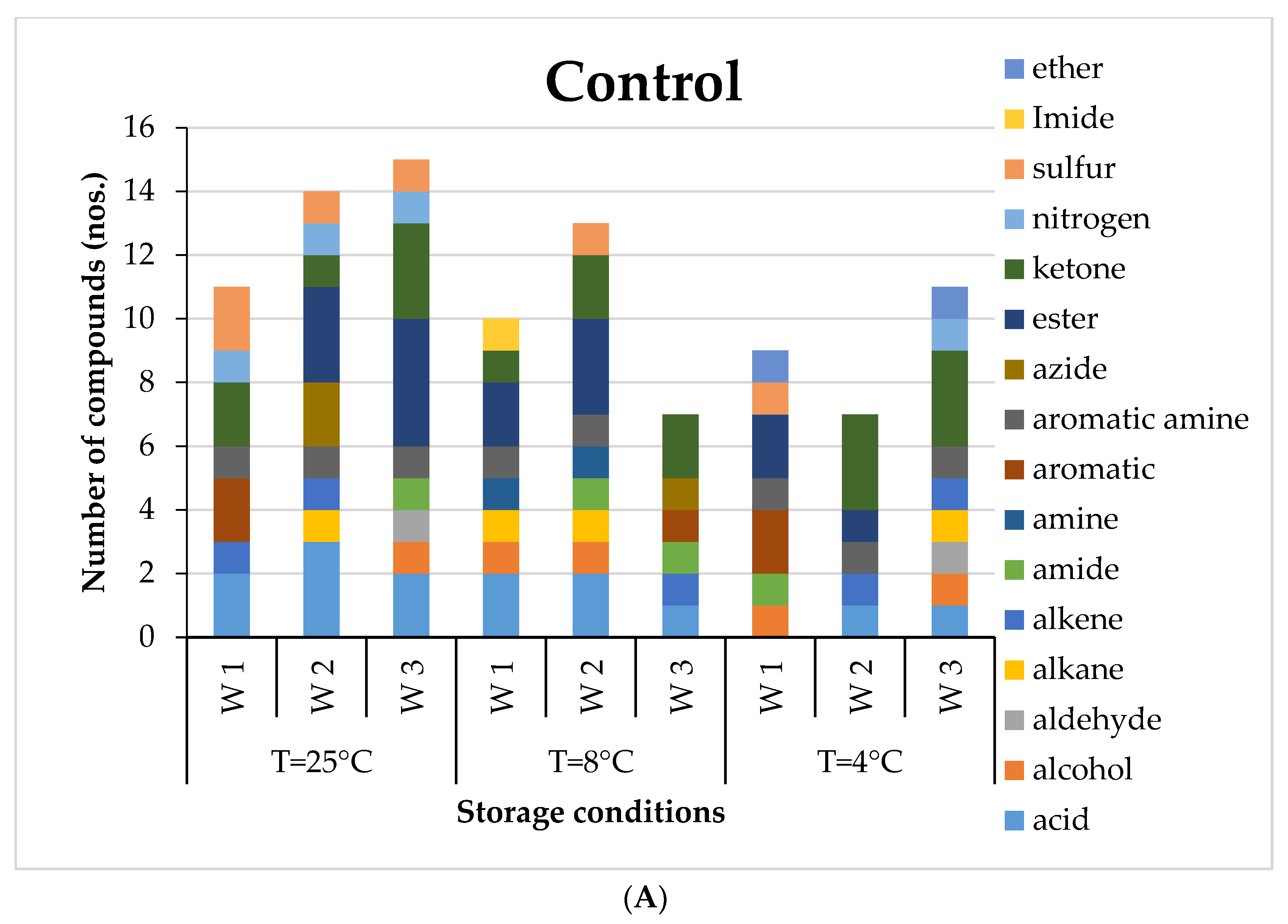
Eleven to twenty-three VOCs were detected in healthy potatoes during storage for three weeks. It can be observed that in the first week of storage, the healthy potatoes had the dominance of aromatic, ester, ketone, sulphur, nitrogen and acid compounds for all storage temperatures (
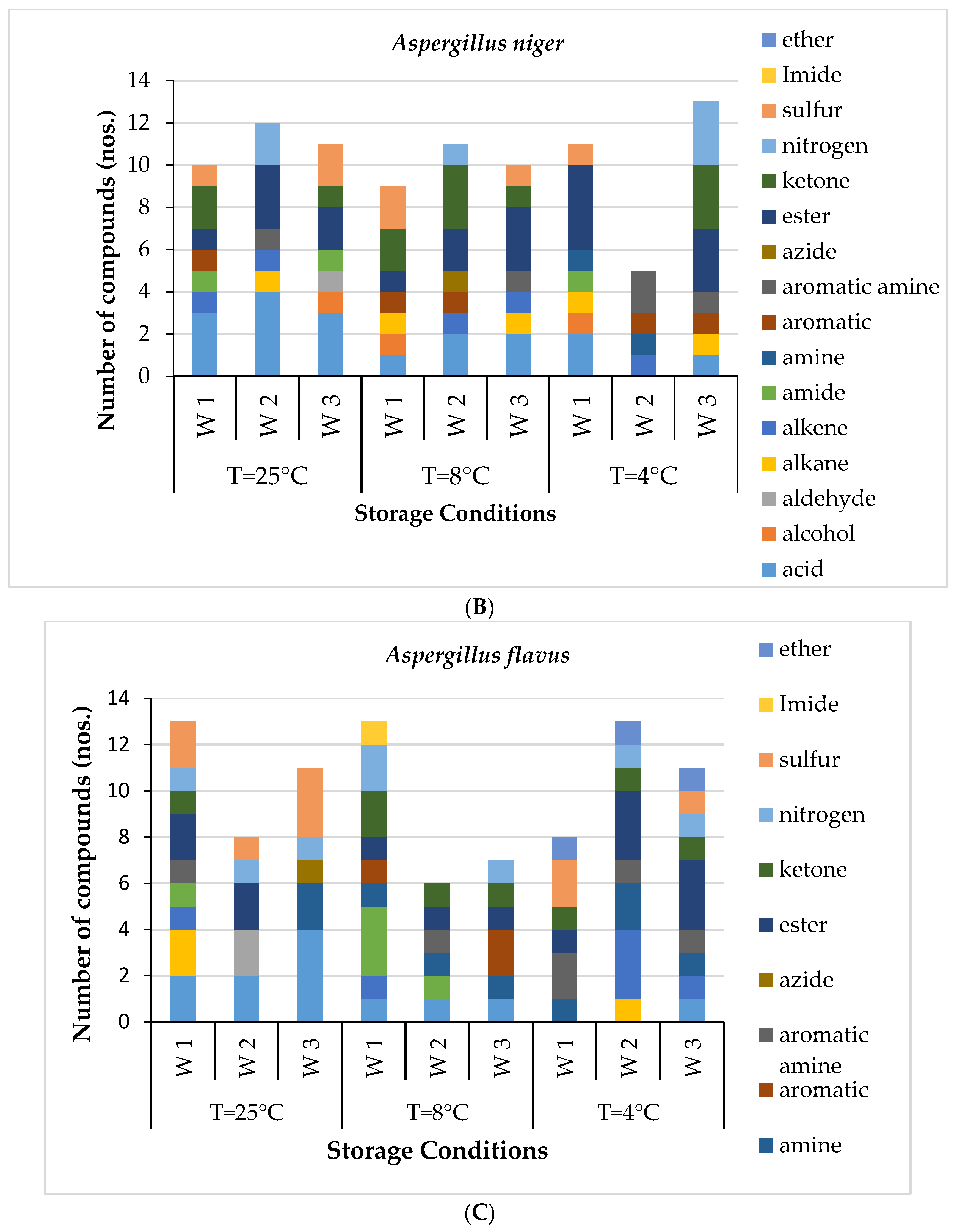
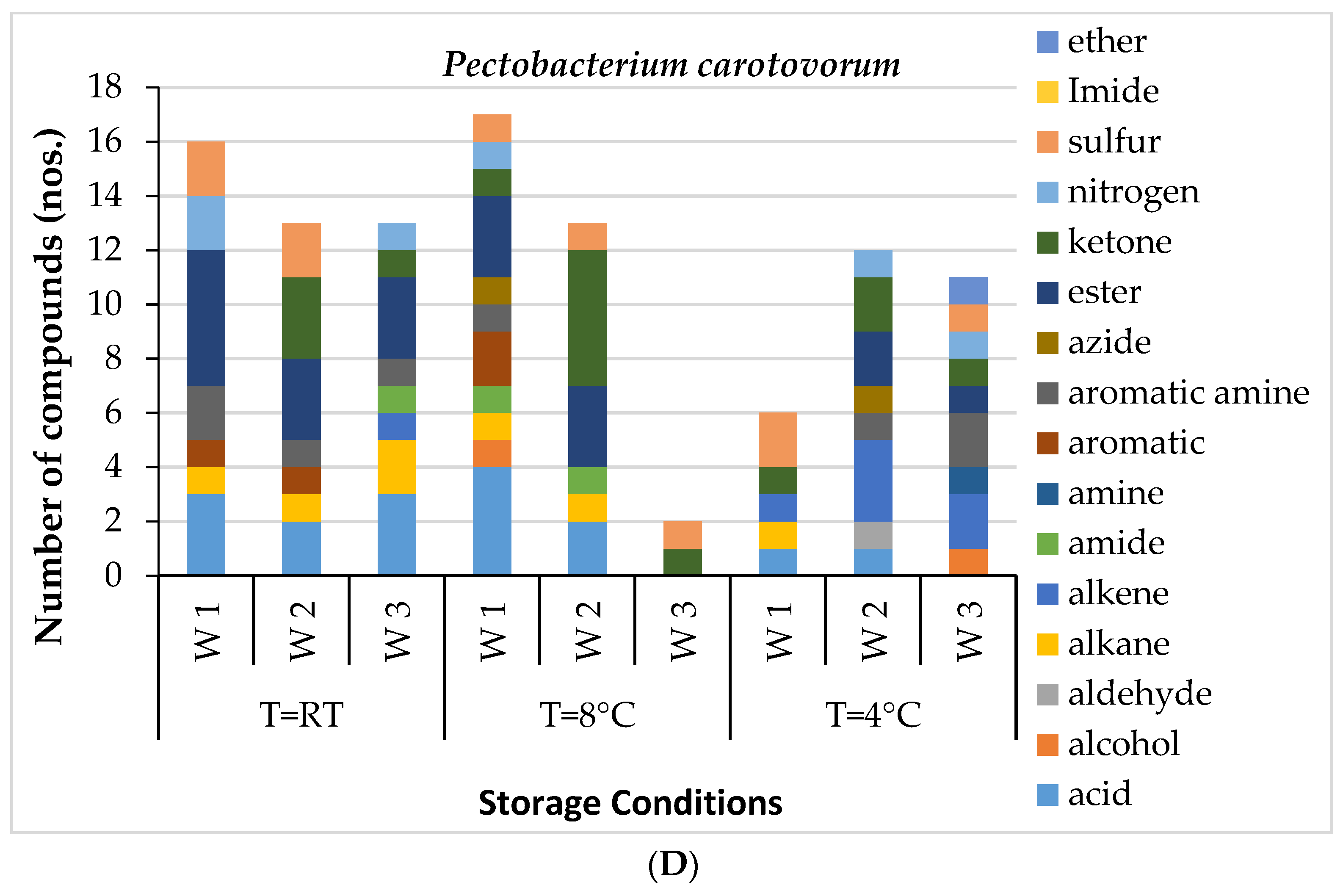
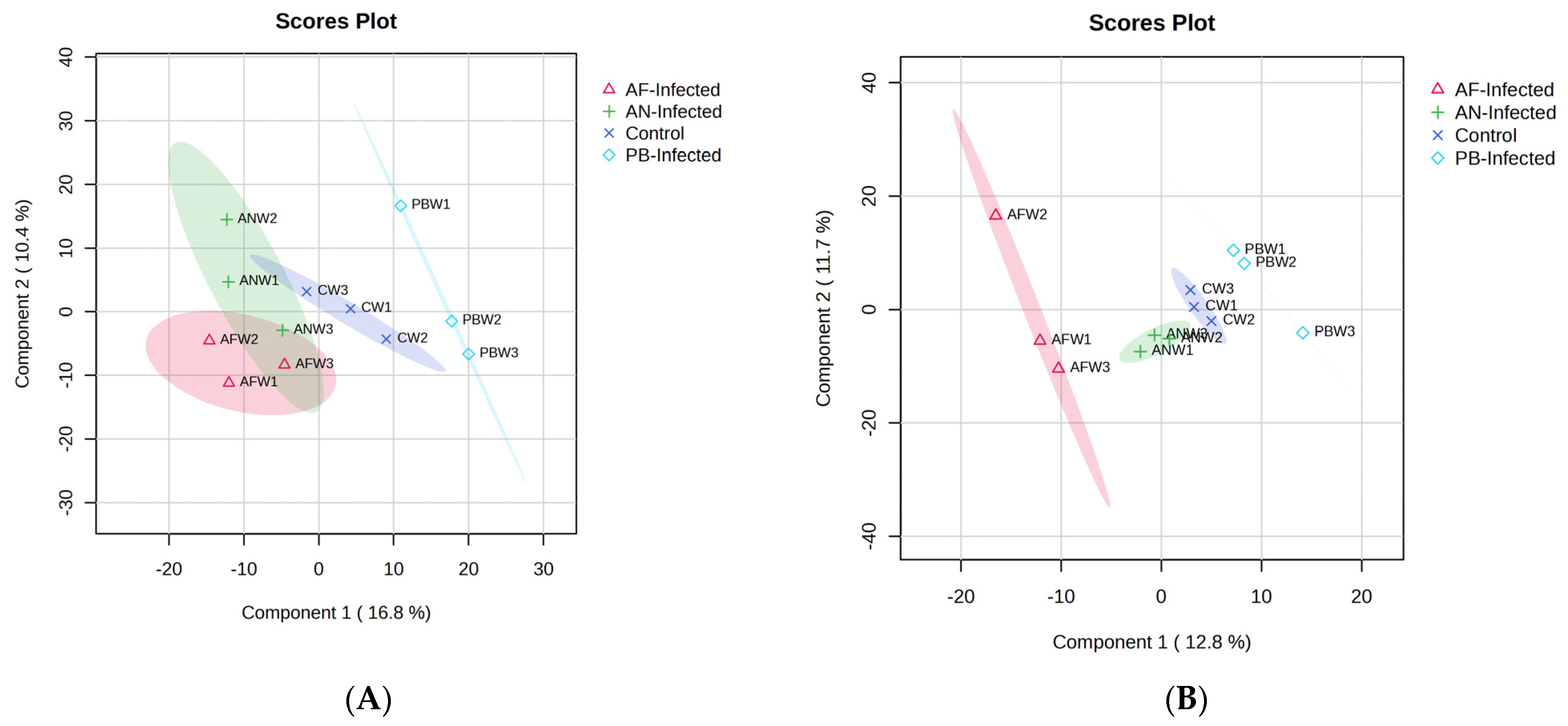
Figure 1A). The most abundant VOC in the healthy potato tubers after the first week of storage was Octadecane, 6-methyl-. However, in the second week, acids, alkane, azide and ester groups were dominant. By the end of three weeks, acids, aldehydes, ketones, alcohol and ester groups had prominence. The GC-MS results of potatoes inoculated with three different microorganisms,
PB,
AN and
AF, revealed various volatile signatures in terms of quantitative and qualitative measures (
Figure 1B–D) with respect to storage duration for different storage temperatures. De Lacy Costello et al. [
12] detected 3,4-dihydro-2H-pyran, 1-methyl-3-propylbenzene, 2-methyl decane, 2,9-dimethyldecane and 2,9-dimethyldodecane in potatoes inoculated with
Erwinia carotovora, which is now known as
Pectobacterium carotovorum; some of these compounds were also detected in the present investigation.
Fungal-inoculated potato tubers easily deteriorated and had early detectable physical symptoms of infection compared to bacterially inoculated potatoes. The volatiles associated with fungal infection were more, and highest (124) in the
AN-inoculated ones. Very high variability of VOCs was observed within the bacterial and fungal infections, as well as two species of selected fungal strains. However, some volatiles that were common to all selected microbial (bacterial and fungal) infections, including butanoic butyl/ethyl ester, hexanoic ethyl/butyl ester and hexadecane, were not detected in healthy potatoes. Some overall (all storage temperatures) discriminative VOCs belonging to the
PB culture were 1-butanol and 1-hexanol, which were also detected by Steglinska et al. [
20]. Their study revealed several VOC biomarkers, such as sesquiterpenes, dimethyl disulfide, 1,2,4-trimethyl benzene, 2,6,11-trimethyl-dodecane, benzothiazole, 3-octanol and 2-butanol, associated with seed potatoes inoculated with
Fusarium sambucinum,
Alternaria tenuissima and
Pectobacterium carotovorum during a three-month storage period. Some volatiles were common to control potato tubers. n,n-dimethylmethylamine, 1-undecene, acetone and styrene were some of those VOCs, which were also the unique VOCs of sweet potato with soft rot disease caused by
Pectobacterium carotovorum [
21].
For AN-inoculated sample, the specific biomarkers observed were alkane, hexadecane, undecane, tetracosane and octadecanoic acid, as well as alkenes such as tridecene and undecene. AF inoculated potato tubers had unique volatile signature acids such as hexadecanoic acid and acetic acid derivatives. Some alcohol derivatives were also observed in the AF-inoculated sample, including undecanol. These observations suggest a correlation between alterations in the internal metabolism of potato tubers inoculated with the selected microbes. For example, high-carbon-number alkanes were associated with the AN-inoculated changes, whereas alcohol groups were associated with the AF-inoculated samples, while in bacterial infection, only low-carbon-number alcohol represented a unique VOC profile. Several dissimilar volatiles were explored between the infections, but only some were considered unique VOC signatures.
The study also revealed volatile discrimination between various storage temperatures (4 ± 1 °C, 8 ± 1 °C, 25 ± 1 °C). In addition, significant variability was observed within different storage conditions in inoculated potato tubers compared to the healthy samples, irrespective of the types of infection.
3.2. Multi-Variate Data Analysis of VOC Using PCA and PLS-DA
The PCA of the multivariable data of VOCs obtained from potato samples inoculated with
PB, AN and
AF and the control group, stored under different storage conditions, is represented in
Figure S1, where the samples were assigned to classify through an unsupervised manner into three distinct classes based on the features of the VOCs belonging to specific groups. Hence, the variability among VOCs was determined in an unsupervised manner, and the fitness of the model was evaluated based on R
2 and Q
2 values. Accordingly, it was observed that the PCA could not classify and distinguish the samples in space through their distribution characteristics, with lower values of both R
2 and Q
2. In the case of the sample stored at 25 °C, the PCA model fitting accounted for 37.4% variance (
Figure S1), in the manner of PC1 contributing 19.1% and PC2 contributing 18.3% of the variance. Further, the model fitting obtained R
2 of 44.7% and Q
2 of 46.4%. Similarly, in the case of VOCs obtained from samples at 8 °C and 4 °C, their respective PC1 explained 16.7% and 25.2%, respectively, while PC2 explained 13.1% and 17.8% of the variance, with R
2 values of 46.4% and 42.9% and Q
2 values of 44.3% and 45.3%, respectively. The VOC clusters of different samples were found to be completely overlapped. However, the extent of overlapping of the clusters was different with respect to the storage conditions.
Further, the data analysis and classification of VOCs were carried out through a supervised model of discriminant features, i.e., partial least squares discriminant analysis (PLS-DA). PLS-DA eliminated the influence of uncontrolled variables, and the model was fitted to the normalized experimental data (
Figure 2).
Figure 2A shows the graphical representation of the PLS-DA model fit with the score to the non-infected and inoculated potatoes with selected microbial species stored at 25 °C. As per the score plot, the selected two principal components explained 27.2% of the data variance. The PLS-DA model shows the best fit with a higher value of R
2 (97.6%) and Q
2 (70.2%) to the VOC data. The position of the points on the graphs represent the respective storage period and a particular type of infection with the samples.
The score plot was drawn to study the classification features among the four distinct groups of the samples. From
Figure 2A, it was observed that there was a distinct separation among all three inoculated samples from the control sample without any infection. Among the infections, the
PB infection of shows a distinct separation from the other two (
AN and
AF) fungal infections. A slight overlap was observed between the data clusters of
AN-inoculated and
AF-inoculated samples, especially among the points representing elapsed storage time. Interestingly, the points representing control, non-inoculated tubers during the initial storage time (first two weeks) were located in the rectangular part of PC1 > 0 and PC2 < 0. With a further increase in storage time, the position shifted to the rectangle representing PC1 < 0 and PC2 > 0. A similar trend of shifting of points was also observed in the
AN-inoculated sample, i.e., points representing the first two weeks were present at PC1 < 0 and PC2 > 0, while at week 3, samples were presented at PC1 < 0 and PC2 < 0. The smallest shift of position was observed in the
AF-inoculated samples among the storage periods, i.e., all points located at PC1 < 0 and PC2 < 0. The highest contribution of PC1 was shown in the non-inoculated control sample, whereas the
PB-inoculated sample contributed the most to PC2.
The best-fit PLS-DA model was further validated through applying a permutation test (200 permutations) to the data and checking its reliability. As per the test results, the model was found to be reliable with R
2 = (0.0, 096.7%) and Q
2 = (0.0, 18.6%) as intercepts [
22].
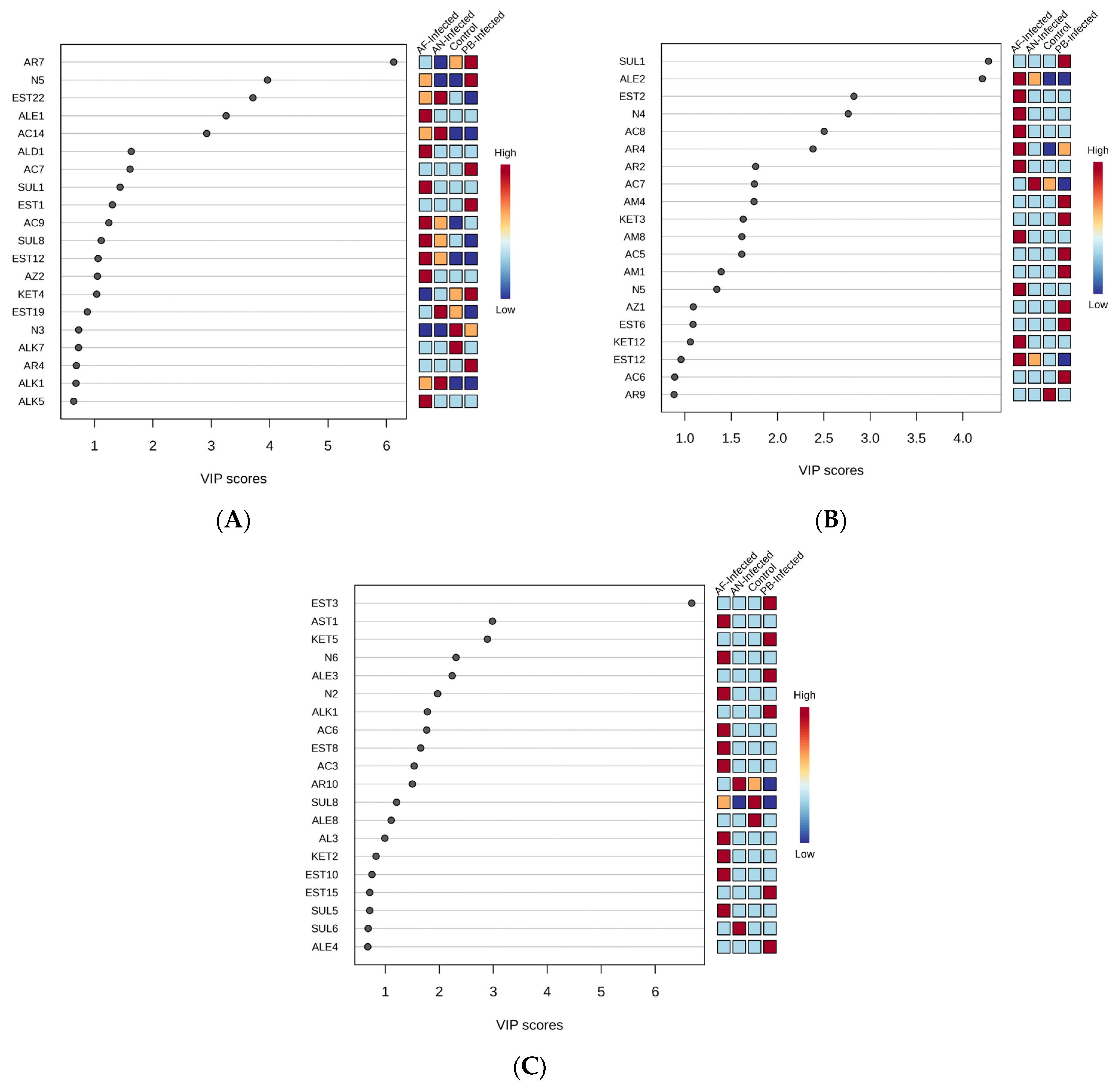
Figure 3A represents the VIP score plot of the VOCs in which the compounds having VIP score > 2 and
p < 0.05 were the key contributors to the discrimination of control and inoculated samples among themselves based on the PLS-DA model.
Similarly, for the experimental potato samples stored at 8 °C,
Figure 2B represents the model fit score plot of the PLS-DA fitted to VOC data recorded from the experimental samples of stored potatoes. It was observed that component 1 of the score plot explained more variance (12.7%) than component 2 (11.7%); both components combined explained about 24.5%. The PLS-DA model best fits the VOC data with an R
2 value of 97.2% and Q
2 value of 41.0%.
Figure 2B reveals that there was a distinct separation among all four types of samples (control and inoculated) taken for storage. The
AF-inoculated sample occupied the largest size of the cluster while it was comparatively very small, with the samples stored at 25 °C. The sample inoculated with
PB showed distinct separation from the other two fungal infections (
AN and
AF) with a defined and unique trend of change in VOC with respect to storage time. The considerable change in PC2 values of
AF-inoculated,
PB-inoculated and control samples, as well as slight changes in PC1 values of
AN-inoculated samples, were observed due to the changes in the profile in terms of concentration as well as the type of VOC with respect to the change in the storage period. The shift of points on the score plot represented the change in the respective PC values. Such a shift of points representing VOCs generated at a particular storage time was found to be the highest in
AF-inoculated samples, followed by
PB-inoculated and control. Accordingly, the points representing
AF-inoculated tubers after the first week of storage time were located in the rectangular part of PC1 < 0 and PC2 < 0. After a further increase in storage time, the position is shifted to the rectangle representing PC1 < 0 and PC2 > 0 and again shifted to the rectangle representing PC1 < 0 and PC2 < 0 with a change in PC values. Such a typical type of shift might be due to the change in the concentration of the group of VOCs as well as new secondary metabolites generated during temperature-influenced changes in metabolic processes. A similar but reverse trend of shifting of points was also shown in the control sample, i.e., points representing the first two weeks were present at PC1 > 0 and PC2 < 0 while the weeks 3 sample was at PC1 > 0 and PC2 > 0. The smallest shift of position was observed in the
AN-inoculated samples among the storage period, i.e., mostly located at PC1 < 0 and PC2 < 0 and slightly shifted to PC1 > 0 and PC2 < 0. The PC-inoculated sample showed the unique trend of a decrease in PC2 value while an increase in PC1 value with respect to an elapsed storage period of up to three weeks. The change in position of the points within the cluster of control and
AN-inoculated samples is found to be much less than the
AF and
PB inoculated samples. On the other hand, the Euclidean distance between the points present in the clusters of
AF and
PB-inoculated samples was higher, which refers to the significantly higher rate of change in the VOC profile of inoculated tubers than control and
AN-inoculated samples over the storage time. The best model was further validated through the 200-permutation-based test in which the model was found to be reliable with an R
2 intercept of (0.0, 99.1%) and a Q
2 intercept of (0.0, 38.4%), and both lines show a slope greater than zero [
23]. The VOC compounds showing VIP score > 2 and
p < 0.05 in
Figure 3B were the key discriminating VOCs for the PLS-DA-based classification of the selected four types of samples.
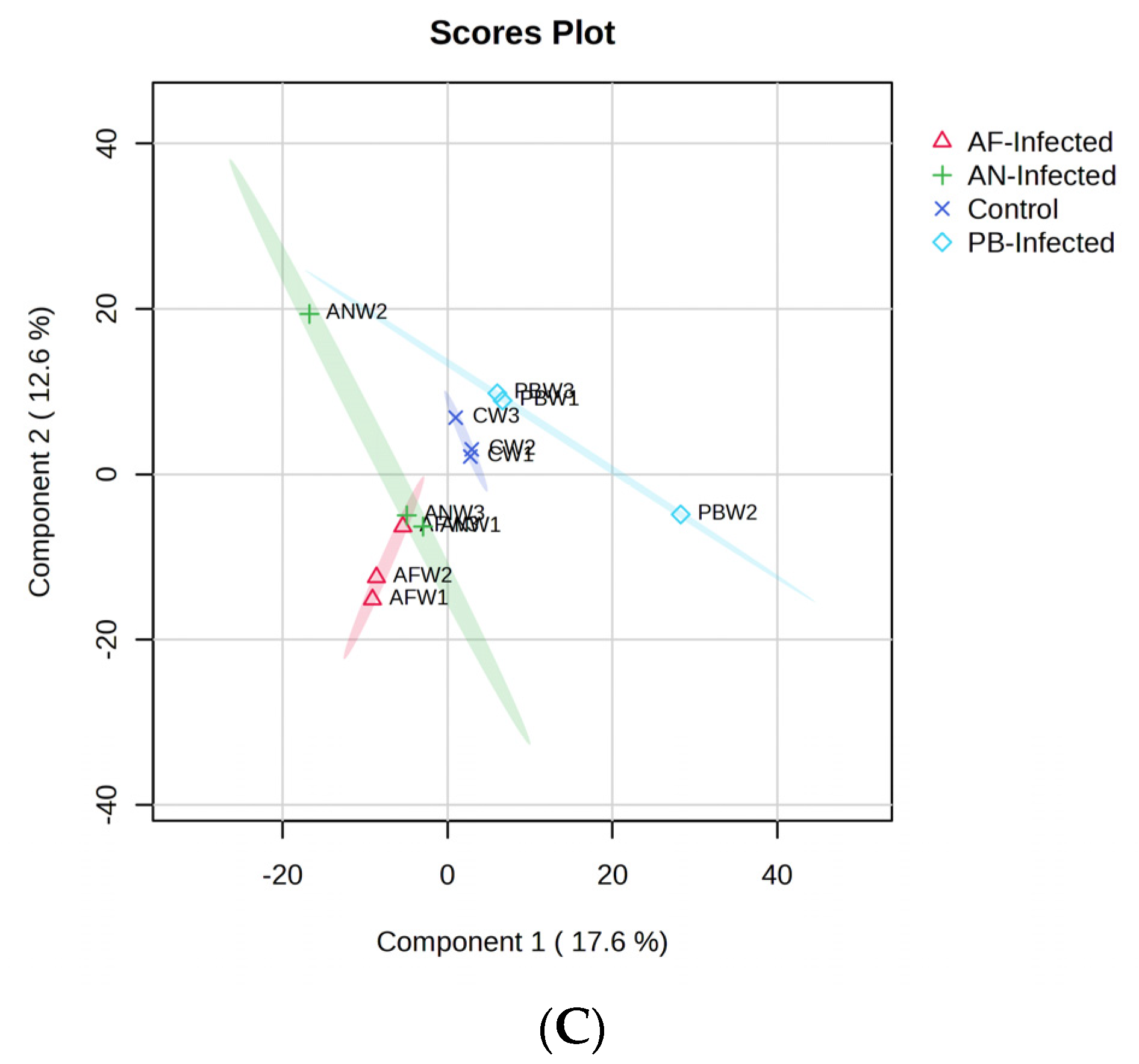
Figure 2C represents the PLS-DA model fit utilized for the VOC-based classification of the potato sample with the selected type of infection. The first two PCs of the score plot represent 30.2% of the total variance. The PLS-DA model was best fitted to the VOC data with higher values of R
2 (96.4%), and Q
2 (65.4%) shows its best reliability and predictability.
Figure 2C shows that there was a distinct separation among all three inoculated samples among themselves as well as with the control. A slight overlap between the clusters of
AN-inoculated and
AF-inoculated samples was observed, especially among the points which represented the end of the storage period, i.e., the third week. Overlapping was also observed within the individual cluster among the points representing VOCs generated with respect to storage time. The overlapping might be due to the similar type of VOC emanating through a similar type of biochemical reaction and metabolite formation because of the same class of infection, i.e., fungal species. Here, for samples stored at 4 °C, the
AN-inoculated sample occupied the largest size of the cluster, while it was comparatively very small when the samples were stored at 8 °C. A similar type of reverse trend was also observed with the
AF- and
PB- inoculated samples compared with 8 °C of storage temperature. The increased size of the cluster of
AN- and
PB-inoculated samples might be due to the higher number of discriminating VOCs obtained when the sample was stored at 4 °C.
A considerable change in PC1 as well as PC2 values of
AN-inoculated,
PB-inoculated and
AF-inoculated samples, compared to slight changes in PC2 values of the control sample, were observed within the cluster due to changes in the VOC profile with respect to the change in storage time. The shift of points on the score plot represents the change in respective PCA values. Such a shift of points representing VOCs generated at a particular storage time was found to be highest in the
AN-inoculated sample, followed by the
PB-inoculated and
AF-inoculated samples. Therefore, in the cluster representing
AN-inoculated tubers, the points representing the first week and third week of storage time were located in the rectangular part of PC1 < 0 and PC2 < 0, while they were shifted into the rectangle representing PC1 < 0 and PC2 > 0 during the storage time of 2 weeks. Such an intermediate shift might be due to the formation of temporary metabolites, which may further be degraded into parent types of metabolites. An exactly similar type of shifting of points was also observed in the case of the sample inoculated with
PB, i.e., points representing the first and third week were present at PC1 > 0 and PC2 > 0, while the second-week sample was present at PC1 > 0 and PC2 < 0. There was no shift of position of the points in the clusters of the
AF-inoculated sample and control sample. The Euclidean distance between the points present in the clusters of
AN- and
PB-inoculated samples was higher, and hence the VOC profile of these samples changed at a significantly higher rate than control and
AF-inoculated samples over the storage time. The best model was further validated through the 200-permutation-based test in which the model was found to be reliable with an R
2 intercept of (0.0, 97%) and Q
2 intercept of (0.0, 25.6%), and both lines show a slope greater than zero [
24]. The VOC compounds that show a VIP score > 2 in
Figure 3C were the key discriminating VOCs for the PLS-DA-based classification of the selected four types of samples.
3.3. Clustered-Heat-Map-Based Prominence of Volatome
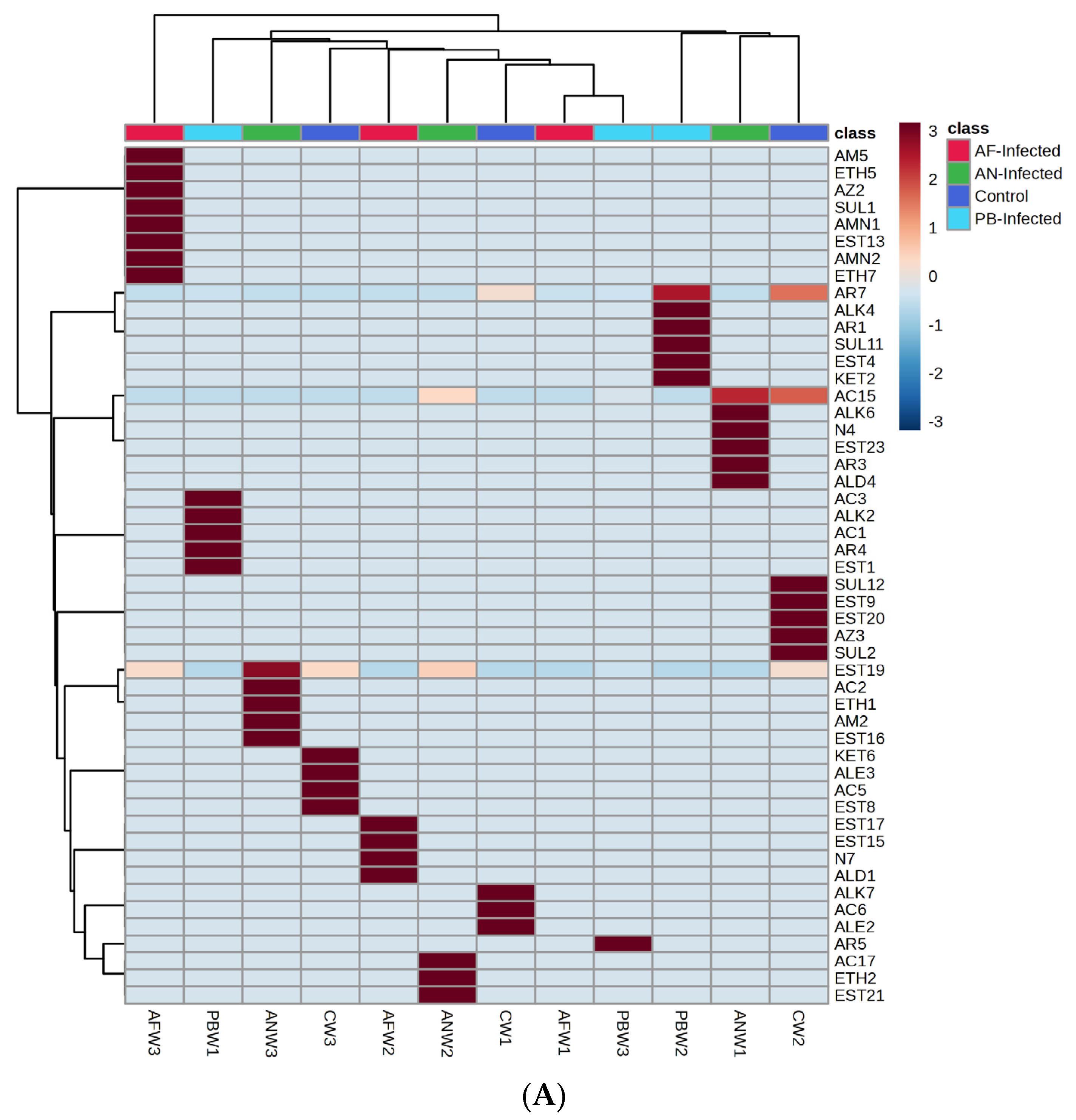
Groups of VOC-based heat maps with hierarchical clustering simultaneously applied to both rows and columns were prepared using Ward’s method and are represented in
Figure 4. The intermediate values of the concentrations were based on their proximity to the maximum or minimum value. For the generation of the said clustered heat map, the VOC data were arranged in the form of their parent group and a unique number of individual VOCs. Further, the numerical data of the VOCs were normalized, and this allowed for clustering and colour scaling based on the concentration of the particular compound. Further, the group of compounds that appeared in a dark brown colour scale on the heat map were considered the dominant group of VOC for instantaneous storage conditions (type of infection, storage temperature and storage period).
Accordingly,
Figure 4A represents the VOC groups’ clustered heat map emanating and mapping from the stored potato samples at 25 °C.
Figure 4A reveals that, for the control non-inoculated samples, the VOCs belonging to the groups of alkane, alkene and acids were dominant in the first week. This dominance was found to be shifted towards the groups of ketone, alkene, acids and esters by the third week of storage. The same group repeated with an increase or decrease in their concentration due to changes in metabolic processes, while the existence of the new dominant group might be due to the formation of new metabolites due to changes in metabolic pathways. In a similar way, in the case of potato samples inoculated with
Pectobacterium carotovorum, acids, alkane, esters and aromatic compounds were mapped as dominant VOC groups at the first week, while at three weeks, the VOCs’ dominance remained for aromatic compounds only.
The dominance of aromatic compounds, acids and esters observed in the first week of storage time shifted to the amide, ether, azide, amine and sulphur groups of compounds in the third week of the storage time when the potato sample was infested with AF. Dominant compounds were also found to be changed with a change in species of fungal infection; likewise, for the AN-inoculated potato sample, acid, alkane, nitrogen, ester, aromatics and aldehydes were the dominant groups of VOC compounds mapped at one week of storage period, while they shifted to ester, acid, ether, amide and ester after three weeks of storage.
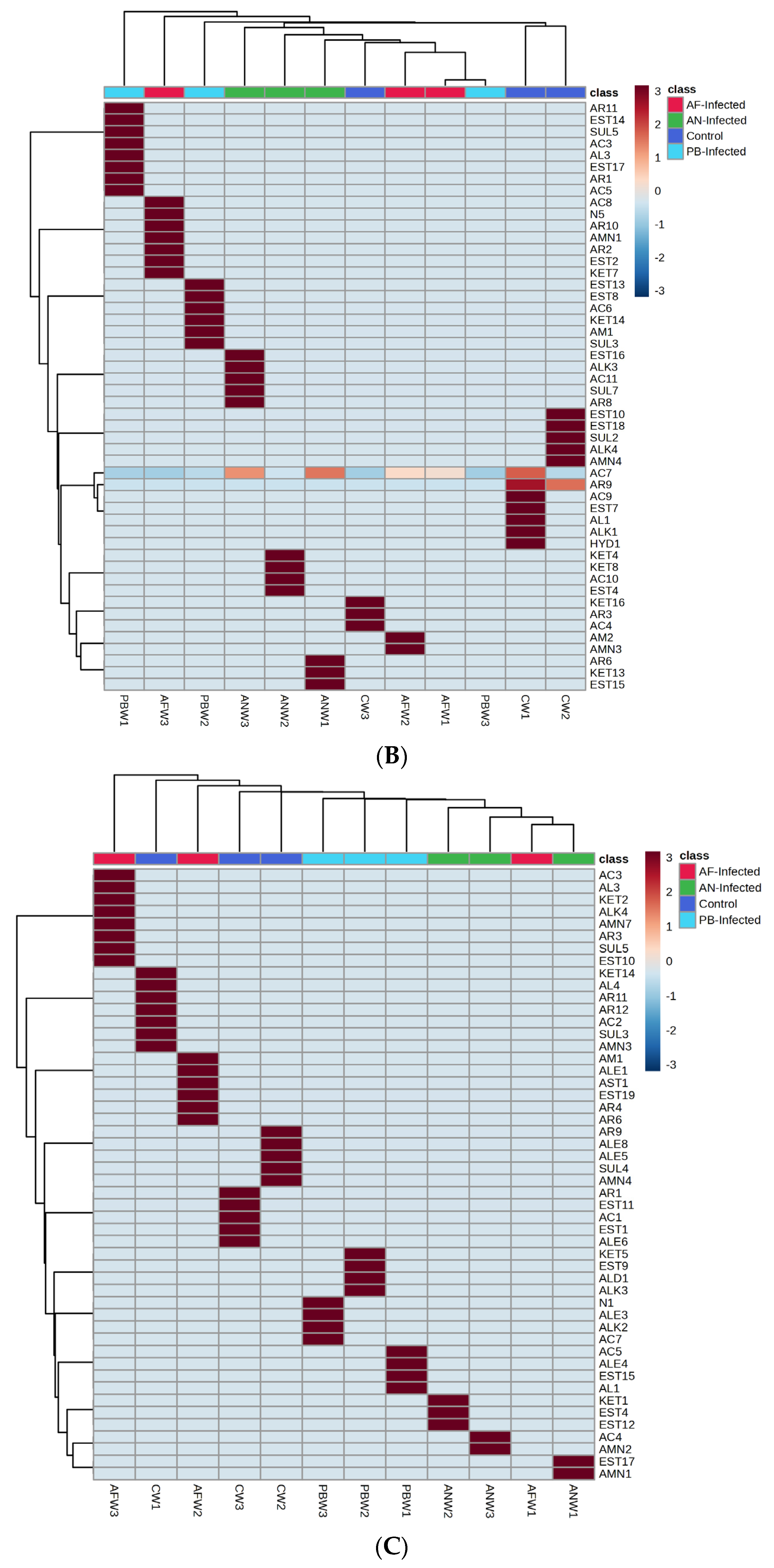
The dominant VOC emanating from the stored samples also varied with respect to the storage temperature.
Figure 4B shows the heat map of the VOC groups mapped from the potato samples stored at 8 °C. The heat map shows that, in the control (CW1) sample, acids, aromatic, ester, alkane, alcohol and hydrazine were the dominant groups in the first week of the storage period, while it was reduced into ketone, aromatic and acids when storage time reached up to three weeks. The sample inoculated with
PB shows aromatic, ester, sulphur, acid and alcohol as dominant VOC groups when mapped at the first week (PBW1) of storage time and gets decomposed due to various metabolic changes in such a way that only acids remained as the dominant VOC group after up to three weeks (PBW3) of storage time. At the same time, the
AN-inoculated sample after one week (ANW1) of storage time especially emitted VOCs belonging to the groups of aromatic, ketone and esters as dominant groups, and after various metabolic processes, they were shifted to esters, alkane, acids, sulphur and aromatic compounds after up to three weeks (ANW3) of storage time. Acid was the only dominant group of VOCs observed at one week (AFW1) of the storage period of the
AF-inoculated potato sample, while after up to three weeks (AFW3) of storage time, nitrogen, aromatic, amine, ester and ketone were added with acids as dominant groups of VOCs.
The smallest number of VOCs was recorded and mapped from the potato samples stored at 4 °C, while the dominance of particular types of VOC groups was found to be different with respect to the type of infection as well as the duration of storage. The profile of the VOC groups recorded from potato samples stored at 4 °C is represented in
Figure 4C in the form of a clustered heat map. From the heat map, it was revealed that for the control sample, ketone, alcohol, acid, sulphur, amines and aromatic compound groups were most dominant in the first week (CW1) of the storage period and further shifted to the groups of esters, acids, alkene and aromatics at three weeks (CW3) of storage time. In a similar way, in the case of potato samples inoculated with
PB, acids, alkene, esters and alcohol were mapped as dominant VOC groups at the first week (PBW1), while at up to three weeks (PBW3) of storage time, the compound dominance remained and shifted towards acid, alkane, alkene and nitrogen groups of VOCs. Among the inoculated fungal samples, the
AF-inoculated sample after one week (AFW1) of storage time shows alcohol only as the dominant VOC group, and due to various metabolic processes after up to three weeks (ANW3), the dominance of VOCs shifted towards acids, alcohol, ketone, alkane, amine, sulphur and aromatic compounds. A highly specific type of group was found in the case of
AN-inoculated samples: likewise, esters and amines at the first week (ANW1) of storage time and acids with amines at the third week (ANW3) of storage time.