Abstract
Herbal and plant extracts are being applied for a wide range of foods against different types of food-borne pathogens. In the present study, ethanolic and aqueous extracts (2% w/v) from cranberry (Vaccinium macrocarpon) and pomegranate (Punica granatum L.) plants were applied alone or in combination with two essential oils (thyme and oregano in a concentration of 0.150 μg/g) in pork meatballs and their antimicrobial activity was estimated. The extracts exhibited promising results (aqueous and ethanolic extracts of pomegranate and cranberry in a food-compatible concentration of 2% w/v) were applied to raw pork meatball production and their antimicrobial activity was recorded versus Enterobacteriaceae, total mesophilic bacteria, yeasts/molds, Staphylococcus spp., Pseudomonas spp. and lactic acid bacteria (LAB). The outcome demonstrated that meatballs containing aqueous extracts of pomegranate were more resistant to spoilage compared to all the other samples since they were preserved for more days. The chemical profiles of plant extracts were determined through LC-QTOF/MS and the chemical composition of the essential oils applied was determined with the use of GC/MS in order to identify the substances involved in the observed antimicrobial activity. Phenolic acids (quinic acid, chlorogenic acid), monoterpenes (p-cymene, carvacrol, thymol, limonene), organic acids (citric acid) and phenols were the main constituents found in the plant extracts and essential oils applied. These extracts of plant origin could be used as natural preservatives in meat products, even in low concentrations.
1. Introduction
Pork meat is one of the most susceptible foods for lipid oxidation, especially in minced form. Along with lipid oxidation, microbial contamination is the main cause of meat deterioration. Moreover, meat and its products are common sources of food-borne pathogens, namely Escherichia coli, Staphylococcus aureus, Salmonella spp. and Listeria spp. [1].
These factors may lead to off-odor and off-flavor development, discoloration, changes in texture and decreased shelf-life [2]. All these alterations affect consumers’ will to buy the meat products that exhibit them and cause economic losses to the meat industry [3].
Products resulting from peroxidation of polysaturated fatty acids (PUFA) and/or possessing increased microbial counts pose a risk to human health, increasing the frequencies of tumors and atherosclerosis [4].
At this exact point, the use of antioxidants is one of the main strategies in order to confront lipid oxidation and microbial contamination in meat products. Synthetic antioxidants are applied with quite satisfying results, but even if they are characterized as GRAS (generally recognized as safe), their extensive use is restricted by legislation, mostly because of potential health risks [2,3].
In addition, during the latest years, consumers’ concern about the impact on health that food consumption may have has led to a demand for more natural foods and preservatives [5].
Plant extracts and essential oils (EOs) are among these alternatives. Among numerous plant origin extracts, cranberry (Vaccinium macrocarpon) and pomegranate (Punica granatum L.) are being used in the food industry, mostly because of their antibacterial and antifungal activity [6].
More specifically, cranberry belongs to the Ericaceae family and has been consumed for centuries mostly because of its medicinal properties and its activity against urinary tract infections, dental decay, stomach ulcers and its antiproliferative action [7]. Several compounds in cranberry, including phenolic acid, tannins, proanthocyanidins and flavonoids, are responsible for its medicinal activities [8].
Pomegranate is characterized by a high concentration of tannins, which are mostly responsible for its antimicrobial properties. The plant belongs to the Punicaceae family and its peel is used in traditional medicine as a remedy for diarrhea and dysentery [9].
According to the literature, it is known that extraction with different kind of solvents, polar or non-polar or even their combination, may provide phytochemicals (flavonols, hydroxy-cinnamic acid), which exhibit bacteriostatic or bactericidal activity against food-borne or pathogenic bacteria [10].
Thyme essential oil (TEO) and oregano essential oil (OEO) are widely used as preservatives in foods and both are recognized as GRAS by the Food and Drug Administration (FDA). They are contained in Thymus vulgaris and Origanum vulgare, respectively, which belong to the Lamiaceae family [11]. Thyme and oregano EOs contain high concentrations of phenolic compounds, such as thymol, carvacrol, p-cymene and γ-terpinene, which cause structural and functional damage to the bacterial cell membrane [12]. Their effectiveness as natural antioxidants and antimicrobials has been previously tested in meat products [13].
The presence of carvacrol and thymol in the composition of these EOs was supposed to be responsible for the decreased lipid peroxidation and radical formation and the extension of shelf-life. Specifically, both oregano and thyme EO display good antimicrobial activity against Gram-negative and Gram-positive bacteria, most of them are common spoilage bacteria or food-borne pathogens within food systems [12].
Plant EOs are generally more active against Gram-positive than Gram-negative bacteria [14], mostly because of their outer membrane, which may restrict the diffusion of hydrophobic compounds through its lipopolysaccharide covering. Combinations of EOs and other extracts of plant origin with high phenolic content have been assessed for synergistic activity at lower concentrations in order to obtain the best antimicrobial and antioxidant activity with the least undesirable impact on the organoleptic properties of food [15].
In the present study, the application of aqueous cranberry and pomegranate extracts (2% w/v) and the application of thyme and oregano EOs (0.150 μg/g), alone or in combination between them, was examined in pork meatballs in refrigerated storage (4 °C), as far as their antimicrobial activity was concerned, since various microbial groups were examined. The total phenolic content (TPC) and the identity of the phenolic substances of the plant extracts were determined. In addition, the composition of the applied essential oils was revealed through gas chromatography-mass spectrometry. The upper goal of our experiments was to examine whether or not these natural preservatives could replace their synthetic counterparts in pork meat with equivalent antimicrobial and antioxidant activity.
2. Materials and Methods
2.1. Raw Materials
Fresh cranberries (Vaccinium macrocarpon) and pomegranates (Punica granatum L.) purchased from a local market of north-eastern Greece (Orestiada, Evros) were applied in the form of aqueous extracts in order to study their impact against several microbial groups.
The oregano essential oil (OEO) and the thyme essential oil (TEO) were commercially purchased from the local market of Nea Orestiada, Evros Regional Unit, Greece.
2.2. Preparation of Extracts
The extraction method applied was an adaptation from the study of Yang, Jia and Zu (2016) [16]. In brief, cranberry and pomegranate fruits were blended for approximately 60 s at 25 °C in a stomacher apparatus (Bagmixer, InterScience, SaintNomlaBretèche, France). Twenty grams of each puree was mixed with 200 mL of water (10% w/v) at 60 °C in an Erlenmeyer flask with agitation for two hours.
A 0.45 mm filter paper under vacuum was used for the filtration of the above aqueous solutions and centrifugation of the filtrates took place at 5000× g (PALL, LifeSciences, Portsmouth, UK) for 20 min at room temperature. Evaporation of the supernatants until a final volume of 5 mL at 50 °C in an evaporator (Model R204B3, Senco Technology Ltd., Shanghai, China) was the next step. Finally, 5 mL of distilled water was added (initial concentration: 2 g/mL) to the previous concentrated solutions. The samples were kept at −4 °C.
2.3. Preparation of Raw Meatballs
Greek traditional techniques were used for the preparation of the meatballs studied. More specifically, a batch consisting of ground pork meat (2.0 kg), lard (0.5 kg), sodium chloride 1.5% w/w (3.0 g) and black pepper (0.2 g) was produced. Homogenization of the mixture took place and 6 different types of meatballs were prepared; namely (a) control samples (C) in which no extract was used, (b) cranberry aqueous extracts (WC) and (c) pomegranate aqueous extracts (WP) with a concentration of 5% v/v in cranberry and pomegranate, respectively, added (all in a concentration of 2% w/v), (d) oregano essential oil (OEO), (e) thyme essential oil (TEO) with a concentration of 0.150 μg/g meatball and (f) respective combinations between cranberry aqueous extracts and TEO and OEO (WC + OEO, WC + TEO, WC + OEO + TEO) and (g) respective combinations of pomegranate aqueous extracts with OEO and TEO (WP + OEO, WP + TEO, WP + OEO + TEO) in the above concentrations. Meatball samples were kept at 4 °C in polystyrene trays for the entire study period. The microbiological experiments were repeated three times for each sample and collection took place at various intervals (1st, 4th, 7th, 8th, 12th day). Data after the 8th day of storage are not shown since the spoilage of meatballs was obvious in odor and appearance.
2.4. Microbiological Analysis of Meatball Portions
Several microbial food-borne pathogens were studied, such as Enterobacteria, total mesophilic bacteria (TMB), yeasts and molds, Staphylococcus spp. and Pseudomonas spp. In addition, the levels of LAB were determined. Initially, 25 g portions of meatball samples were homogenized in 225 mL of sterilized 1/4 Ringers solution (Sigma-Aldrich, St. Louis, MO, USA) from which decimal dilutions were prepared in tubes with sterilized 9 mL ¼ Ringers. An amount of 0.1 mL of homogenate was spread-plated on the surface of the following media: plate count agar (Oxoid Ltd., Hampshire, UK) for the detection of total mesophilic counts, acidified MRS agar (Oxoid Ltd., Hampshire, UK) for LAB, violet red bile glucose agar (Oxoid Ltd., Hampshire, UK) for enterobacteria, pseudomonas CFC selective agar for pseudomonads, Baird-Parker egg yolk tellurite agar for staphylococci and acidified (pH 4.5) malt agar (Oxoid Ltd., Hampshire, UK) for yeast and molds. Incubation periods varied from 24, 48 and 72 h according to the medium and temperature kept at 30 °C for TMC, pseudomonads and yeast/molds or at 37 °C for LAB, enterobacteria and staphylococci. A representative number of isolated colonies were Gram-stained and tested for the presence of catalase, particularly for LAB confirmation.
2.5. Determination of Total Phenolic Content (TPC)
The total phenolic content of aqueous cranberry and pomegranate extracts was determined according to A. Waterhouse’s method [17]. More specifically, a calibration curve was prepared with solutions of gallic acid diluted in ethanol and afterwards in water. The concentrations varied from 0–1000 mg/L gallic acid.
After the preparation of the calibration curve, 20 μL from each extract was pipetted into cuvette with 1.58 mL water and 100 μL of Folin-Ciocalteau reagent and mixed well. After 5 min, 300 μL of sodium carbonate solution (20% w/v) was added. The solutions were left at 20 °C for 2 h and the absorbance of each solution was determined at 765 nm. Results are reported as gallic acid equivalent (GAE).
2.6. GC/MS Analysis
An amount of 1 mL of each essential oil (OEO and TEO) was diluted with 9 mL of pure hexane and filtrated using 0.22 μm filters. The mixtures’ chemical composition was determined by GC-MS analysis (6890N GC, 5973NetworkedMS MSD (Agilent Technologies, Santa Clara, CA, USA)) as previously described [18], with some modifications. In brief, 1 μL of each sample was directly injected into an HP-5MS column (30 m, 0.25 mm i.d., 0.25 μm film thickness). Carrier gas (He) flow was 1.5 mL/min in split mode (1:50). The injector and the detector temperatures were set at 240 °C. Oven temperature was set at 35 °C, held constant for 6 min, followed by an increase to 60 °C at a 2 °C/min rate and held constant for 5 min. A new increase to 200 °C at a 5 °C/min rate was followed by a final increase to 250 °C at a 25 °C/min rate, held constant for 5 min. The mass spectrometer was operated in the electron impact mode (electron energy at 70 eV and scan range at 45 to 400 m/z). Results acquired were processed by ChemStation integrated software (Agilent Technologies), and compounds were identified by comparing the retention times and mass spectra to NBS75K and Wiley275 reference libraries and in-house libraries, and by determining Kovats retention indexes (KIs). Both samples’ analyses were carried out in triplicate and the mean data are presented (standard deviation for all values was about ±5%).
2.7. LC-QTOF/MS Analysis
The determination of target analytes was performed using an ultrahigh-performance liquid chromatography (UHPLC) system with an HPG-3400 pump (Dionex Ultimate 3000 RSLC, Thermo Fischer Scientific, Dreieich, Germany) coupled to a QTOF mass spectrometer (Maxis Impact, Bruker Daltonics, Bremen, Germany). The method has been described previously [19]. QTOF external calibration was realized every day using sodium formate in a mixture of isopropanol:water (50:50 v/v). Moreover, internal calibration was performed by calibrant injection starting each run (1st segment, 0.1–0.25 min). There was a typical resolving power (full-width at half-maximum, FWHM) between 35,000 and 40,000 at m/z 226.1593 of 430.91.
2.8. Statistical Analysis
Bacterial populations in ground pork meat are presented as mean ± standard deviation of Log CFU/g. Bacterial counts between various treatments (fruit extracts) and also between consecutive days (in each species) were estimated by using the ANOVA procedure with Tukey’s HSD post hoc comparisons. Statistical analysis was performed with SPSS v20® (IBM Corp. Armonk, NY, USA) at a 95% significance level.
3. Results
3.1. Microbiological Analyses
The bacterial counts in ground beef meatballs during storage (4 °C/7 days) treated with various combinations of pomegranate and cranberry aqueous extract (1% w/v) as well as oregano and thyme essential oils (0.150 μg/g of meatball) are presented in Table 1. The respective zones of inhibition (ZOIs) measured (in mm) are included.

Table 1.
Bacterial counts (Log CFU/g) in ground beef meatballs during storage (4 °C/7 days) treated with various combinations of pomegranate and cranberry aqueous extract (2% w/v) as well as oregano and thyme essential oils (0.150 μg/g of meatball).
Enterobacteriaceae were abundant in almost all of our samples and average populations ranged from undetected to 6.79 Log CFU/g. In control samples Enterobacteriaceae were found in a mean population of 3.09 ± 0.5 Log CFU/g during the first day and up to 7.49 ± 0.4 Log CFU/g after 7 days. Even from the first day, all treatments statistically differed from the control regarding the counts of Enterobacteriaceae (ANOVA F = 10.06, p < 0.05) indicating an immediate effect of their antimicrobial potential against this particular group of pathogens.
However, this effectiveness was not observed during the 4th day of storage (ANOVA F = 2.14, p > 0.05) but reappeared by the end of the observation period on the 7th day (ANOVA F = 18.43, p < 0.05). The combination of oregano EO, thyme EO and pomegranate aqueous extract resulted in the most notable delay of Enterobacteriaceae growth (Figure 1a).
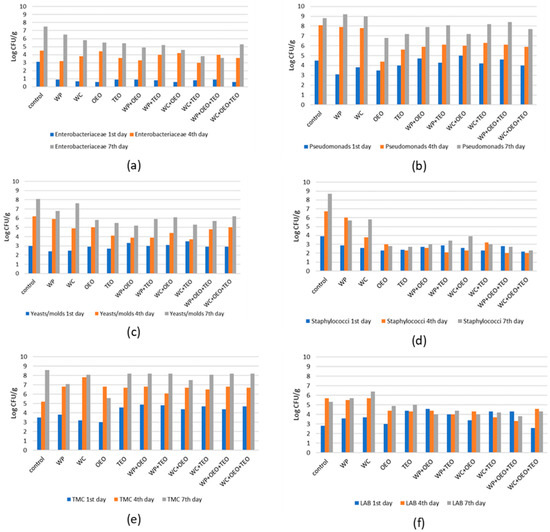
Figure 1.
Total counts of (a) Enterobacteriaceae, (b) Pseudomonas spp., (c) yeasts and molds, (d) Staphylococcus spp., (e) total mesophilic counts (TMCs), and (f) LAB detected on meatballs after 1, 4 and 7 days of storage at 4 °C.
Pseudomonads were also recovered from all samples. Mean control counts during the 1st day were 4.49 ± 0.3 Log CFU/g and 8.79 ± 0.3 Log CFU/g on the 7th day, while in all other treatments counts ranged from minus 2 Log to plus 0.5 Log of the above value. Although some statistically significant differences during the consequent days of storage were observed among the various treatments, the lowest counts were found when the oregano EO was solely used (Figure 1b).
Yeasts and molds were the bacterial group with the highest increase (over 6 Logs) in our samples during the 7 days of observation. Mean control counts were initially 2.99 ± 0.1 Log CFU/g and rose to 8.09 ± 0.5 Log CFU/g on the last day. Significant differences during the study period were observed between the control counts and all treatments, except those with pomegranate or cranberry aqueous extracts (Figure 1c).
Staphylococcus spp. in our samples was counted from 2.29 ± 0.1 Log CFU/g to 8.69 ± 0.6 Log CFU/g. During the 1st day, the lowest bacterial populations were observed in the treatment with oregano EO (average 2.29 ± 0.1 Log CFU/g) and the one with thyme EO (TEO) combined with cranberry aqueous extract (WC + TEO). Both of these counts differed statistically from those of the control group (ANOVA F = 1.76, p < 0.05). However, during the 7th day, all treatments were statistically different from the control regarding the Staphylococcus spp. population (ANOVA, F = 33.56, p < 0.05), indicating a strong antibacterial action of the various extracts and essential oils, which appeared to be stronger when combinations of the above were used, lowering the final counts up to 6.4 Logs (as in the case of WC + OEO + TEO with an average 2.29 ± 0.4 Log CFU/g) (Figure 1d).
In all treatments, total mesophilic counts ranged from an average of 4.25 ± 0.7 Log CFU/g during the 1st day (control 3.5 ± 0.7 Log CFU/g) to 7.74 ± 0.8 Log CFU/g by the end of the study period (control 8.6 ± 0.3 Log CFU/g). There was no particular antimicrobial effectiveness of essential oils or fruit extracts against that microbial group besides the one observed for oregano EO during the 7th day of analysis, when 5.59 ± 0.8 Log CFU/g was observed in comparison with 8.59 ± 0.3 Log CFU/g for the control group (Figure 1e).
As shown in Table 1, initially (1st day) LABs were recovered in populations from 2.59 ± 0.7 Log CFU/g in WC + OEO + TEO samples to 4.59 ± 0.2 Log CFU/g in WP + OEO samples and reached as high as 6.39 ± 0.2 Log CFU/g in WC samples during the 7th day. During the observation period LABs showed a similar growth pattern in all treatments with the exception of the WP + OEO + TEO samples where their final counts were statistically lower than others (3.79 ± 0.7 Log CFU/g, ANOVA F = 9.82, p < 0.05) (Figure 1f).
3.2. Total Phenolic Content (TPC)
From the above analytical results (Table 2), it is obvious that the aqueous cranberry extract exhibits three-fold phenolic content in comparison with the respective pomegranate extract. This fact was verified by the following chemical profile of the extracts with the LC-QTOF/MS analysis, where the quantity and the number of phenolic compounds in the cranberry extract definitely increased.

Table 2.
Total phenolic content of the aqueous pomegranate and cranberry extracts expressed as mg/mL of gallic acid.
3.3. Determination of Chemical Composition of Oregano and Thyme Essential Oils by GC/MS
Table 3 presents the chemical profile of the two essential oils used, thyme and oregano.

Table 3.
Chemical profile of the two essential oils used, oregano (OEO) and thyme (TEO).
From the above, it becomes obvious that the main components of the oregano essential oil (OEO) applied are p-cymene (29.4%), carvacrol (26.6%), thymol (12.7%) and limonene (9.3%).
As far as thyme essential oil (TEO) is concerned, its chemical profile mostly consists of thymol (29.8%), p-cymene (27.3%), limonene (9.5%) and carvacrol (6.4%).
Other substances such as a-pinene, linalool, a-terpineol, γ-terpineol, caryophyllene and caryoplyllene oxide were detected in lower percentages ranging from 1.1% to 3.5% in both essential oils.
In OEO 24 compounds were determined, which constitute 92% of the volatile oil, and in TEO 23 compounds were detected, corresponding to 97.7% of the volatile oil.
The analysis revealed that in both essential oils, monoterpene hydrocarbons and oxygenated monoterpenes were the main group of chemicals.
3.4. Determination of Chemical Composition of Plant Extracts Using LC-QTOF/MS Analysis
From the above analytical results (Table 4), aqueous cranberry extract mainly consists of malic acid (96 mg/L) and citric acid (74.59 mg/L). Benzoic acid (3.21 mg/L) and chlorogenic acid (2.22 mg/L) follow. All the other components exist in quantities smaller than 0.3 mg/L and include substances such as p-coumaric acid (0.02 mg/L), quercetin (0.21 mg/L), hydroxytyrosol (0.04 mg/L), 4-hydroxybenzoic acid (0.03 mg/L) and galangin (0.02 mg/L).

Table 4.
Chemical composition of aqueous extracts of cranberry and pomegranate expressed in mg/L.
Aqueous pomegranate extract consists mostly of citric acid (109.02 mg/L) and malic acid (28 mg/L) as major components. Several substances like galangin, quercetin, kaempferide, protocatechuic acid and kaempferol exist in this extract as in the aqueous cranberry extract. Nevertheless, components like 4-hydroxybenzoic acid, benzoic acid and hydroxytyrosol in this case are absent.
4. Discussion
Fundamentally, the antimicrobial mode of action of polyphenolic rich plant extracts includes the following:
(a) Molecules that disrupt the cell membrane, like the OH-group. These molecules induce increased membrane permeability and consequently leakage of cell content.
(b) Decreased pH of the medium because of the accumulated proton concentration and finally induced depolarization impacting the proton motive force leading to cell death.
(c) Organic acids that impact on the NADH oxidation [20].
Recent research concerning the antimicrobial activity of plant extracts demonstrated that the number of OH-groups of the phenolic ring plays important role in the observed activity [21]. The position of the OH-group on the aromatic ring is of equal importance. Research data reveal that meta-thesis is more active compared to ortho-thesis [22] and OH-groups in position 2′ of chalcones and position 5’ of flavanones and flavones increase their antimicrobial properties probably by the delocalization of electrons from the cytoplasmic membrane [23].
In addition, the presence of alkyl groups in the aromatic nucleus has been reported to interfere in the antimicrobial activity of plant-derived phenols and affect the distribution ratio between polar and non-polar phases, bacterial phases included [24].
As a general rule, it can be noted that Gram-negative bacteria are more resistant to phenolics than Gram-positive bacteria [25]. This is probably because of the cell wall that they possess and it is linked to the polysaccharide envelope, which prevents the entrance of phytochemicals [26].
A similar mode of antimicrobial and antioxidant activity was observed for essential oils. Although a certain amount of leakage from bacterial cells may not result in loss of viability, an extensive loss of cytoplasmic content will lead to cellular death [27]. Disturbance of the cytoplasmic membrane or disrupting the proton motive force are two other pathways of activity. The importance of the presence of OH-groups in phenolic compounds such as carvacrol and thymol is already known [23,28]. Other antimicrobial pathways of EO components include binding on membrane proteins, stimulation of pseudomycelia formation, inhibition of certain necessary metabolic enzymes, etc. [14].
Furthermore, these observations were also verified in our study. The growth of Enterobacteriaceae seemed to be inhibited more extensively when aqueous pomegranate extract combined with oregano essential oil (OEO) and thyme essential oil (TEO) was applied to the pork meat. From the chemical profile of the aqueous pomegranate extract, it can be concluded that citric and malic acid, two classic organic acids, are its major components. Their presence leads to a reduction in the pH value, creating an unpleasant environment for Enterobacteria and gradually leading them to death, depression of the internal pH of microbial cells by ionization of undissociated acid molecules and disruption of substrate transport by altering cell membrane permeability or reduction of the proton motive force [29].
In addition to the acidic conditions, both EOs with major components p-cymene, carvacrol and thymol appear to induce cell membrane permeability [30] and simultaneously disintegrate the outer membrane of Gram-negative bacteria, releasing lipopolysaccharides (LPSs) [31].
In general, the antimicrobial activity of these two EOs increases when the pH decreases. The inhibitory effect of plant extracts becomes greater at acidic pH values. Pseudomonas spp. seemed to be inhibited mostly when OEO was applied solely. Strains of this family are known to show consistently high resistance to plants’ antimicrobial activity [32]. Limonene has also been found to be more active than p-cymene [23]. Essential oils from different oregano species are known to exhibit high levels of antimicrobial activity against Pseudomonas strains. The fact that Pseudomonas genera do not exhibit the ability to tolerate the conditions generated by the presence of OEO may be associated with a disturbance in the proteinosynthesis of several metabolic enzymes [33]. The necessary concentration levels could be lower than the MIC (minimum inhibitory concentration).
As far as yeasts/molds are concerned, our results are in accordance with data from the literature. Rasooli et al. [34] analyzed the effect of A. niger exposure to MIC levels of TEO and they revealed severe damage to the cell wall, cell membrane and cellular organelles. The mycelium exposed to TEO exhibited morphological changes in the hyphae, disruption in the plasma membrane and mitochondrial destruction.
The fact that the aqueous pomegranate extract in combination with OEO and the aqueous cranberry combined with TEO are the most effective treatments against yeasts in the meat system studied should be attributed to the synergistic interaction between organic acids (malic and citric acid) from the plant extracts and p-cymene, carvacrol and thymol from the essential oils applied. It has been suggested that some hydrophobic compounds present in plant extracts could change the permeability of the microbial membranes for cations modifying cell pH and affecting cell activity. Nevertheless, higher solubility does not always mean greater antimicrobial action. Bagheri-Gavkosh et al. [35] showed that plant extracts suppressed mycotoxin production by A. parasiticus. This inhibition was explained by the presence of flavonoid compounds such as p-coumaric acid and quercetin in plant extracts. These substances were detected in the essential oils and plant extracts applied in our study as well.
Staphylococcus strains were mostly inhibited by the combination of the aqueous cranberry extracts with OEO and TEO. From previous studies, it has been established that carvacrol and thymol have additive effects obtained by interactions between volatile oils [36]. These two substances are isomers, suggesting a similar mechanism of action, which includes functional and structural damage to the outer and inner membrane and membrane proteins. The additive antimicrobial effect of these two EOs against S. aureus strains has been already demonstrated in several studies [11,37], verifying our findings.
Total mesophilic bacteria (TMB) were found to be more efficiently controlled by OEO when used solely. This fact was confirmed other studies as well, implying that synergistic inhibitory effects on food-borne bacteria are present when plant extracts are applied [38]. Other researchers suggest that TMB in meat and meat products can be limited during refrigerated storage by plant extracts and EOs or a combination of both [24,38].
More specifically, p-cymene and γ-terpinene as constituents of OEO, along with carvacrol and thymol, induce the strongest antimicrobial effect and control the population of total mesophilic bacteria [36]. In another study [39], similar findings were resumed when TEO and beet juice were applied to meat sausage and total mesophiles were estimated in refrigerated storage. Then, partial inhibition of TMB was observed when TEO in the highest concentration tested (0.95% w/v) was applied, replaced by 50% by nitrite.
Finally, the population of lactic acid bacteria (LAB) was determined. It is known that the presence of LAB on meats usually ensures that shelf-life is prolonged. After the inhibition of aerobic spoilage bacteria, LAB may become the dominant bacteria group. The population of LAB reached the lowest values when aqueous pomegranate extract and both EOs were applied in the meat samples (3.8 Log CFU/g during the 7th day) compared to the control (5.3 Log CFU/g during the 7th day). Notably, in every application including pomegranate or cranberry extract combined with one or both EOs, the population of LAB natural microbiota decreased compared to the respective day of refrigerated storage. The only exception was the case where the two aqueous extracts were tested solely in the meat sample. This can be attributed to the relatively low pH, especially in the case of aqueous cranberry extract. These results are in agreement with Menezes et al. [40]. Aminzare et al. [41] confirmed that the combination of Zataria multiflora EO, a thyme-like medicinal plant from Iran, and grape seed extract, when applied in cooked sausage, caused a reduction of total mesophilic and psychrotrophic viable counts and LAB. This reduction was attributed to the synergistic effect between thymol and carvacrol. The same conclusions can be made in our treatments.
5. Conclusions
In summary, the present study examined the antimicrobial activity of aqueous pomegranate and cranberry extracts alone or in combination with oregano and thyme essential oils solely or simultaneously. The food system used was minced pork meatballs and the microbial groups tested were Enterobacteriaceae, total mesophilic bacteria, yeasts/molds, Staphylococcus spp., Pseudomonas spp. and lactic acid bacteria (LAB). All the microbial group populations were reduced after the application of particular EOs and/or plant extracts in each case. The chemical profile of plant extracts and TEO-OEO was determined in order to clarify the interactions between the organic acids, phenolic acids and phenols with monoterpenes and triterpenes.
On this basis, the combinations of plant-derived extracts and EOs could replace synthetic antioxidants, which are widely used in meat and meat-product preservation as antimicrobials and antioxidants. Further investigation should take place in order for more alternative combinations and their possible sensorial attributes to be determined.
Author Contributions
Conceptualization, I.M.; methodology, M.D. (Maria Daoutidou), M.D. (Marilena Dasenaki) and A.N.; validation, I.M.; formal analysis, A.A.; investigation, M.D. (Maria Daoutidou) and I.M.; resources, S.P.; data curation, A.A. and I.M.; writing—original draft preparation, I.M. and A.T.; writing—review and editing, S.P., A.T. and A.A.; visualization, I.M.; supervision, I.M. and N.T.; project administration, I.M.; funding acquisition, I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank Yiannis Kourkoutas and the Laboratory of Applied Microbiology & Bio-technology, Department of Molecular Biology & Genetics, Democritus University of Thrace, 68100 Alexandroupolis, Greece for the technical support of our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sofos, J.N. Challenges to meat safety in the 21st century. Meat Sci. 2008, 78, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Int. Food Res. J. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Abushelaibi, A.; Manheem, K.; Al Rashedi, A.; Kadim, I.T. Lipid oxidation, protein degradation, microbial and sensorial quality of camel meat as influenced by phenolic compounds. LWT-Food Sci. Technol. 2015, 63, 953–959. [Google Scholar] [CrossRef]
- Campbell, F.; Dickinson, H.O.; Critchley, J.A.; Ford, G.A.; Bradburn, M. A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur. J. Prev. Cardiol. 2013, 20, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287. [Google Scholar] [CrossRef]
- Viljanen, K.; Heiniö, R.L.; Juvonen, R.; Kössö, T.; Puupponen-Pimiä, R. Relation of sensory perception with chemical composition of bioprocessed lingonberry. Food Chem. 2014, 157, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Välimaa, A.L.; Honkalampi-Hämäläinen, U.; Pietarinen, S.; Willför, S.; Holmbom, B.; von Wright, A. Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int. J. Food Microbiol. 2007, 115, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Caillet, S.; Côté, J.; Sylvain, J.F.; Lacroix, M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control 2012, 23, 419–428. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 53, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Diop, A.; St-Pierre, A.; Tardif, M.; Vialle, A.; Barnabé, S. Comparing polyphenolic yields from the crowberry Empetrum nigrum L. on the Basse-Cote-Nord du Québec via solvent and microwave-assisted extractions. Ind. Biotechnol. 2019, 15, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent Oil Bear Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006, 73, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Daoutidou, M.; Plessas, S.; Alexopoulos, A.; Mantzourani, I. Assessment of antimicrobial activity of pomegranate, cranberry, and black chokeberry extracts against foodborne pathogens. Foods 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Chebli, B.; Bounimi, S. Synergistic antioxidant activity of three essential oils of Lamiaceae family from Morocco. Appl. J. Environ. Eng. Sci. 2017, 3, 2–3. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Li, S.; Jia, J.; Gu, H.; Yang, L. An efficient homogenate-microwave-assisted extraction of flavonols and anthocyanins from blackcurrant marc: Optimization using combination of Plackett-Burman design and Box-Behnken design. Ind. Crops Prod. 2016, 94, 834–847. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1–I11. [Google Scholar]
- Nikolaou, A.; Tsakiris, A.; Kanellaki, M.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Kourkoutas, Y. Wine production using free and immobilized kefir culture on natural supports. Food Chem. 2019, 272, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Dasenaki, M.; Drakopoulou, S.; Aalizadeh, R.; Thomaidis, N. Targeted and Untargeted Metabolomics as an Enhanced Tool for the Detection of Pomegranate Juice Adulteration. Foods 2019, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Agents that inhibit bacterial biofilm formation. Future Med. Chem 2015, 7, 647–671. [Google Scholar] [CrossRef]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Alcaraz, L.E.; Blanco, S.E.; Puig, O.N.; Tomas, F.; Ferretti, F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, J.E.; Liew, Y.C.; Chew, S.; Markham, J.; Bell, H.C.; Wyllie, S.G.; Warmington, J.R. Effects of tea tree oil on Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 194–198. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R.J.A.E.M. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Eswaranandam, S.; Hettiarachchy, N.S.; Johnson, M.G. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157: H7, and Salmonella gaminara. J. Food Sci. 2004, 69, FMS79–FMS84. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ultee, A.; Kets, E.P.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Malik, A. Antimicrobial action of essential oil vapours and negative air ions against Pseudomonas fluorescens. Int. J. Food Microbiol. 2010, 143, 205–210. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.P.; de Araújo Torres, R.; de Azerêdo, G.A.; Figueiredo, R.C.B.Q.; da Silva Vasconcelos, M.A.; de Souza, E.L. Carvacrol and 1, 8-cineole alone or in combination at sublethal concentrations induce changes in the cell morphology and membrane permeability of Pseudomonas fluorescens in a vegetable-based broth. Int. J. Food Microbiol. 2012, 158, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rasooli, I.; Rezaei, M.B.; Allameh, A. Growth inhibition and morphological alterations of Aspergillus niger by essential oils from Thymus eriocalyx and Thymus x-porlock. Food Control 2006, 17, 359–364. [Google Scholar] [CrossRef]
- Bagheri-Gavkosh, S.; Bigdeli, M.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Inhibitory effects of Ephedra major host on Aspergillus parasiticus growth and aflatoxin production. Mycopathologia 2009, 168, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Netopilova, M.; Houdkova, M.; Urbanova, K.; Rondevaldova, J.; Kokoska, L. Validation of Qualitative Broth Volatilization Checkerboard Method for Testing of Essential Oils: Dual-Column GC–FID/MS Analysis and In Vitro Combinatory Antimicrobial Effect of Origanum vulgare and Thymus vulgaris against Staphylococcus aureus in Liquid and Vapor Phases. Plants 2021, 10, 393. [Google Scholar] [CrossRef]
- Burt, S.A.; Vlielander, R.; Haagsman, H.P.; Veldhuizen, E.J. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157: H7 by addition of food stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, H.; Zhang, W.; Mu, H.; Sun, C.; Duan, J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiol. 2015, 60, 421–430. [Google Scholar] [CrossRef]
- Lages, L.Z.; Radünz, M.; Gonçalves, B.T.; da Rosa, R.S.; Fouchy, M.V.; Gularte, M.A.; Gandra, E.A. Microbiological and sensory evaluation of meat sausage using thyme (Thymus vulgaris, L.) essential oil and powdered beet juice (Beta vulgaris L., Early Wonder cultivar). LWT 2021, 148, 111794. [Google Scholar] [CrossRef]
- Menezes, N.M.C.; Martins, W.F.; Longhi, D.A.; de Aragão, G.M.F. Modeling the effect of oregano essential oil on shelf-life extension of vacuum-packed cooked sliced ham. Meat Sci. 2018, 139, 113–119. [Google Scholar] [CrossRef]
- Aminzare, M.; Tajik, H.; Aliakbarlu, J.; Hashemi, M.; Raeisi, M. Effect of cinnamon essential oil and grape seed extract as functional-natural additives in the production of cooked sausage-impact on microbiological, physicochemical, lipid oxidation and sensory aspects, and fate of inoculated Clostridium perfringens. J. Food Saf. 2018, 38, e12459. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).