Inhibitory Effect of Lactiplantibacillusplantarum and Lactococcus lactis Autochtonous Strains against Listeria monocytogenes in a Laboratory Cheese Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Antimicrobial Activity Assay
2.3. Technological Characteristics
2.4. Preparation of the Inoculums
2.5. Miniature Fresh Cheese Manufacture and “In Situ” Antilisterial Activity of LAB
2.6. Statistical Analysis
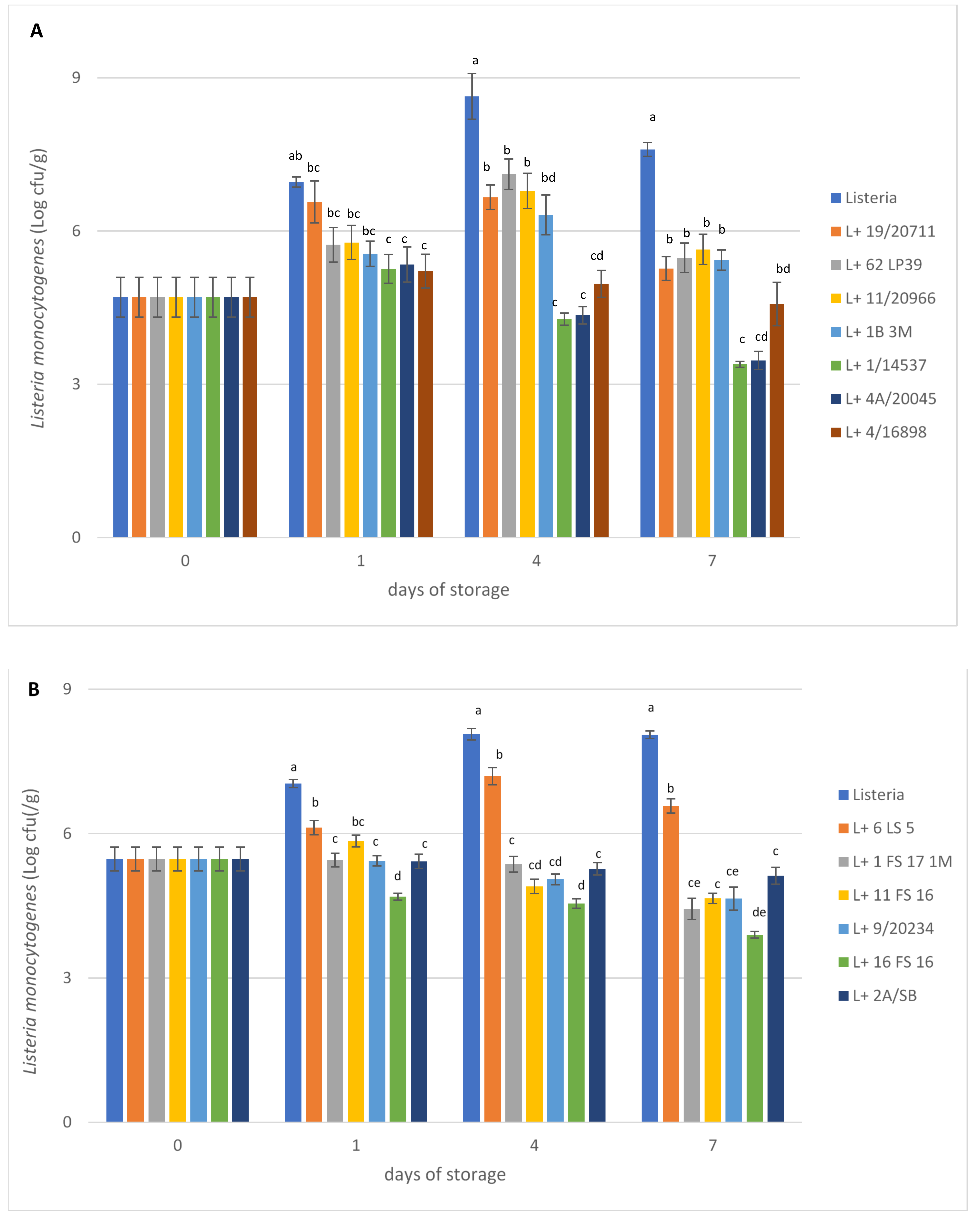
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul Ross, R.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, D.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Cosentino, S.; Viale, S.; Deplano, M.; Fadda, M.E.; Pisano, M.B. Application of autochthonous Lactobacillus strains as biopreservatives to control fungal spoilage in caciotta cheese. Hindawi BioMed Res. Int. 2018, 2018, 3915615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015, 13, 4138. [Google Scholar]
- National Research Council 1978. 1975 Resurvey of the Annual Poundage of Food Chemicals Generally Recognized as Safe (GRAS); National Academies Press: Washington, DC, USA, 1978. [Google Scholar]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grosu-Tudor, S.S.; Stancu, M.M.; Pelinescu, D.; Zamfir, M. Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World J. Microbiol. Biotechnol. 2014, 30, 2459–2469. [Google Scholar] [CrossRef]
- Rezpkowska, A.; Zielińska, D.; Ołdak, A.; Kołożyn-Krajewska, D. Organic whey as a source of Lactobacillus strains with selected technological and antimicrobial properties. Int. J. Food Sci. Technol. 2017, 52, 1983–1994. [Google Scholar] [CrossRef]
- Favaro, L.; Penna, A.L.B.; Todorov, S.D. Bacteriocinogenic LAB from cheeses—Application in biopreservation? Trends Food Sci. Technol. 2015, 41, 37–48. [Google Scholar] [CrossRef]
- Blana, V.A.; Grounta, A.; Tassou, C.C.; Nychas, G.-J.E.; Panagou, E.Z. Inoculated fermentation of green olives with potential probiotic Lactobacillus pentosus and Lactobacillus plantarum starter cultures isolated from industrially fermented olives. Food Microbiol. 2014, 38, 208–218. [Google Scholar] [CrossRef]
- Nieto-Arribas, P.; Poveda, J.M.; Seseña, S.; Palop, L.; Cabezas, L. Technological characterization of Lactobacillus isolates from traditional Manchego cheese for potential use as adjunct starter cultures. Food Control 2009, 20, 1092–1098. [Google Scholar] [CrossRef]
- Gálvez, A.; López, R.L.; Abriouel, H.; Valdivia, E.; Omar, N.B. Application of bacteriocins in the control of food borne pathogenic and spoilage bacteria. Crit. Rev. Biotechnol. 2008, 28, 125–152. [Google Scholar] [CrossRef]
- Coelho, M.C.; Silva, C.C.G.; Ribeiro, S.C.; Dapkevicius, M.L.N.E.; Rosa, H.J.D. Control of Listeria monocytogenes in fresh cheese using protective lactic acid bacteria. Food Microbiol. 2014, 191, 53–59. [Google Scholar] [CrossRef]
- Gonzáles, L.; Sandoval, H.; Sacristán, N.; Castro, J.; Fresno, J.; Tornadijo, M. Identification of lactic acid bacteria isolated from Genestoso cheese throughout ripening and study of their antimicrobial activity. Food Control 2007, 18, 716–722. [Google Scholar] [CrossRef]
- Stiles, M.E.; Holzapfel, W.H. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Teixeira, P. Pediococcus acidilactici as a potential probiotic to be used in food industry. Int. J. Food Sci. Technol. 2015, 50, 1151–1157. [Google Scholar] [CrossRef]
- Valenzuela, J.F.; Pinuer, L.A.; Cancino, A.G.; Yáñez, R.B. Metabolic fluxes in Lactic acid bacteria—A review. Food Biotechnol. 2015, 29, 185–217. [Google Scholar] [CrossRef]
- Siezen, R.J.; Tzeneva, V.A.; Castioni, A.; Wels, M.W.W.; Phan, H.T.; Rademaker, J.L.W.; Starrenburg, M.J.C.; Kleerebezem, M.; Molenaar, D.; van Hylckama Vlieg, J.E.T. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ. Microbiol. 2010, 12, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Guidone, A.; Zotta, T.; Ross, R.P.; Stanton, C.; Rea, M.C.; Parente, E.; Ricciardi, A. Functional properties of Lactobacillus plantarum strains: A multivariate screening study. LWT Food Sci. Technol. 2014, 56, 69–76. [Google Scholar] [CrossRef]
- Rodríguez-Pazo, N.; Vázquez-Araújo, L.; Pérez-Rodríguez, N.; Cortés-Diéguez, S.; Domínguez, J.M. Cell-free supernatants obtained from fermentation of cheese whey hydrolyzates and phenylpyruvic acid by Lactobacillus plantarum as a source of antimicrobial compounds, bacteriocins, and natural aromas. Appl. Biochem. Biotechnol. 2013, 171, 1042–1060. [Google Scholar] [CrossRef]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of organic acids by probiotic Lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Lo, R.; Bansal, N.; Turner, M.S. Characterisation of Lactococcus lactis isolates from herbs, fruits and vegetables for use as biopreservatives against Listeria monocytogenes in cheese. Food Control 2018, 85, 472–483. [Google Scholar] [CrossRef] [Green Version]
- López-Díaz, T.M.; Alonso, C.; Román, C.; García-López, M.L.; Moreno, B. Lactic acid isolates from a hand-made blue cheese. Food Microbiol. 2000, 17, 23–32. [Google Scholar] [CrossRef]
- Dal Bello, B.; Cocolin, L.; Zeppa, G.; Field, D.; Cotter, P.D.; Hill, C. Technological characterization of bacteriocin producing Lactococcus lactis strains employed to control Listeria monocytogenes in Cottage cheese. Int. J. Food Microbiol. 2012, 153, 58–65. [Google Scholar] [CrossRef]
- Hanning, I.B.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Food safety and food security. Nat. Educ. Knowl. 2012, 3, 9. [Google Scholar]
- Brooks, J.C.; Martinez, B.; Stratton, J.; Bianchini, A.; Krokstrom, R.; Hutkins, R. Survey of raw milk cheeses for microbiological quality and prevalence of foodborne pathogens. Food Microbiol. 2012, 31, 154–158. [Google Scholar] [CrossRef]
- De Castro, V.; Escudero, J.M.; Rodriguez, J.L.; Muniozguren, N.; Uribarri, J.; Saez, D.; Vazquez, J. Listeriosis outbreak caused by Latin-style fresh cheese, Bizkaia, Spain. Eurosurveillance 2012, 17, 8–10. [Google Scholar] [CrossRef]
- Schlech, W.F., III; Acheson, D. Foodborne listeriosis. Clin. Infect. Dis. 2000, 31, 770–775. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.B.; Jones, M.V.; Holyoak, C. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J. Appl. Bacteriol. 1990, 69, 63–72. [Google Scholar] [CrossRef]
- McIntyre, L.; Wilcott, L.; Naus, M. Listeriosis outbreaks in British Columbia, Canada, caused by soft ripened cheese contaminated from environmental sources. BioMed Res. Int. 2015, 2015, 131623. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Dworak, R.; Prager, R.; Becker, B.; Brockmann, S.; Wicke, A.; Wichmann-Schauer, H.; Hof, H.; Werber, D.; Stark, K. Large listeriosis outbreak linked to cheese made from pasteurized milk, Germany, 2006–2007. Foodborne Pathog. Dis. 2010, 7, 1581–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, S.-I.; Kawamoto, K.; Takeshi, K.; Okada, Y.; Yamasaki, M.; Yamamoto, S.; Igimi, S. An outbreak of food-borne listeriosis due to cheese in Japan, during 2001. Int. J. Food Microbiol. 2005, 104, 189–196. [Google Scholar] [CrossRef]
- Rogga, K.J.; Samelis, J.; Kakouri, A.; Katsiari, M.C.; Savvaidis, I.N.; Kontominas, M.G. Survival of Listeria monocytogenes in Galotyri, a traditional Greek soft acid-curd cheese, stored aerobically at 4 °C and 12 °C. Int. Dairy J. 2005, 15, 59–67. [Google Scholar] [CrossRef]
- Kabuki, D.Y.; Kuaye, A.Y.; Wiedmann, M.; Boor, K.J. Molecular subtyping and tracking of Listeria monocytogenes in Latin-style fresh-cheese processing plants. J. Dairy Sci. 2004, 87, 2803–2812. [Google Scholar] [CrossRef] [Green Version]
- Jackson, K.A.; Gould, L.H.; Hunter, J.C.; Kucerova, Z.; Jackson, B. Listeriosis outbreaks associated with soft cheeses, United States, 1998–2014. Emerg. Infect. Dis. 2018, 24, 1116–1118. [Google Scholar] [CrossRef]
- Cosentino, S.; Fadda, M.E.; Deplano, M.; Melis, R.; Pomata, R.; Pisano, M.B. Antilisterial activity of nisin-like bacteriocin producing Lactococcus lactis subsp. lactis isolated from traditional sardinian dairy products. J. Biomed. Biotechnol. 2012, 2012, 376428. [Google Scholar]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of antibacterial activity of Lactobacillus plantarum strains isolated from two different kinds of regional cheeses from Poland: Oscypek and korycinski cheese. BioMed Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef] [Green Version]
- Pisano, M.B.; Viale, S.; Conti, S.; Fadda, M.E.; Deplano, M.; Melis, M.P.; Deiana, M.; Cosentino, S. Preliminary evaluation of probiotic properties of Lactobacillus strains isolated from Sardinian dairy products. BioMed Res. Int. 2014, 2014, 286390. [Google Scholar] [CrossRef] [Green Version]
- Pisano, M.B.; Patrignani, F.; Cosentino, S.; Guerzoni, M.E.; Franz, C.M.A.P.; Holzapfel, W.H. Diversity and functional properties of Lactobacillus plantarum-group strains isolated from Italian cheese products. Dairy Sci. Technol. 2010, 91, 65–76. [Google Scholar]
- Scano, P.; Pisano, M.B.; Murgia, A.; Cosentino, S.; Caboni, P. GC-MS metabolomics and antifungal characteristics of autochthonous Lactobacillus strains. Dairy 2021, 2, 326–335. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Deplano, M.; Corda, A.; Cosentino, S. Microbiological and chemical characterization of Fiore Sardo, a traditional Sardinian cheese made from ewe’s milk. Int. J. Dairy Technol. 2006, 59, 171–179. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Melis, R.; Ciusa, M.L.; Viale, S.; Deplano, M.; Cosentino, S. Molecular identification of bacteriocins produced by Lactococcus lactis dairy strains and their technological and genotypic characterization. Food Control 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Siroli, L.; Camprini, L.; Pisano, M.B.; Patrignani, F.; Lanciotti, R. Volatile molecule profiles and anti-Listeria monocytogenes activity of nisin producers Lactococcus lactis strains in vegetable drinks. Front. Microbiol. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Bukvicki, D.; Siroli, L.; D’Alessandro, M.; Cosentino, S.; Fliss, I.; Said, L.B.; Hassan, H.; Lanciotti, R.; Patrignani, F. Unravelling the potential of Lactococcus lactis strains to be used in cheesemaking production as biocontrol agents. Foods 2020, 9, 1815. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Pisano, B.; Piras, C.; Deplano, M.; Palmas, F. Chatacterization of lactic acid bacteria isolated from traditional Fiore Sardo cheese. In Proceedings of the International Conference SFAM, Wageningen, The Netherlands, 9–11 January 2002; pp. 104–107. [Google Scholar]
- Pisano, M.B.; Casula, M.; Corda, A.; Fadda, M.E.; Deplano, M.; Cosentino, S. In vitro probiotic characteristics of Lactobacillus strains isolated from Fiore Sardo cheese. Ital. J. Food Sci. 2008, 20, 505–516. [Google Scholar]
- Schillinger, U.; Lücke, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef] [Green Version]
- Galeslod, T.E.; Hassing, F.; Stadhouders, J. Agar medium for the isolation and enumeration of aroma bacteria in starters. Neth. Milk Dairy J. 1961, 15, 127–129. [Google Scholar]
- Perez, G.; Cardell, E.; Zarate, V. Technological characterization of lactic acid bacteria from Tenerife cheese. Int. J. Food Sci. Technol. 2003, 38, 537–546. [Google Scholar] [CrossRef]
- Georgieva, R.N.; Iliev, I.N.; Chipeva, V.A.; Dimitonova, S.P.; Samelis, J.; Danova, S.T. Identification and in vitro characterization of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J. Basic Microbiol. 2008, 48, 234–244. [Google Scholar] [CrossRef]
- Zago, M.; Fornasari, M.E.; Carminati, D.; Burns, P.; Suàrez, V.; Vinderola, G.; Reinheimer, J.; Giraffa, G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011, 28, 1033–1040. [Google Scholar] [CrossRef]
- Nero, L.A.; Mattos, M.R.; Beloti, V.; Barros, M.A.F.; Ortolani, M.B.T.; Franco, B.D.G.M. Autochthonous microbiota of raw milk with antagonistic activity against Listeria monocytogenes and Salmonella enteritidis. J. Food Saf. 2009, 29, 261–270. [Google Scholar] [CrossRef]
- Kondrotiene, K.; Kasnauskyte, N.; Serniene, L.; Gölz, G.; Alter, T.; Kaskoniene, V.; Maruska, A.S.; Malakauskas, M. Characterization and application of newly isolated nisin producing Lactococcus lactis strains for control of Listeria monocytogenes growth in fresh cheese. LWT—Food Sci. Technol. 2018, 87, 507–514. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacterial against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falardeau, J.; Trmčić, A.; Wang, S. The occurrence, growth, and biocontrol of Listeria monocytogenes in fresh and surface-ripened soft and semisoft cheeses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4019–4048. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, B.; Zeppa, G.; Bianchi, D.M.; Decastelli, L.; Traversa, A.; Gallina, S.; Coisson, J.D.; Locatelli, M.; Travaglia, F.; Cocolin, L. Effect of nisin-producing Lactococcus lactis starter cultures on the inhibition of two pathogens in ripened cheeses. Int. J. Dairy Technol. 2013, 66, 468–477. [Google Scholar]
- Furtado, D.N.; Todorov, S.D.; Landgraf, M.; Destro, M.T.; Franco, B.D.G.M. Bacteriocinogenic Lactococcus lactis subsp. lactis DF04Mi isolated from goat milk: Application in the control of Listeria monocytogenes in fresh Minas-type goat cheese. Braz. J. Microbiol. 2015, 46, 201–206. [Google Scholar]
- Samelis, J.; Giannou, E.; Pappa, E.C.; Bogović-Matijašić, B.; Lianou, A.; Parapouli, M.; Drainas, C. Behavior of artificial listerial contamination in model Greek Graviera cheeses manufactured with the indigenous nisin A-producing strain Lactococcus lactis subsp. cremoris M104 as costarter culture. J. Food Saf. 2017, 37, e12326. [Google Scholar]
- Martinez-Rios, V.; Gkogka, E.; Dalgaard, P. New term to quantify the effect of temperature on pHmin-values used in cardinal parameter growth models for Listeria monocytogenes. Front. Microbiol. 2019, 10, 1510. [Google Scholar] [CrossRef] [Green Version]
- Campagnollo, F.B.; Margalho, L.P.; Kamimura, B.A.; Feliciano, M.D.; Freire, L.; Lopes, L.S.; Alvarenga, V.O.; Cadavez, V.A.P.; Gonzales-Barron, U.; Schaffner, D.W.; et al. Selection of indigenous lactic acid bacteria presenting anti-listerial activity, and their role in reducing the maturation period and assuring the safety of traditional Brazilian cheeses. Food Microbiol. 2018, 73, 288–297. [Google Scholar] [CrossRef]
- Henderson, L.O.; Erazo Flores, B.J.; Skeens, J.; Kent, D.; Murphy, S.I.; Wiedmann, M.; Guariglia-Oropeza, V. Nevertheless, she resisted—Role of the environment on Listeria monocytogenes sensitivity to nisin treatment in a laboratory cheese model. Front. Microbiol. 2020, 11, 635. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, E.; Calzada, J.; Arqués, J.L.; Rodríguez, J.M.; Nuñez, M.; Medina, M. Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 in cheese. Int. Dairy J. 2005, 15, 51–57. [Google Scholar] [CrossRef]
- Ahmadzadeh Nia, S.; Hanifian, S. Survival of Listeria monocytogenes strains in ultra-filtered white cheese: Effect of Lactobacillus plantarum and incubation period. J. Food Process. Preserv. 2017, 41, e13283. [Google Scholar] [CrossRef]
- Panebianco, F.; Giarratana, F.; Caridi, A.; Sidari, R.; De Bruno, A.; Giuffrida, A. Lactic acid bacteria isolated from traditional Italian dairy products: Activity against Listeria monocytogenes and modelling of microbial competition in soft cheese. LWT—Food Sci. Technol. 2021, 137, 110446. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Moreno, I.; Kuaye, A.Y. Applicability of bacteriocin-producing Lactobacillus plantarum, Enterococcus faecium and Lactococcus lactis ssp. lactis as adjunct starter in Minas Frescal cheesemaking. Int. J. Dairy Technol. 2008, 61, 352–357. [Google Scholar]
| Strains | Casein Hydrolysis | Lipolytic Activity § | Citrate Utilization | Acidifying Activity * | |
|---|---|---|---|---|---|
| ΔpH (6 h) | ΔpH (24 h) | ||||
| Lactococcus lactis | |||||
| 16FS16 | + | - | - | 0.91 ± 0.05 | 1.84 ± 0.13 |
| 11FS16 | - | - | + | 0.73 ± 0.11 | 1.22 ± 0.10 |
| 6LS5 | + | - | - | 0.71 ± 0.08 | 1.44 ± 0.07 |
| 1FS171M | + | - | - | 0.64 ± 0.08 | 1.24 ± 0.15 |
| 2A/SB | + | - | - | 0.41 ± 0.06 | 1.90 ± 0.03 |
| 9/20234 | + | - | - | 0.57 ± 0.09 | 1.39 ± 0.12 |
| Lactiplantibacillus plantarum | |||||
| 62LP39b | + | - | + | 0.66 ± 0.10 | 1.14 ± 0.14 |
| 11/20966 | + | - | + | 0.67 ± 0.09 | 1.29 ± 0.07 |
| 4A/20045 | - | - | + | 0.50 ± 0.11 | 1.14 ± 0.13 |
| 19/20711 | + | - | + | 0.60 ± 0.03 | 1.08 ± 0.12 |
| 1B3M | - | - | + | 0.63 ± 0.12 | 1.15 ± 0.16 |
| 4/16898 | + | - | + | 1.09 ± 0.09 | 2.22 ± 0.11 |
| 1/14537 | - | - | + | 1.02 ± 0.05 | 1.76 ± 0.09 |
| Strains | S. aureus ATCC 25923 | E. coli ATCC 25922 | L. monocytogenes ATCC 7644 |
|---|---|---|---|
| Lactococcus lactis | |||
| 16FS16 | + | - | + |
| 11FS16 | + | - | + |
| 6LS5 | + | - | + |
| 1FS171M | + | + | + |
| 2A/SB | + | + | + |
| 9/20234 | + | - | + |
| Lactiplantibacillus plantarum | |||
| 62LP39b | + | + | + |
| 11/20966 | + | + | + |
| 4A/20045 | + | + | + |
| 19/20711 | + | + | + |
| 1B3M | + | + | + |
| 4/16898 | + | + | + |
| 1/14537 | + | + | + |
| Cheese pH Days of Storage at 10 °C | ||||
|---|---|---|---|---|
| Cheese Made with Strain | 1 | 4 | 7 | |
| Lactococcus lactis | pH of milk: 6.57 | |||
| Control * | 6.42 ± 0.03 a | 5.75 ± 0.08 a | 5.37 ± 0.14 a | |
| 16FS16 | 5.22 ± 0.08 b | 4.59 ± 0.09 b | 4.50 ± 0.12 b | |
| 11FS16 | 5.48 ± 0.04 bce | 4.65 ± 0.02 b | 4.51 ± 0.07 b | |
| 6LS5 | 5.65 ± 0.04 c | 4.68 ± 0.01 c | 4.28 ± 0.04 b | |
| 1FS17 1M | 5.12 ± 0.06 b | 4.55 ± 0.07 b | 4.29 ± 0.09 b | |
| 2A/SB | 4.33 ± 0.16 d | 4.10 ± 0.08 d | 4.08 ± 0.11 b | |
| 9/20234 | 5.74 ± 0.08 e | 5.28 ± 0.04 a | 5.06 ± 0.06 a | |
| Lactiplantibacillus plantarum | pH of milk: 6.68 | |||
| Control * | 6.25 ± 0.37 ab | 5.84 ± 0.15 a | 5.47 ± 0.10a | |
| 62LP39b | 6.28 ± 0.04 a | 4.44 ± 0.04 b | 4.23 ± 0.09 b | |
| 11/20966 | 6.32 ± 0.12 a | 4.61 ± 0.05 bc | 4.22 ± 0.04 b | |
| 4A/20045 | 6.04 ± 0.08 ab | 4.88 ± 0.10 cd | 4.36 ± 0.03 b | |
| 19/20711 | 6.36 ± 0.04 a | 4.41 ± 0.04 b | 4.27 ± 0.08 b | |
| 1B 3M | 6.37 ± 0.05 a | 4.49 ± 0.02 bc | 4.26 ± 0.06 b | |
| 4/16898 | 5.44 ± 0.23 b | 4.64 ± 0.09 bcd | 4.29 ± 0.02 b | |
| 1/14537 | 5.97 ± 0.22 ab | 5.03 ± 0.13 d | 4.61 ± 0.04 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, M.B.; Fadda, M.E.; Viale, S.; Deplano, M.; Mereu, F.; Blažić, M.; Cosentino, S. Inhibitory Effect of Lactiplantibacillusplantarum and Lactococcus lactis Autochtonous Strains against Listeria monocytogenes in a Laboratory Cheese Model. Foods 2022, 11, 715. https://doi.org/10.3390/foods11050715
Pisano MB, Fadda ME, Viale S, Deplano M, Mereu F, Blažić M, Cosentino S. Inhibitory Effect of Lactiplantibacillusplantarum and Lactococcus lactis Autochtonous Strains against Listeria monocytogenes in a Laboratory Cheese Model. Foods. 2022; 11(5):715. https://doi.org/10.3390/foods11050715
Chicago/Turabian StylePisano, Maria Barbara, Maria Elisabetta Fadda, Silvia Viale, Maura Deplano, Federica Mereu, Marijana Blažić, and Sofia Cosentino. 2022. "Inhibitory Effect of Lactiplantibacillusplantarum and Lactococcus lactis Autochtonous Strains against Listeria monocytogenes in a Laboratory Cheese Model" Foods 11, no. 5: 715. https://doi.org/10.3390/foods11050715
APA StylePisano, M. B., Fadda, M. E., Viale, S., Deplano, M., Mereu, F., Blažić, M., & Cosentino, S. (2022). Inhibitory Effect of Lactiplantibacillusplantarum and Lactococcus lactis Autochtonous Strains against Listeria monocytogenes in a Laboratory Cheese Model. Foods, 11(5), 715. https://doi.org/10.3390/foods11050715






