Lower Health Risks of Potentially Toxic Metals after Transplantation of Aquacultural Farmed Mussels from a Polluted Site to Unpolluted Sites: A Biomonitoring Study in the Straits of Johore
Abstract
1. Introduction
2. Materials and Methods
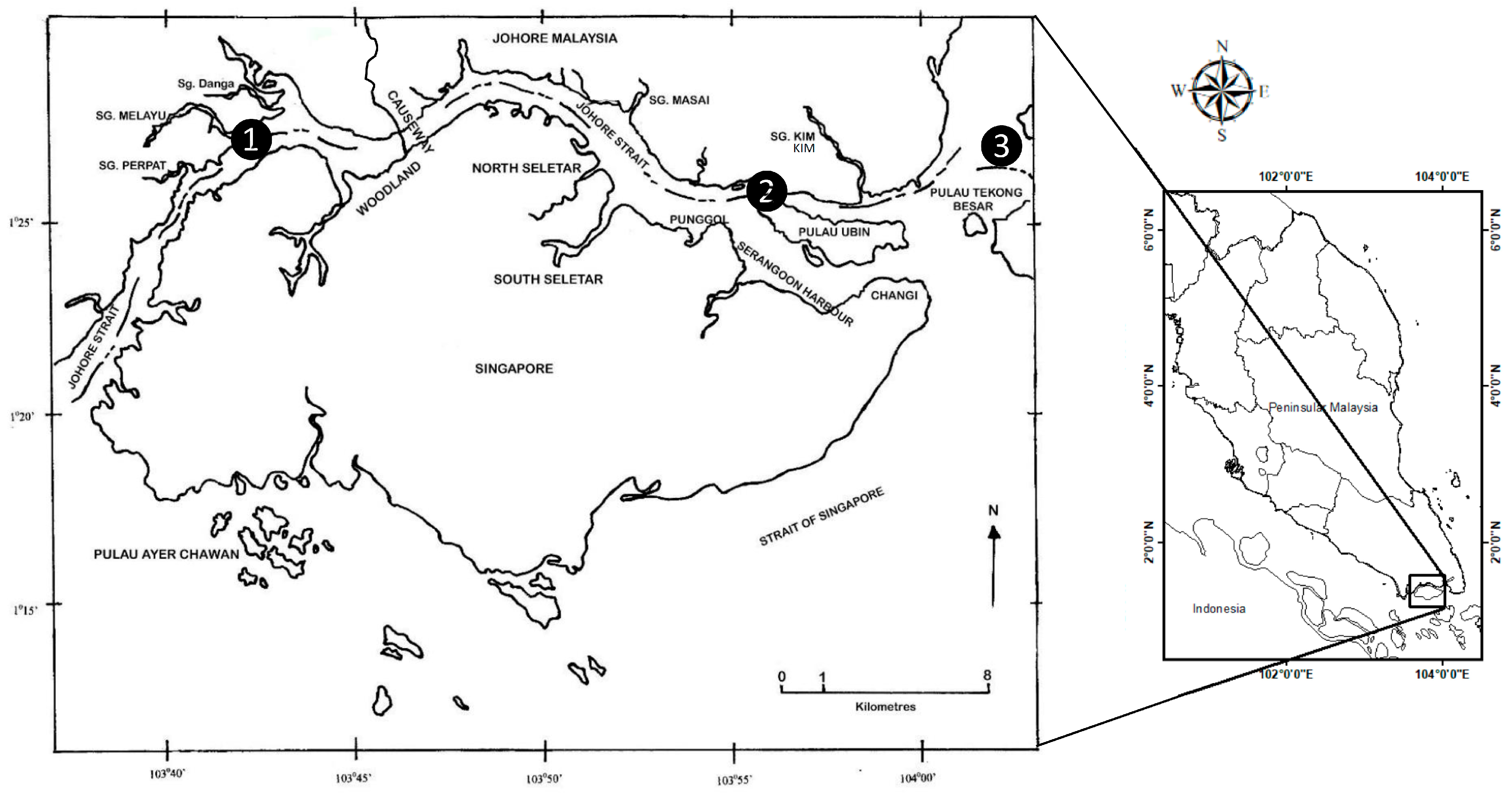
2.1. Study Sites of Transplantations
2.2. Mussel Population Transplants
2.3. Quality Monitoring and Assurance
2.4. Data Treatment
Human Health Risk Assessments
- (a)
- Direct comparisons with seafood safety guidelines
- (b)
- Target hazard quotient
- (c)
- Comparisons between estimated weekly intake (EWI) and provisional tolerable weekly intake (PTWI):
2.5. Statistics Analysis
3. Results
3.1. Ni
3.1.1. Comparison with Reported Studies and Food Safety Guidelines of Nickel
3.1.2. Comparisons of Nickel Target Hazard Quotients
3.1.3. Comparisons between Estimated Weekly Intake (EWI) and Provisional Tolerable Weekly Intake (PTWI)
3.2. Cd
3.2.1. Cd Safety Guidelines
3.2.2. Comparisons of Cadmium Target Hazard Quotients
3.2.3. Comparisons between Cadmium Estimated Weekly Intake (EWI) and Provisional Tolerable Weekly Intake (PTWI)
3.3. Pb
3.3.1. Pb Safety Guidelines and Comparison with Reported Studies
3.3.2. Comparisons of Lead Target Hazard Quotients
3.3.3. Comparisons between Pb Estimated Weekly Intake (EWI) and Provisional Tolerable Weekly Intake (PTWI)
3.4. Cu
3.4.1. Cu Safety Guidelines and Comparison with Reported Studies
3.4.2. Comparisons of Copper Target Hazard Quotients
3.4.3. Comparisons between Cu Estimated Weekly Intake (EWI) and Provisional Tolerable Weekly Intake (PTWI)
3.5. Fe
3.5.1. Fe Safety Guidelines and Comparison with Reported Studies
3.5.2. Comparisons of Fe Target Hazard Quotients
3.5.3. Comparisons between Fe Estimated Weekly Intake (EWI) and Provisional Tolerable Weekly Intake (PTWI)
3.6. Zn
3.6.1. Zn Safety Guidelines and Comparison with Reported Studies
3.6.2. Comparisons of Zinc Target Hazard Quotients
3.6.3. Comparisons between Zn Estimated Weekly Intake (EWI) and Provisional Tolerable Weekly Intake (PTWI)
4. General Discussion
4.1. Lower Metal Levels Are Expected and Well-Supported by the Literature
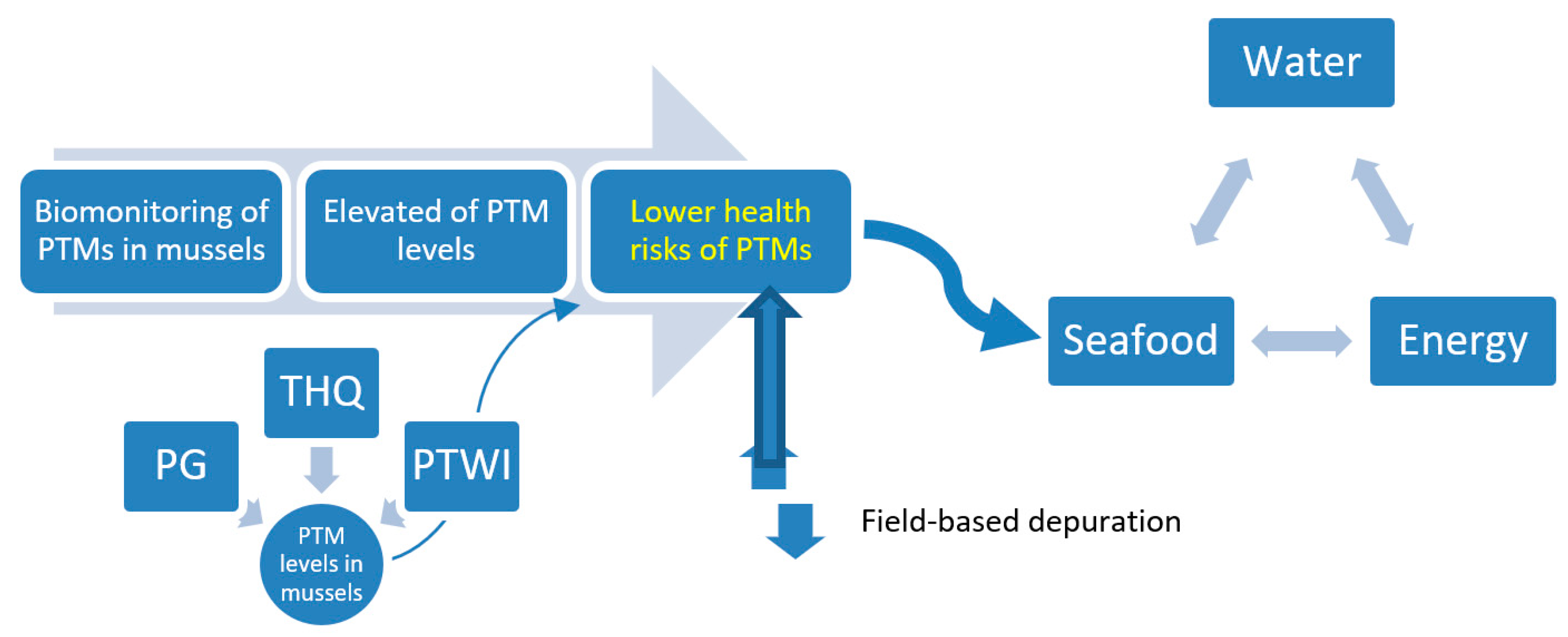
4.2. Seafood–Energy–Water Nexus: A Food Safety Concern in the Straits of Johore
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AFS. Annual Fisheries Statistics of Malaysia Department of Fisheries Malaysia. 2021. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 25 February 2023).
- AFS. Annual Fisheries Statistics of Malaysia Department of Fisheries Malaysia. 2001. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 25 February 2023).
- Malaysia Mussel Prices. 2023. Available online: https://www.selinawamucii.com/insights/prices/malaysia/mussels/ (accessed on 27 April 2023).
- Nordin, R. January Harvest for Sungai Johor Mussel Farm. The Star. 30 November 2022. Available online: https://www.thestar.com.my/metro/metro-news/2022/11/30/january-harvest-for-sungai-johor-mussel-farm (accessed on 25 February 2023).
- Yap, C.K.; Ismail, A.; Tan, S.G.; Omar, H. Correlations between speciation of Cd, Cu, Pb and Zn in sediment and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environ. Int. 2002, 28, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K. Mussel Watch in Malaysia Past, Present and Future; UPM Press: Serdang, Malaysia, 2012. [Google Scholar]
- Faverney, C.R.; Guibbolini-Sabatier, M.E.; Francour, P. An ecotoxicological approach with transplanted mussels (Mytilus galloprovincialis) for assessing the impact of tyre reefs immersed along the NW Mediterranean Sea. Mar. Environ. Res. 2010, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Emmanouil, C. Advanced PAH pollution monitoring by bivalves. Environ. Chem. Lett. 2015, 13, 395–411. [Google Scholar] [CrossRef]
- Phillips, D.J.H. The chemistries and environmental fates of trace metals and organchlorines in aquatic ecosystems. Mar. Pollut. Bull. 1995, 31, 193–200. [Google Scholar] [CrossRef]
- Regoli, F.; Orlando, E. Seasonal variation of trace metal concentrations (Cu, Fe, Mn, Pb, Zn) in the digestive gland of the Mediterranean mussel Mytilus galloprovincialis: Comparison between a polluted and a non polluted site. Arch. Environ. Contam. Toxicol. 1994, 27, 36–43. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, T.; Wang, J.; Wang, Z.; Li, P.; Lai, Y.; Sanganyado, E.; Liu, W. Integrated assessment of heavy metal pollution using transplanted mussels in eastern Guangdong, China. Environ. Pollut. 2018, 243, 601–609. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Grilo, T.F.; Reis, A.T.; Coelho, J.P.; Pereira, E.; Pardal, M.A. Field transplantation of the bivalve Scrobicularia plana along a mercury gradient in Ria de Aveiro (Portugal): Uptake and depuration kinetics. Sci. Tot. Environ. 2015, 512–513, 55–61. [Google Scholar] [CrossRef]
- Schintu, M.; Durante, L.; Maccioni, A.; Meloni, P.; Degetto, S.; Contu, A. Measurement of environmental trace-metal levels in Mediterranean coastal areas with transplanted mussels and DGT techniques. Mar. Pollut. Bull. 2008, 57, 832–837. [Google Scholar] [CrossRef]
- Andral, B.; Galgani, F.; Tomasino, C.; Bouchoucha, M.; Blottiere, C.; Scarpato, A.; Benedicto, J.; Deudero, S.; Calvo, M.; Cento, A.; et al. Chemical contamination baseline in the Western basin of the Mediterranean sea based on transplanted mussels. Arch. Environ. Contam. Toxicol. 2011, 61, 261–271. [Google Scholar] [CrossRef]
- Tsangaris, C.; Kaberi, H.; Catsiki, V.A. Metal levels in sediments and transplanted mussels in Pagassitikos Gulf (Aegean Sea, Eastern Mediterranean). Environ. Monitor. Assess. 2013, 185, 6077–6087. [Google Scholar] [CrossRef]
- Turja, R.; Soirinsuo, A.; Budzinski, H.; Devier, M.H.; Lehtonen, K.K. Biomarker responses and accumulation of hazardous substances in mussels (Mytilus trossulus) transplanted along a pollution gradient close to an oil terminal in the Gulf of Finland (Baltic Sea). Comp. Biochem. Physiol C Toxicol. Pharmacol. 2013, 157, 80–92. [Google Scholar] [CrossRef]
- Zimmer, L.A.; Asmund, G.; Johansen, P.; Mortensen, J.; Hansen, B.W. Pollution from mining in South Greenland: Uptake and release of Pb by blue mussels (M. edulis L.) documented by transplantation experiments. Polar Biol. 2011, 34, 431–439. [Google Scholar] [CrossRef]
- Hedouin, L.; Pringault, O.; Bustamante, P.; Fichez, R.; Warnau, M. Validation of two tropical marine bivalves as bioindicators of mining contamination in the New Caledonia lagoon: Field transplantation experiments. Water Res. 2011, 45, 483–496. [Google Scholar] [CrossRef]
- Hunt, C.D.; Slone, E. Long-term monitoring using resident and caged mussels in Boston Harbor yield similar spatial and temporal trends in chemical contamination. Mar. Environ. Res. 2010, 70, 343–357. [Google Scholar] [CrossRef]
- Giarratano, E.; Duarte, C.A.; Amin, O.A. Biomarkers and heavy metal bioaccumulation in mussels transplanted to coastal waters of the Beagle Channel. Ecotoxicol. Environ. Saf. 2010, 73, 270–279. [Google Scholar] [CrossRef]
- Yap, C.K.; Ismail, A.; Edward, F.B.; Tan, S.G.; Siraj, S.S. Use of different soft tissues of Perna viridis as biomonitors of bioavailability and contamination by heavy metals (Cd, Cu, Fe, Pb, Ni, and Zn) in a semi-enclosed intertidal water, the Johore Straits. Toxicol. Environ. Chem. 2006, 88, 683–695. [Google Scholar] [CrossRef]
- Bayen, S.; Thomas, G.O.; Lee, H.K.; Jeffrey, P.O. Organochlorine, pesticides and heavy metals in green-lipped mussel, Perna viridis in Singapore. Water Air Soil Pollut. 2003, 155, 103–116. [Google Scholar] [CrossRef]
- Yap, C.K.; Ismail, A.; Tan, S.G. Background concentrations of Cd, Cu, Pb and Zn in the green-lipped mussel Perna viridis (Linnaeus) from Peninsular Malaysia. Mar. Pollut. Bull. 2003, 46, 1035–1048. [Google Scholar] [CrossRef]
- Shahbazi, A.; Zakaria, M.P.; Yap, C.K.; Tan, S.G.; Surif, S.; Mohamed, A.R.; Sakari, M.; Bakhtiari, A.R.; Bahry, P.S.; Chandru, K.; et al. Use of different tissues of Perna viridis as biomonitors of polycyclic aromatic hydrocarbons in the coastal water of Peninsular Malaysia. Environ. Forensics 2010, 11, 248–263. [Google Scholar] [CrossRef]
- Zulkifli, S.Z.; Ismail, A.; Mohamat-Yusuff, F.; Arai, T.; Miyazaki, N. Johor Strait as a hotspot for trace elements contamination in Peninsular Malaysia. Bull. Environ. Contam. Toxicol. 2010, 84, 568–573. [Google Scholar] [CrossRef]
- Yap, C.K.; Tan, S.G. Iron (Fe) concentrations in the byssus and soft tissues of the green-lipped mussel Perna viridis (L.): Byssus as an excretion route of Fe and Fe bioavailability in the coastal waters. Ind. J. Mar. Sci. 2007, 36, 227–234. [Google Scholar]
- Yap, C.K.; Ismail, A.; Tan, S.G. Water content, conversion factor, shell thickness and condition index of the green-lipped mussel Perna viridis (Linnaeus) from Malaysian coastal waters. Malayan Nat. J. 2003, 56, 387–396. [Google Scholar]
- USFDA/ISSC National Shellfish Sanitation Program. Guide for the Control of Molluscan Shellfish. Guidance Documents Chapter II. Growing Areas: 04. Action Levels, Tolerances, and Guidance Levels for Poisonous or Deleterious Substances in Seafood; Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2007.
- Nauen, C. Compilation of Legal Limits for Hazardous Substances in Fish and Fishery Products. FAO Fish. Circ. 1983, 464, 108. [Google Scholar]
- CXS 193-1995; Codex Alimentarius. International Food Standard. In General Standard Contaminants and Toxins in Food and Feed; Adopted in 1995 Revised in 1997, 2006, 2008, 2009 Amended in 2010, 2012, 2013, 2014, 2015, 2016, 2017, 2018, 2019. FAO: Rome, Italy; WHO: Geneva, Switzerland, 2019; 66p. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 26 February 2023).
- European Commission. Commission Regulation (EC) No 1881/2006 of the European Parliament and the Council of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Official Journal of the European Communities, L364/18. 2006. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=EN (accessed on 12 June 2015).
- ANZFSC, Australian New Zealand Food Standards Code, Standard 1.4.1-Contaminants and Natural Toxicants (F2011C00052). 2015. Available online: https://www.comlaw.gov.au/Details/F2015C00052/Download (accessed on 21 September 2015).
- Malaysian Food Regulation. Malaysian Law on Food and Drugs; Malaysian Law Publishers: Kuala Lumpur, Malaysia, 1985. [Google Scholar]
- JECFA. Evaluation of Certain Food Additives and Contaminants (Twenty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives); WHO Technical Report Series, No. 696; Corrigenda: Fareham, UK, 1983. [Google Scholar]
- Nurul Izzah, A.; Wan Rozita, W.M.; Tengku Rozaina, T.M.; Cheong, Y.L.; Siti, F.; Nasriyah, C.H.; Nor Aini, A.; Rafiza, S.; Lokman, H.S. Fish Consumption Pattern among Adults of Different Ethnics in Peninsular Malaysia. Food Nutr. Res. 2016, 60, 32697. [Google Scholar] [CrossRef]
- US EPA Human Health Risk Assessment. Regional Screening Level (RSL)—Summary Table November 2021. Available online: https://semspub.epa.gov/work/HQ/401635.pdf (accessed on 26 December 2021).
- JECFA. Summary and Conclusions of the Seventy-Third Meeting of the JECFA; Joint FAO/WHO Expert Committee on Food Additives, Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2010. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality. Volume 1, Recommendations, 2nd ed.; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- JECFA. Evaluation of Certain Food Additives and Contaminants (Twenty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives); WHO Technical Report Series, No. 683; WHO: Geneva, Switzerland, 1982. [Google Scholar]
- JECFA. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Includes All Updates up to the 89th JECFA (June 2020). 2021. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/search.aspx?fcc=2 (accessed on 7 January 2022).
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Update of the Risk Assessment of Nickel in Food and Drinking Water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- JECFA. Safety Evaluation of Certain Contaminants in Food/Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series; 63; FAO JECFA Monographs; FAO United Nations: Rome, Italy, 2011. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1996; p. 662. [Google Scholar]
- Yap, C.K.; Cheng, W.H.; Ali, K.; Ismail, A. Health risk assessments of heavy metal exposure via consumption of marine mussels collected from anthropogenic sites. Sci. Total Environ. 2016, 553, 285–296. [Google Scholar] [CrossRef]
- Sasikumar, G.; Krishnakumar, P.K.; Bhat, G.S. Monitoring trace metal contaminants in green mussel, Perna viridis from the coastal waters of Karnataka, southwest coast of India. Arch. Environ. Toxicol. 2006, 51, 206–214. [Google Scholar] [CrossRef]
- Liu, J.H.; Kueh, C.S.W. Biomonitoring of heavy metals and trace organics using the intertidal mussel Perna viridis in Hong Kong coastal waters. Mar. Pollut. Bull. 2005, 51, 857–875. [Google Scholar] [CrossRef]
- De Astudillo, L.R.; Yen, I.C.; Agard, J.; Bekele, I.; Hubbard, R. Heavy metals in green mussel (Perna viridis) and oysters (Crassostrea sp.) from Trinidad and Venezuela. Arch. Environ. Contam. Toxicol. 2002, 42, 410–415. [Google Scholar] [CrossRef]
- Nicholson, S.; Szefer, P. Accumulation of metals in the soft tissues, byssus and shell of the mytilid mussel Perna viridis (Bivalvia: Mytilidae) from polluted and uncontaminated locations in Hong Kong coastal waters. Mar. Pollut. Bull. 2003, 46, 1040–1043. [Google Scholar] [CrossRef]
- Fung, C.N.; Lam, J.C.W.; Zheng, G.J.; Connell, D.W.; Monirith, I.; Tanabe, S.; Richardson, B.J.; Lam, P.K.S. Mussel-based monitoring of trace metal and organic contaminants along the east coast of China using Perna viridis and Mytilus edulis. Environ. Pollut. 2004, 127, 203–216. [Google Scholar] [CrossRef]
- Chinnadurai, S.; de Campos, C.J.A.; Geethalakshmi, V.; Kripa, V.; Mohamed, K.S. Baseline health risk assessment of trace metals in bivalve shellfish from commercial growing areas in the estuaries of Ashtamudi and Vembanad (Kerala, India). Environ. Sci. Pollut. Res. 2021, 28, 68338–68348. [Google Scholar] [CrossRef]
- Yap, C.K.; Al-Mutairi, K.A. Comparative study of potentially toxic nickel and their potential human health risks in seafood (fish and mollusks) from Peninsular Malaysia. Biology 2022, 11, 376. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity—An overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and rcotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef]
- Aktar, M.; Khan, M.A.A.; Siddique, M.A.M. Trace metal concentrations in the green lipped mussel Perna viridis (Linnaeus, 1758) collected from Maheshkhali Channel, Cox’s Bazar, Bangladesh. J. Fish. Sci. Com. 2014, 8, 42–51. [Google Scholar] [CrossRef]
- Kamaruzzaman, B.Y.; Ong, M.C.; Kasim, Z.; Shahbudin, S. Levels of heavy metals in green-lipped mussel Perna viridis (Linnaeus) from Muar Estuary, Johore, Malaysia. Pakistan J. Biol. Sci. 2008, 11, 2249–2253. [Google Scholar] [CrossRef]
- Kamaruzzaman, B.Y.; Mohd Zahir, M.S.; Akbar John, B.; Jalal, K.C.A.; Shahbudin, S.; Al-Barwani, S.M.; Goddard, J.S. Bioaccumulation of some metals by green mussel Perna viridis (Linnaeus 1758) from Pekan, Pahang, Malaysia. Int. J. Biol. Chem. 2011, 5, 54–60. [Google Scholar] [CrossRef]
- Sheng, T.L.; Han, P.J.; Tan, E.; Hing, Y.T.; Ramli, M.H.; Kassim, Z.; Ong Meng Chuan, M.C. Concentration of heavy metals in Perna viridis collected from Straits of Johor, Malaysia. Malays. J. Anal. Sci. 2021, 25, 930–939. [Google Scholar]
- Mahat, N.A.; Muktar, N.K.; Ismail, R.; Abdul Razak, F.I.; Abdul Wahab, R.; Abdul Keyon, A.S. Toxic metals in Perna viridis mussel and surface seawater in Pasir Gudang coastal area, Malaysia, and its health implications. Environ. Sci. Poll. Res. 2018, 25, 30224–30235. [Google Scholar] [CrossRef]
- Soegianto, A.; Putranto, T.W.C.; Payus, C.M.; Zarqasi, F.R.; Syafitrirulla, P.P.; Muchlisin, M.I.; Ramdhani, S.; Nosafandra, A.S.; Wibisono, A.D. Metals in the tissues of the East Java Coast Indonesian green mussel (Perna viridis Linnaeus, 1758) and associated health risks. Reg. Stud. Mar. Sci. 2021, 48, 102045. [Google Scholar] [CrossRef]
- Montojo, U.M.; Baldoza, B.J.S.; Cambia, F.D.; Benitez, K.C.D.; Perelonia, K.B.S.; Rivera, A.T.F. Levels and health risk assessment of mercury, cadmium, and lead in green mussel (Perna viridis) and oyster (Crassostrea iredalei) harvested around Manila Bay, Philippines. Food Control 2021, 124, 107890. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Dietary exposure to cadmium at close to the current provisional tolerable weekly intake does not affect renal function among female Japanese farmers. Environ. Res. 2004, 95, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.I. Pulmonary deposition, clearance and effects of inhalation soluble and insoluble cadmium compounds. IARC Sci. Publ. 1992, 118, 189–204. [Google Scholar]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Okubo, H.; Murakami, K.; Miyamoto, K.; Hosoi, Y.; Murata, K.; Kayama, F. Age-relevant renal effects of cadmium exposure through consumption of home-harvested rice in female Japanese farmers. Environ. Int. 2013, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Aoshima, K.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Hosoi, Y.; Katoh, T.; Kayama, F. Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int. Arch. Occup. Environ. Health 2010, 83, 953–970. [Google Scholar] [CrossRef]
- Jovic, M.; Stankovic, S. Human exposure to trace metals and possible public health risks via consumption of mussels Mytilus galloprovincialis from the Adriatic coastal area. Food Chem. Toxicol. 2014, 70, 241–251. [Google Scholar] [CrossRef]
- Zhu, F.; Fan, W.; Wang, X.; Qu, L.; Yao, S. Health risk assessment of eight heavy metals in nine varieties of edible vegetable oils consumed in China. Food Chem Toxicol. 2011, 49, 3081–3085. [Google Scholar] [CrossRef]
- Riani, E.; Cordova, M.R.; Arifin, Z. Heavy metal pollution and its relation to the malformation of green mussels cultured in muara Kamal Waters, Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2018, 133, 664–670. [Google Scholar] [CrossRef]
- Garcia-Leston, J.; Mendez, J.; Pasaro, E.; Laffon, B. Genotoxic effects of lead: An updated review. Environ. Int. 2010, 36, 623–636. [Google Scholar] [CrossRef]
- Canfield, R.L.; Henderson, C.R.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual impairment in children with blood lead concentrations below 10 lg per deciliter. N. Engl. J. Med. 2003, 348, 1517–1526. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Kortsha, G.X.; Brown, G.G. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology 1997, 48, 650–658. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Nordberg, G.F.; Nogawa, K.; Nordberg, M.; Friberg, L.T. Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Academic Press: Burlington, VT, USA, 2007; pp. 445–486. [Google Scholar]
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef]
- Oyaro, N.; Juddy, O.; Murago, E.N.M.; Gitonga, E. The contents of Pb, Cu, Zn and Cd in meat in Nairobi, Kenya. J. Food Agric. Environ. 2007, 5, 119–121. [Google Scholar]
- Simpson, R.D. Uptake and loss of zinc and lead by mussels (Mytilus edulis) and relationships with body weight and reproductive cycle. Mar. Poll. Bull. 1979, 10, 74–78. [Google Scholar] [CrossRef]
- Geffard, A.; Amiard, J.C.; Amiard-Triquet, C. Kinetics of metal elimination in oysters from a contaminated estuary. Comp. Biochem. Physiol. 2002, 131, 281–293. [Google Scholar] [CrossRef]
- Behrens, W.J.; Duedall, I.W. The behaviour of heavy metals in transplanted hard clams, Mercenaria mercenaria. ICES J. Mar. Sci. 1981, 39, 223–230. [Google Scholar] [CrossRef]
- Andres, S.; Baudrimont, M.; Lapaquellerie, Y.; Ribeyre, F.; Maillet, N.; Latouche, C.; Boudou, A. Field transplantation of the freshwater bivalve Corbicula fluminea along a polymetallic contamination gradient (River Lot, France): I. Geochemical characteristics of the sampling sites and cadmium and zinc bioaccumulation kinetics. Environ. Toxicol. Chem. 1999, 18, 2462–2471. [Google Scholar] [CrossRef]
- Wallner-Kersanach, M.; Theede, H.; Eversberg, U.; Lobo, S. Accumulation and Elimination of trace metals in a transplantation experiment with Crassostrea rhizophorae. Arch. Environ. Contam. Toxicol. 2000, 38, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Burt, A.; Maher, W.; Roach, A.; Krikowa, F.; Honkoop, P.; Bayne, B. The accumulation of Zn, Se, Cd, and Pb and physiological condition of Anadara trapezia transplanted to a contamination gradient in Lake Macquarie, New South Wales, Australia. Mar. Environ. Res. 2007, 64, 54–78. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Palmero, S.; Zanicchi, G.; Capelli, R.; Vaissiere, R.; Orunesu, M. Role of metallothioneins in Cu and Cd accumulation and elimination in the gill and digestive gland cells of Mytilus galloprovincialis (Lam.). Mar. Environ. Res. 2003, 16, 23–36. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal accumulation in marine invertebrates: Marine biology or marine chemistry. J. Mar. Biol. Assoc. 1997, 77, 195–210. [Google Scholar] [CrossRef]
- Kim, C.-K.; Choi, M.S. Biomonitoring of trace metals using transplanted mussels, Mytilus galloprovincialis, in coastal areas around Ulsan and Onsan Bays, Korea. Ocean Sci. J. 2017, 52, 31–42. [Google Scholar] [CrossRef]
- Hickey, C.W.; Roper, D.S.; Buckland, S.J. Metal concentrations of resident and transplanted freshwater mussels Hyridella menziesi (Unionacea: Hyriidae) and sediments in the Waikato River, New Zealand. Sci. Tot. Environ. 1995, 175, 163–177. [Google Scholar] [CrossRef]
- Odzak, N.; Zvonarić, T.; Kljaković Gašpić, Z.; Barić, A. Biomonitoring of copper, cadmium, lead, zinc and chromium in the Kastela Bay using transplanted mussels. Fresenius Environ. Bull. 2001, 10, 37–41. [Google Scholar]
- Yap, C.K.; Rashiq, M.; Edward, F.B. Is a mussel processing site a point source of Zn contamination? Evidence of Zn remobilization from boiled Mussel, Perna viridis. Pertanika J. Trop. Agric. Sci. 2012, 35, 199–207. [Google Scholar]
- Liu, G.; Arthur, M.; Viglia, S.; Xue, J.; Meng, F.; Lombardi, G.V. Seafood-energy-water nexus: A study on resource use efficiency and the environmental impact of seafood consumption in China. J. Clean. Prod. 2020, 277, 124088. [Google Scholar] [CrossRef]
- Bai, X.; Fu, Z.; Li, N.; Stankovski, S.; Zhang, X.; Li, X. Water environmental nexus-based quality and safety risk assessment for fish (Carassius auraus) in aquaculture. J. Clean. Prod. 2021, 288, 125633. [Google Scholar] [CrossRef]
- Abisha, R.; Krishnani, K.K.; Sukhdhane, K.; Verma, A.K.; Brahmane, M.; Chadha, N.K. Sustainable development of climate-resilient aquaculture and culture-based fisheries through adaptation of abiotic stresses: A review. J. Water Clim. Chang. 2022, 13, 2671–2689. [Google Scholar] [CrossRef]
- Keener, L. Food Safety Objectives: The Nexus among Preventive Controls, Validation, and Food Safety Assurance. Food Safety Magazine. 2022. Available online: https://www.food-safety.com/articles/8336-food-safety-objectives-the-nexus-among-preventive-controls-validation-and-food-safety-assurance (accessed on 24 February 2023).
- Orimoloye, I.R. Water, energy and food nexus: Policy relevance and challenges. Front. Sustain. Food Syst. 2022, 5, 824322. [Google Scholar] [CrossRef]
- Lai, Q.; Ma, J.; He, F.; Zhang, A.; Pei, D.; Yu, M. Current and Future Potential of Shellfish and Algae Mariculture Carbon Sinks in China. Int. J. Environ. Res. Public Health 2022, 19, 8873. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, C.; Zhao, L. Effect of the marine system on the pressure of the food–energy–water nexus in the coastal regions of China. J. Clean. Prod. 2021, 319, 128753. [Google Scholar] [CrossRef]
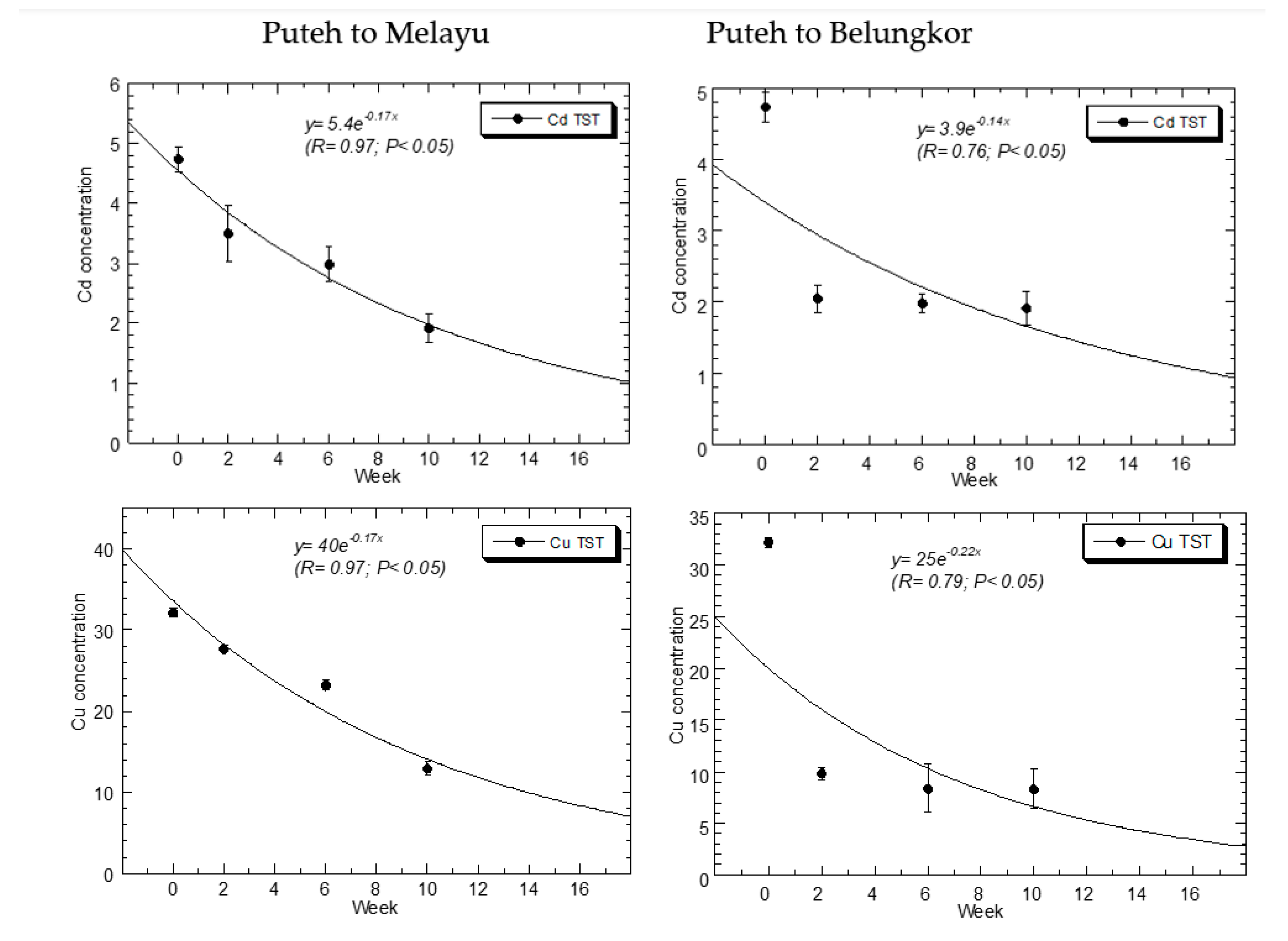
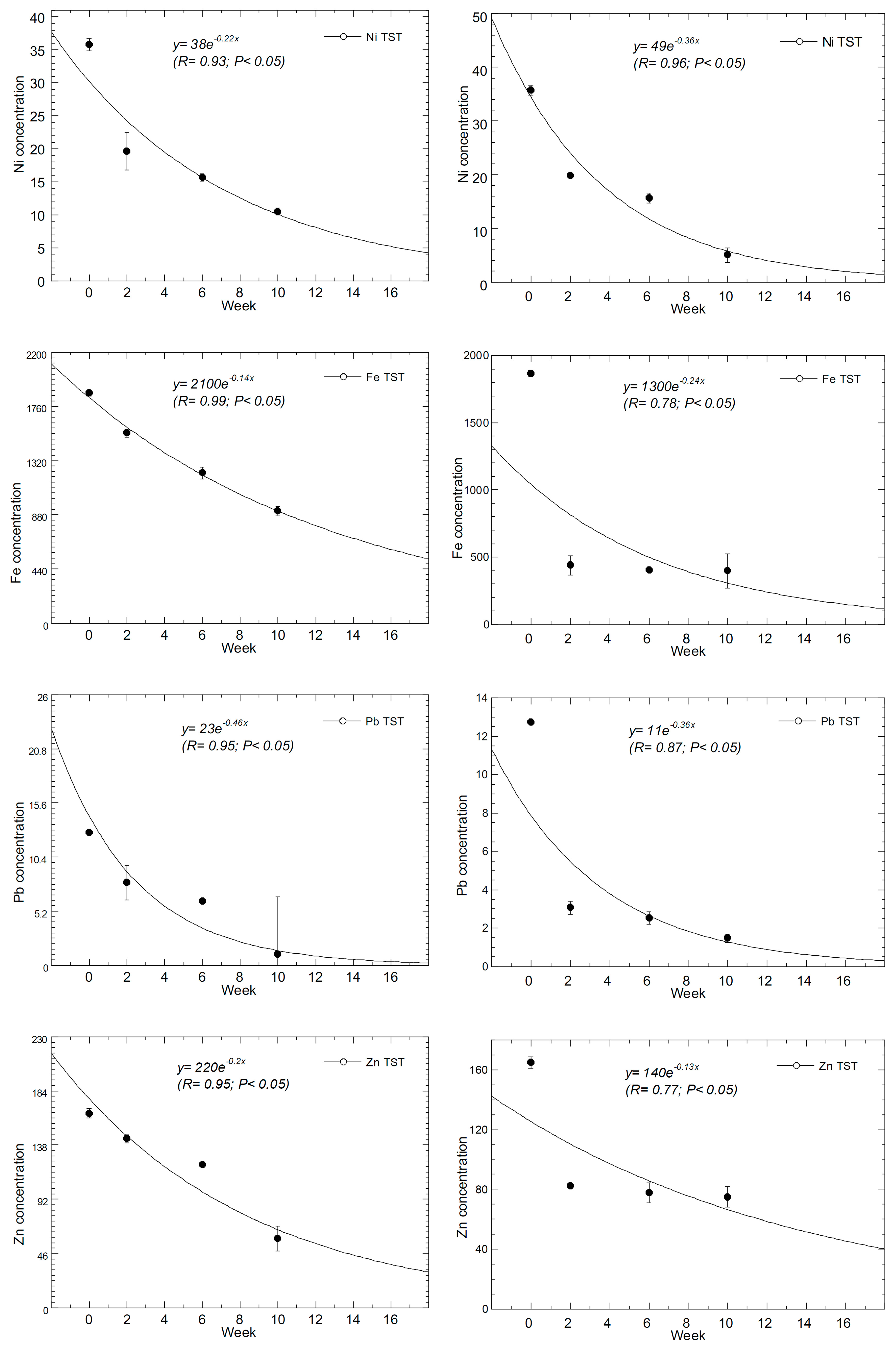
| P-B | Cd | Cu | Ni | Fe | Pb | Zn |
|---|---|---|---|---|---|---|
| Minimum | 1.87 | 8.29 | 5.05 | 395 | 1.48 | 74.89 |
| Maximum | 4.74 | 32.16 | 35.77 | 1867 | 12.74 | 164.92 |
| Mean | 2.67 | 14.66 | 19.07 | 775 | 4.95 | 99.98 |
| Median | 2.02 | 9.09 | 17.73 | 419 | 2.80 | 80.06 |
| Std Error | 0.69 | 5.84 | 6.37 | 364 | 2.62 | 21.70 |
| P-M | Cd | Cu | Ni | Fe | Pb | Zn |
| Minimum | 1.92 | 12.92 | 10.51 | 909 | 1.06 | 58.68 |
| Maximum | 4.74 | 32.16 | 35.77 | 1867 | 12.74 | 164.92 |
| Mean | 3.29 | 23.99 | 20.40 | 1384 | 6.97 | 122.11 |
| Median | 3.25 | 25.43 | 17.66 | 1381 | 7.05 | 132.43 |
| Std Error | 0.59 | 4.12 | 5.45 | 206 | 2.41 | 22.94 |
| Puteh to Belungkor | Cd | Cu | Ni | Fe | Pb | Zn |
|---|---|---|---|---|---|---|
| Week 2 | 56.74 | 69.51 | 44.67 | 76.57 | 75.93 | 50.02 |
| Week 6 | 58.23 | 73.97 | 56.19 | 78.53 | 80.21 | 52.90 |
| Week 10 | 59.70 | 74.22 | 85.88 | 78.81 | 88.35 | 54.59 |
| Puteh to Melayu | Cd | Cu | Ni | Fe | Pb | Zn |
| Week 2 | 26.16 | 13.96 | 45.09 | 17.38 | 37.66 | 13.01 |
| Week 6 | 36.92 | 27.89 | 56.14 | 34.69 | 51.71 | 26.40 |
| Week 10 | 59.49 | 59.83 | 70.62 | 51.29 | 91.68 | 64.42 |
| Puteh to Melayu | Cd | Cu | Ni | Fe | Pb | Zn | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTWI | 361.5 | 217,000 | 5642 | 347,200 | 1302 | 434,000 | ||||||||||||
| Week | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI |
| 0 | 0.52 | 0.52 | 3.64 | 3.53 | 0.09 | 24.7 | 3.92 | 0.20 | 27.5 | 205 | 0.29 | 1434 | 1.40 | 0.399 | 9.78 | 18.1 | 0.06 | 127 |
| 2 | 0.38 | 0.38 | 2.69 | 3.04 | 0.08 | 21.2 | 2.15 | 0.11 | 15.1 | 169 | 0.24 | 1185 | 0.87 | 0.249 | 6.10 | 15.7 | 0.05 | 110 |
| 6 | 0.33 | 0.33 | 2.30 | 2.54 | 0.06 | 17.8 | 1.72 | 0.09 | 12.0 | 134 | 0.19 | 936 | 0.68 | 0.193 | 4.72 | 13.3 | 0.04 | 93.2 |
| 10 | 0.21 | 0.21 | 1.47 | 1.42 | 0.04 | 9.92 | 1.15 | 0.06 | 8.07 | 99.8 | 0.14 | 698 | 0.12 | 0.033 | 0.81 | 6.44 | 0.02 | 45.1 |
| Puteh to Belungkor | Cd | Cu | Ni | Fe | Pb | Zn | ||||||||||||
| PTWI | 361.5 | 217,000 | 5642 | 347,200 | 1302 | 434,000 | ||||||||||||
| Week | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI | EDI | THQ | EWI |
| 0 | 0.52 | 0.52 | 3.64 | 3.53 | 0.09 | 24.7 | 3.92 | 0.20 | 27.5 | 205 | 0.29 | 1434 | 1.40 | 0.399 | 9.78 | 18.1 | 0.06 | 127 |
| 2 | 0.23 | 0.23 | 1.57 | 1.08 | 0.03 | 7.53 | 2.17 | 0.11 | 15.2 | 48.0 | 0.07 | 336 | 0.34 | 0.096 | 2.35 | 9.04 | 0.03 | 63.3 |
| 6 | 0.22 | 0.22 | 1.52 | 0.92 | 0.02 | 6.43 | 1.72 | 0.09 | 12.0 | 44.0 | 0.06 | 308 | 0.28 | 0.079 | 1.94 | 8.52 | 0.03 | 59.6 |
| 10 | 0.21 | 0.21 | 1.47 | 0.91 | 0.02 | 6.37 | 0.55 | 0.03 | 3.88 | 43.4 | 0.06 | 304 | 0.16 | 0.046 | 1.14 | 8.21 | 0.03 | 57.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, C.K.; Al-Mutairi, K.A. Lower Health Risks of Potentially Toxic Metals after Transplantation of Aquacultural Farmed Mussels from a Polluted Site to Unpolluted Sites: A Biomonitoring Study in the Straits of Johore. Foods 2023, 12, 1964. https://doi.org/10.3390/foods12101964
Yap CK, Al-Mutairi KA. Lower Health Risks of Potentially Toxic Metals after Transplantation of Aquacultural Farmed Mussels from a Polluted Site to Unpolluted Sites: A Biomonitoring Study in the Straits of Johore. Foods. 2023; 12(10):1964. https://doi.org/10.3390/foods12101964
Chicago/Turabian StyleYap, Chee Kong, and Khalid Awadh Al-Mutairi. 2023. "Lower Health Risks of Potentially Toxic Metals after Transplantation of Aquacultural Farmed Mussels from a Polluted Site to Unpolluted Sites: A Biomonitoring Study in the Straits of Johore" Foods 12, no. 10: 1964. https://doi.org/10.3390/foods12101964
APA StyleYap, C. K., & Al-Mutairi, K. A. (2023). Lower Health Risks of Potentially Toxic Metals after Transplantation of Aquacultural Farmed Mussels from a Polluted Site to Unpolluted Sites: A Biomonitoring Study in the Straits of Johore. Foods, 12(10), 1964. https://doi.org/10.3390/foods12101964






