Olfactory and Gustatory Supra-Threshold Sensitivities Are Linked to Ad Libitum Snack Choice
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Overview of the Study
2.3. Sensory Experiments
2.3.1. Stimuli
2.3.2. Sensitivity Tests
2.4. Laboratory-Based Ad Libitum Snack Choice Task
2.5. Data Analysis
3. Results
3.1. Descriptive Statistics for Participant Characteristics and Snack Intake
3.2. Comparison of Sweet and Savoury Snack Intake between “High” and “Low” Sensitivity Groups
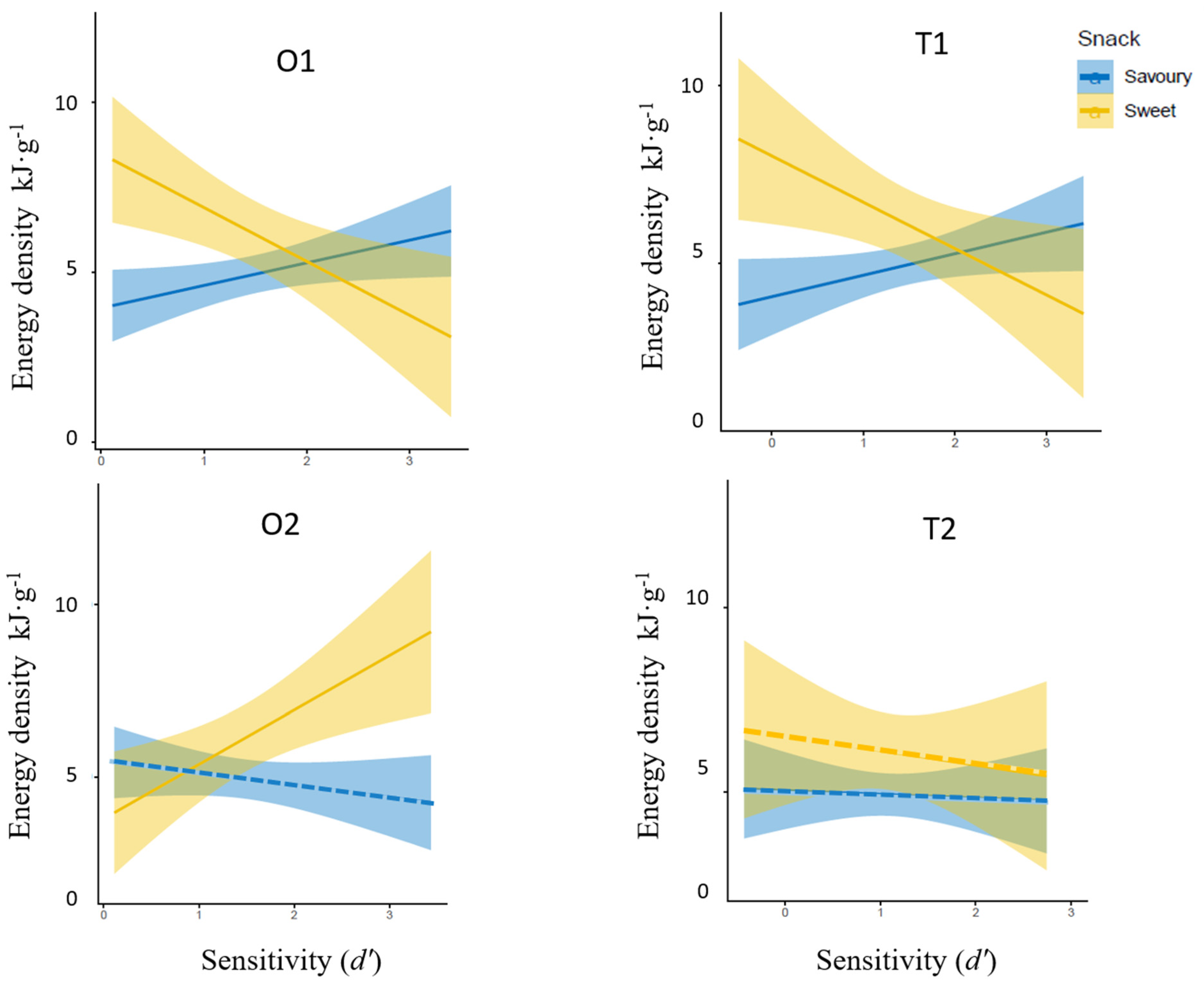
3.3. Relationship between Odour and Taste Sensitivities and Variabilities in Energy Density of Snack Choices
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zizza, C.; Siega-Riz, A.M.; Popkin, B.M. Significant Increase in Young Adults’ Snacking between 1977–1978 and 1994–1996 Represents a Cause for Concern! Prev. Med. 2001, 32, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, E.; Hutchinson, J.M.; Krystia, O.; Mirotta, J.A.; Mutch, D.M.; Buchholz, A.C.; Ma, D.W. Single Nucleotide Polymorphisms in Taste Receptor Genes Are Associated with Snacking Patterns of Preschool-Aged Children in the Guelph Family Health Study: A Pilot Study. Nutrients 2018, 10, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.J.; Popkin, B.M. Patterns and trends in food portion sizes, 1977–1998. JAMA 2003, 289, 450–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, R.; Cleveland, L.; Goldman, J.; Moshfegh, A. Snacking Behavior of Children and Teenagers in the United States; Federation of American Societies for Experimental Biology: Bethesda, MD, USA; Wiley Online Library: Hoboken, NJ, USA, 2006. [Google Scholar]
- Murakami, K.; Livingstone, M.B.E. Eating frequency is positively associated with overweight and central obesity in US adults. J. Nutr. 2015, 145, 2715–2724. [Google Scholar] [CrossRef] [Green Version]
- Macdiarmid, J.; Loe, J.; Craig, L.C.A.; Masson, L.; Holmes, B.; McNeill, G. Meal and snacking patterns of school-aged children in Scotland. Eur. J. Clin. Nutr. 2009, 63, 1297–1304. [Google Scholar] [CrossRef] [Green Version]
- Duffey, K.J.; Rivera, J.A.; Popkin, B.M. Snacking is prevalent in Mexico. J. Nutr. 2014, 144, 1843–1849. [Google Scholar] [CrossRef] [Green Version]
- Ovaskainen, M.-L.; Tapanainen, H.; Pakkala, H. Changes in the contribution of snacks to the daily energy intake of Finnish adults. Appetite 2010, 54, 623–626. [Google Scholar] [CrossRef]
- Mielmann, A.; Brunner, T.A. Consumers’ snack choices: Current factors contributing to obesity. Br. Food J. 2018, 121, 347–358. [Google Scholar] [CrossRef]
- O’Connor, L.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. The cross-sectional association between snacking behaviour and measures of adiposity: The Fenland Study, UK. Br. J. Nutr. 2015, 114, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- Raptou, E. The Role of Snack Choices, Body Weight Stereotypes and Smoking Behavior in Assessing Risk Factors for Adolescent Overweight and Obesity. Foods 2021, 10, 557. [Google Scholar] [CrossRef]
- Bolhuis, D.P.; Costanzo, A.; Newman, L.P.; Keast, R.S. Salt promotes passive overconsumption of dietary fat in humans. J. Nutr. 2015, 146, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleobury, L.; Tapper, K. Reasons for eating ‘unhealthy’snacks in overweight and obese males and females. J. Hum. Nutr. Diet. 2014, 27, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veling, H.; Aarts, H.; Stroebe, W. Using stop signals to reduce impulsive choices for palatable unhealthy foods. Br. J. Health Psychol. 2013, 18, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Lopez-Garcia, E.; Leon-Munoz, L.M.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Buckland, G.; Chirlaque, M.-D.; et al. Consumption of fried foods and risk of coronary heart disease: Spanish cohort of the European Prospective Investigation into Cancer and Nutrition study. BMJ 2012, 344, e363. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Cruz, A.; Bacardí-Gascón, M.; Jones, E.G. Consumption of fruits, vegetables, soft drinks, and high-fat-containing snacks among Mexican children on the Mexico-US border. Arch. Med. Res. 2002, 33, 74–80. [Google Scholar] [CrossRef]
- Julián-Almárcegui, C.; Vandevijvere, S.; Gottrand, F.; Beghin, L.; Dallongeville, J.; Sjöstrom, M.; Leclercq, C.; Manios, Y.; Widhalm, K.; De Morares, A.F.; et al. Association of heart rate and blood pressure among European adolescents with usual food consumption: The HELENA study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Siudikiene, J.; Maciulskiene, V.; Nedzelskiene, I. Dietary and oral hygiene habits in children with type I diabetes mellitus related to dental caries. Stomatologija 2005, 7, 58–62. [Google Scholar]
- Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Tanaka, M.; Fukui, M. Impact of different timing of consuming sweet snack on postprandial glucose excursions in healthy women. Diabetes Metab. 2019, 45, 369–374. [Google Scholar] [CrossRef]
- Keller, K.L.; English, L.K.; Fearnbach, S.N.; Lasschuijt, M.; Anderson, K.; Bermudez, M.; Fisher, J.O.; Rolls, B.J.; Wilson, S.J. Brain response to food cues varying in portion size is associated with individual differences in the portion size effect in children. Appetite 2018, 125, 139–151. [Google Scholar] [CrossRef]
- Peng, M.; Cahayadi, J.; Geng, X.; Eidels, A. Mixed messages: Assessing interactions between portion-size and energy-density perceptions in different weight and sex groups. Appetite 2020, 144, 104462. [Google Scholar] [CrossRef] [PubMed]
- Labbe, D.; Rytz, A.; Godinot, N.; Ferrage, A.; Martin, N. Is portion size selection associated with expected satiation, perceived healthfulness or expected tastiness? A case study on pizza using a photograph-based computer task. Appetite 2017, 108, 311–316. [Google Scholar] [PubMed] [Green Version]
- Feyzabadi, V.Y.; Mohammadi, N.K.; Omidvar, N.; Karimi-Shahanjarini, A.; Nedjat, S.; Rashidian, A. Factors associated with unhealthy snacks consumption among adolescents in Iran’s schools. Int. J. Health Policy Manag. 2017, 6, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdenzi, C.; Roberts, C.; Schirmer, A.; Delplanque, S.; Cekic, S.; Porcherot, C.; Cayeux, I.; Sander, D.; Grandjean, D. Variability of affective responses to odors: Culture, gender, and olfactory knowledge. Chem. Senses 2013, 38, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Rabin, M.D.; Cain, W.S. Determinants of measured olfactory sensitivity. Percept. Psychophys. 1986, 39, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic review of olfactory shifts related to obesity. Obes. Rev. 2019, 20, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Schriever, V.A.; Gudziol, V.; Hummel, C.; Hähner, A.; Iannilli, E.; Sinding, C.; Aziz, M.; Seo, H.S.; Negoias, S.; et al. Changes of olfactory abilities in relation to age: Odor identification in more than 1400 people aged 4 to 80 years. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 1937–1944. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.; Hempstead, M.; Gomez, I.A.; Gilbert, A.N.; Vosshall, L.B. An olfactory demography of a diverse metropolitan population. BMC Neurosci. 2012, 13, 122. [Google Scholar] [CrossRef] [Green Version]
- Abeywickrema, S.; Ginieis, R.; Oey, I.; Peng, M. An empirical evaluation of supra-threshold sensitivity measures for decremental and incremental stimulus intensity: Data from gustatory and olfactory performance. Food Qual. Prefer. 2022, 97, 104457. [Google Scholar] [CrossRef]
- Jaeger, S.; McRae, J.F.; Bava, C.M.; Beresford, M.K.; Hunter, D.; Jia, Y.; Chheang, S.L.; Jin, D.; Peng, M.; Gamble, J.C.; et al. A Mendelian Trait for Olfactory Sensitivity Affects Odor Experience and Food Selection. Curr. Biol. 2013, 23, 1601–1605. [Google Scholar] [CrossRef] [Green Version]
- Stafford, L.D.; Whittle, A. Obese Individuals Have Higher Preference and Sensitivity to Odor of Chocolate. Chem. Senses 2015, 40, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Vander Woude, E.A.; Sudan, R.; Thompson, J.S.; Leopold, D.A. Altered olfactory acuity in the morbidly obese. Obes. Surg. 2004, 14, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, N.; Johnson, M.C.; Butler, J.L.; Bell, G.A.; Ferguson, K.L.; Fadool, A.R.; Fadool, J.C.; Gale, A.M.; Gale, D.S.; Fadool, D.A. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J. Neurosci. 2014, 34, 6970–6984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, S.; Holthuysen, N.; Boesveldt, S. The influence of olfactory impairment in vital, independently living older persons on their eating behaviour and food liking. Food Qual. Prefer. 2014, 38, 30–39. [Google Scholar] [CrossRef]
- Boesveldt, S.; Bobowski, N.; McCrickerd, K.; Maître, I.; Sulmont-Rossé, C.; Forde, C. The changing role of the senses in food choice and food intake across the lifespan. Food Qual. Prefer. 2018, 68, 80–89. [Google Scholar] [CrossRef]
- Ginieis, R.; Abeywickrema, S.; Oey, I.; Franz, E.A.; Perry, T.; Keast, R.S.; Peng, M. The role of an individual’s olfactory discriminability in influencing snacking and habitual energy intake. Appetite 2021, 167, 105646. [Google Scholar] [CrossRef]
- Stewart, J.; Keast, R. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.; Newman, L.P.; Keast, R.S. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef]
- Heinze, J.M.; Costanzo, A.; Baselier, I.; Fritsche, A.; Frank-Podlech, S.; Keast, R. Detection thresholds for four different fatty stimuli are associated with increased dietary intake of processed high-caloric food. Appetite 2018, 123, 7–13. [Google Scholar] [CrossRef]
- Kim, G.; Lee, H. Frequent consumption of certain fast foods may be associated with an enhanced preference for salt taste. J. Hum. Nutr. Diet. 2009, 22, 475–480. [Google Scholar] [CrossRef]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is sweet taste perception associated with sweet food liking and intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Cordero, E.; Malacara-Hernandez, J.M.; Martinez-Cordero, C. Taste perception in normal and overweight Mexican adults. Appetite 2015, 89, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Duizer, L. The effect of frequency of consumption of artificial sweeteners on sweetness liking by women. J. Food Sci. 2007, 72, S714–S718. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Keast, R.S.; Roura, E. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br. J. Nutr. 2017, 118, 763–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keast, R.S.; Roper, J. A complex relationship among chemical concentration, detection threshold, and suprathreshold intensity of bitter compounds. Chem. Senses 2007, 32, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, N.; Beno, N.; Septier, C.; Salles, C.; Thomas-Danguin, T. Cross-modal interactions between taste and smell: Odour-induced saltiness enhancement depends on salt level. Food Qual. Prefer. 2011, 22, 678–682. [Google Scholar] [CrossRef]
- Ginieis, R.; Abeywickrema, S.; Oey, I.; Keast, R.S.; Peng, M. Searching for individual multi-sensory fingerprints and their links with adiposity—New insights from meta-analyses and empirical data. Food Qual. Prefer. 2022, 104574. [Google Scholar] [CrossRef]
- Dravnieks, A.; Masurat, T.; Lamm, R.A. Hedonics of odors and odor descriptors. J. Air Pollut. Control Assoc. 1984, 34, 752–755. [Google Scholar] [CrossRef]
- Harper, R.; Smith, E.; Land, D.G. Odour Description and Odour Classification: A Multidisciplinary Examination; American Psychological Association: Washington, DC, USA, 1968. [Google Scholar]
- Stevenson, R.J.; Prescott, J.; Boakes, R.A. The acquisition of taste properties by odors. Learn. Motiv. 1995, 26, 433–455. [Google Scholar] [CrossRef]
- Boesveldt, S.; de Graaf, K. The differential role of smell and taste for eating behavior. Perception 2017, 46, 307–319. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Boakes, S. That smells filling: Effects of pairings of odours with sweetness and thickness on odour perception and expected satiety. Food Qual. Prefer. 2016, 54, 128–136. [Google Scholar] [CrossRef]
- Yin, W.; Hewson, L.; Linforth, R.; Taylor, M.; Fisk, I.D. Effects of aroma and taste, independently or in combination, on appetite sensation and subsequent food intake. Appetite 2017, 114, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol. Behav. 2005, 85, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Williamson, A.M.; Hasted, A.; Hort, J. Exploring the relationships between taste phenotypes, genotypes, ethnicity, gender and taste perception using Chi-square and regression tree analysis. Food Qual. Prefer. 2020, 83, 103928. [Google Scholar] [CrossRef]
- Sorokowski, P.; Karwowski, M.; Misiak, M.; Marczak, M.K.; Dziekan, M.; Hummel, T.; Sorokowska, A. Sex differences in human olfaction: A meta-analysis. Front. Psychol. 2019, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Van Strien, T.; Frijters, J.E.; Bergers, G.P.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- World Health Organization. Body Mass Index (BMI) Standards. 2021. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 8 February 2022).
- Aliani, M.; Ryland, D.; Pierce, G.N. Effect of flax addition on the flavor profile of muffins and snack bars. Food Res. Int. 2011, 44, 2489–2496. [Google Scholar] [CrossRef]
- Vehmas, K.; Calton, A.; Grenman, K.; Aisala, H.; Sozer, N.; Nordlund, E. Development and Consumer Perception of a Snack Machine Producing Customized Spoonable and Drinkable Products Enriched in Dietary Fiber and Protein. Foods 2020, 9, 1454. [Google Scholar] [CrossRef]
- Parnell, W.; Wilson, N.; Thomson, C.; Mackay, S.; Stefanogiannis, N. A Focus on Nutrition: Key Findings of the 2008/09 New Zealand Adult Nutrition Survey; Ministry of Health: Wellington, New Zealand, 2011.
- Koubaa, Y. Odour-induced taste enhancement and consumption of low-sugar pastry. Int. J. Mark. Res. 2017, 59, 749–765. [Google Scholar] [CrossRef]
- Zoon, H.F.; de Graaf, C.; Boesveldt, S. Food odours direct specific appetite. Foods 2016, 5, 12. [Google Scholar] [CrossRef]
- Macmillan; Creelman, C. Detection Theory: A User’s Guide; Lawrence Earlbaum Associates Inc.: Mahwah, NJ, USA, 2005. [Google Scholar]
- Beck, K.L.; Jones, B.; Ullah, I.; McNaughton, S.A.; Haslett, S.J.; Stonehouse, W. Associations between dietary patterns, socio-demographic factors and anthropometric measurements in adult New Zealanders: An analysis of data from the 2008/09 New Zealand Adult Nutrition Survey. Eur. J. Nutr. 2018, 57, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.; Casswell, S.; Maskill, C.; Jones, S.; Wyllie, A. Fruit and vegetables as adolescent food choices in New Zealand. Health Promot. Int. 1998, 13, 55–65. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Wright, S.M.; Paluch, R.A.; Leddy, J.; Hawk, L.W., Jr.; Jaroni, J.L.; Saad, F.G.; Crystal-Mansour, S.; Lermane, C. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol. Behav. 2004, 81, 511–517. [Google Scholar] [CrossRef]
- Feig, E.H.; Piers, A.D.; Kral, T.V.; Lowe, M.R. Eating in the absence of hunger is related to loss-of-control eating, hedonic hunger, and short-term weight gain in normal-weight women. Appetite 2018, 123, 317–324. [Google Scholar] [CrossRef]
- Halford, J.C.; Boyland, E.J.; Hughes, G.; Oliveira, L.P.; Dovey, T.M. Beyond-brand effect of television (TV) food advertisements/commercials on caloric intake and food choice of 5–7-year-old children. Appetite 2007, 49, 263–267. [Google Scholar] [CrossRef]
- Stinson, E.J.; Piaggi, P.; Ibrahim, M.; Venti, C.; Krakoff, J.; Votruba, S.B. High Fat and Sugar Consumption During Ad Libitum Intake Predicts Weight Gain. Obesity 2018, 26, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.M.; Choi, I.S. A study on obesity and food habit of adolescents in Yeosu, Jeonnam area. Korean J. Community Nutr. 2003, 8, 129–137. [Google Scholar]
- Mulder, L.B.; Rupp, D.E.; Dijkstra, A. Making snacking less sinful: (Counter-) moralising obesity in the public discourse differentially affects food choices of individuals with high and low perceived body mass. Psychol. Health 2015, 30, 233–251. [Google Scholar] [CrossRef]
- Smith, J.M.; Ditschun, T.L. Controlling satiety: How environmental factors influence food intake. Trends Food Sci. Technol. 2009, 20, 271–277. [Google Scholar] [CrossRef]
- Edwards, J.S.; Gustafsson, I.B. The room and atmosphere as aspects of the meal: A review. J. Foodserv. 2008, 19, 22–34. [Google Scholar] [CrossRef]
- Cicerale, S.; Riddell, L.J.; Keast, R.S. The association between perceived sweetness intensity and dietary intake in young adults. J. Food Sci. 2012, 77, H31–H35. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalil Mozhdehi, F.; Abeywickrema, S.; Bremer, P.J.; Peng, M. Comparing Taste Detection Thresholds across Individuals Following Vegan, Vegetarian, or Omnivore Diets. Foods 2021, 10, 2704. [Google Scholar] [CrossRef]
- Smith, S.L.; Ludy, M.-J.; Tucker, R.M. Changes in taste preference and steps taken after sleep curtailment. Physiol. Behav. 2016, 163, 228–233. [Google Scholar] [CrossRef]
- Low, J.Y.; Lacy, K.E.; McBride, R.L.; Keast, R.S. The associations between oral complex carbohydrate sensitivity, BMI, liking, and consumption of complex carbohydrate based foods. J. Food Sci. 2018, 83, 2227–2236. [Google Scholar] [CrossRef]
- Vandenbroele, J.; Van Kerckhove, A.; Zlatevska, N. Portion size effects vary: The size of food units is a bigger problem than the number. Appetite 2019, 140, 27–40. [Google Scholar] [CrossRef]
- Robinson, E.; Oldham, M.; Cuckson, I.; Brunstrom, J.M.; Rogers, P.J.; Hardman, C.A. Visual exposure to large and small portion sizes and perceptions of portion size normality: Three experimental studies. Appetite 2016, 98, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Oliver, G.; Wardle, J.; Gibson, E.L. Stress and food choice: A laboratory study. Psychosom. Med. 2000, 62, 853–865. [Google Scholar] [CrossRef]
- Vatanparast, H.; Islam, N.; Masoodi, H.; Shafiee, M.; Patil, R.P.; Smith, J.; Whiting, S.J. Time, location and frequency of snack consumption in different age groups of Canadians. Nutr. J. 2020, 19, 85. [Google Scholar] [CrossRef]
- Elena, F.; Maria, B. Snack patterns of Greek adults 20–50 years of age. J. Foodserv. 2006, 17, 197–204. [Google Scholar] [CrossRef]
- Simmons, J.P.; Nelson, L.D.; Simonsohn, U. False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol. Sci. 2011, 22, 1359–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, E.; Bevelander, K.E.; Field, M.; Jones, A. Methodological and reporting quality in laboratory studies of human eating behavior. Appetite 2018, 125, 486–491. [Google Scholar] [CrossRef] [PubMed]

| Sensory Modality | Code | Chemical Name | Supplier | Descriptor | Concentration Range | Reference Concentration/Log Step |
|---|---|---|---|---|---|---|
| Olfactory | O1 | 4-Hydroxy-3-methoxybenzaldehyde | Vanesse, Camlin Fine Sciences, Mumbai, India | Vanillin; Sweet-eliciting odour | 0.657 to 5.000 g·L−1 | 0.395 g·L−1 0.221 log |
| O2 | 3-(Methylthio) propionaldehyde | Sigma-Aldrich, Burlington, MA, USA | Methional Potato odour; Savoury-eliciting odour | 0.260 to 1.000 μL·L−1 | 0.186 μL·L−1 0.146 log | |
| Gustatory | T1 | Sucrose | Chelsea, Auckland, New Zealand | Sweet | 9.537 to 15.100 g·L−1 | 8.5 g·L−1 0.050 log |
| T2 | Salt (NaCl) | Labochem International, Frankfurt, Germany | Salty/savoury | 1.683 to 2.667 g·L−1 | 1.5 g·L−1 0.050 log |
| Snack Category | Snack Item | Brand Details | Energy Content (kJ per 100 g) | Sugar Content in g (per 100 g) |
|---|---|---|---|---|
| Sweet | Chocolate | Cadbury® Old Gold Chocolate, Mondelez Pty Ltd., Auckland, New Zealand | 2372 | 56 |
| Chocolate chip cookies | CookieTime® Choco Chunk, CookieTime, Christchurch, New Zealand | 2010 | 39 | |
| Crunchy granola | Muerli O & G® Blueberry and Coconut, Nestle, Auckland, New Zealand | 1710 | 23 | |
| Red apples (Royal gala) | Fresh Produce Group, Auckland, New Zealand | 218 | 10 | |
| NaCl in mg (per 100 g) | ||||
| Savoury | Salted peanut | ETA® foods, Auckland, New Zealand | 2440 | 620 |
| Corn chips | Mexicano® Tasty Salsa, Wellington, New Zealand | 1777 | 525 | |
| Rice crackers | Pekish® sour cream and chives, Monde Nissin, Mulgrave, Australia | 1775 | 430 | |
| Steamed broccoli—with 0.05% w/w added salt | Fresh produce group, Auckland, New Zealand | 142 | 261 |
| Mean ± Standard Deviation | Range | |
|---|---|---|
| Age (years) | 26 ± 6 | 21–39 |
| BMI (kg∙m−2) | 27.1 ± 5.1 | 20.5–40.5 |
| DEBQ * score | ||
| Restrained eating | 2.2 ± 0.7 | 1.0–3.9 |
| Emotional eating | 2.3 ± 0.9 | 0.8–4.7 |
| External eating | 3.3 ± 0.6 | 2.0–4.7 |
| Baseline hunger level | 20.2 ± 11.3 | 3.1–50.4 |
| Snack Category | Weight (in Grams) | Weight Percentage | Energy (in kJ) | Energy Density (kJ·g−1) |
|---|---|---|---|---|
| Sweet | 99.7 ± 56.7 | 61.1 ± 22.5 | 610 ± 426 | 6.1 ± 4.6 |
| Savoury | 63.7 ± 46.6 | 38.9 ± 22.5 | 311 ± 278 | 5.5 ± 4.6 |
| t-statistic | −4.07 | −5.82 | −4.88 | −0.90 |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.368 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeywickrema, S.; Ginieis, R.; Oey, I.; Peng, M. Olfactory and Gustatory Supra-Threshold Sensitivities Are Linked to Ad Libitum Snack Choice. Foods 2022, 11, 799. https://doi.org/10.3390/foods11060799
Abeywickrema S, Ginieis R, Oey I, Peng M. Olfactory and Gustatory Supra-Threshold Sensitivities Are Linked to Ad Libitum Snack Choice. Foods. 2022; 11(6):799. https://doi.org/10.3390/foods11060799
Chicago/Turabian StyleAbeywickrema, Sashie, Rachel Ginieis, Indrawati Oey, and Mei Peng. 2022. "Olfactory and Gustatory Supra-Threshold Sensitivities Are Linked to Ad Libitum Snack Choice" Foods 11, no. 6: 799. https://doi.org/10.3390/foods11060799
APA StyleAbeywickrema, S., Ginieis, R., Oey, I., & Peng, M. (2022). Olfactory and Gustatory Supra-Threshold Sensitivities Are Linked to Ad Libitum Snack Choice. Foods, 11(6), 799. https://doi.org/10.3390/foods11060799







