Abstract
The world faces numerous problems and two of them are global food shortages and the dwindling number of pollinating insects. Plant products that do not arise from pollination are plant galls, which as in the case of oak apples, can resemble fruits and be the size of a cherry. It is suggested that once research has understood how chemical signals from gall-inducing insects program a plant to produce a gall, it should be possible to mimic and to improve nature and “bioengineer” designer galls of different sizes, colorations and specific contents to serve as food or a source of medicinally useful compounds. To achieve this objective, the genes involved in the formation of the galls need to be identified by RNA-sequencing and confirmed by gene expression analyses and gene slicing. Ultimately the relevant genes need to be transferred to naïve plants, possibly with the aid of plasmids or viruses as practiced in crop productivity increases. There is then even the prospect of engineered plant galls to be produced by plant tissue culture via genetic manipulation without the involvement of insects altogether.
1. Introduction: The Problem
Although populations have been decreasing in the majority of the EU-countries [1,2] and in several East Asian countries as well [3], the global population is still rising and expected to reach 10.4 × 109 people by the end of the 21st century [4]. What has been of concern is whether there will be sufficient food for this increase in population and already in 1975 Meyer-Rochow [5] addressed this problem. He wrote in the Australian and New Zealand Association for the Advancement of Science journal Search an article with the title “Can Insects Help to Ease the Problem of World Food Shortage?”. In that article he recommended that organizations, such as the FAO and WHO back his idea. Despite the skepticism and even ridicule that this suggestion initially received (Figure 1) [6], it eventually became accepted and over the last 20 years has seen an extraordinary jump in public support—last but not least because of the backing from an FAO publication by Van Huis et al. [7].

Figure 1.
One of many cartoons that appeared in newspapers and magazines [6] after it had been suggested in 1975 that insects could ease the problem of global food shortages.
Now, nearly 50 years after that pivotal earlier publication on edible insects appeared in Search, I should like to follow up that idea with a suggestion to use molecularly engineered plant galls as a novel food item that does not require the services of pollinating insects and that can be used as a resource from which to extract therapeutically valuable chemicals.
Why should it be important to produce crops that do not require an input from pollinating insects? For a multitude of reasons: in the anthropocene pollinating insect numbers are on the decline worldwide [8,9]. It was noticed already in 2013 that losses of winter bee hives had gone up significantly in both Europe and the USA, and honey harvests in France, for example, had dropped to one third in 2017 of what they used to be in the 1990s [10]. An estimated 87.5% (approximately 300,000 species) of the world’s flowering plants are pollinated by insects [10], and it has been predicted by Giannini et al. in 2017 [11], that disruptions of the pollinating process could lead to crop losses in nearly 90% of the regions of Brazil that were analysed in that study.
Biotic pollination is not only efficient, it actually improves fruit and seed quantity of tropical crops by 70% and crops cultivated in Europe by 85% [12] and although, for example, rice and wheat are wind pollinated, the global economic value of insect pollination was already 153 billion Euros in 2005 [13]. Production losses that can be attributed directly to the absence of flower visitors are of the order of 5% for the developed and 8% for the developing world superimposed on global agricultural production increases of 140% between 1961 and 2006 [12]. This may not sound too bad, but pollinator dependency and the deficits for the developed and the developing regions between 1961 and 2006 rose by 50% and 62%, respectively, and that is quite alarming [12].
There are, of course, other factors that can affect beekeeping and bee populations, as in the case of Romania during the transition period after 1989 to a market economy, when beekeeping experienced a severe decline in that country. For reasons explained in detail by Popescu et al. in 2019 [14], it was largely because of the long history of beekeeping in Romania and the experience in economic, social and environmental contexts that apicultural practices recovered in that country. However, even that country is not immune to the global concerns of declining pollinator populations.
Pollination, of course, in contrast to vegetative reproduction, involves the transfer of pollen, i.e., highly reduced microgametophytes, from the male flower (in case of a dioecious species with separate male and female plants) or the male parts known as the anthers of a bisexual plant, to a flower’s female component, the stigma. What follows, is the fertilisation of the flower’s ovules by the gametes from the pollen grain and the subsequent growth of a fruit with its seed or seeds. Self-fertilisation occurs when pollen from within the same flower or from different flowers but of the same plant get transferred to the female. This can lead to inbreeding and ultimately to a reduced resistance of the plant to diseases and environmental stressors. Therefore it is to be avoided. Cross-pollination in which pollen stems from different plant individuals (or at least different flowers) involves to a very large extent pollinating insects and the latter depend for their survival on a host of different plant species and an intact environment. Bees are the most important pollinators of our fruits, vegetables, nuts, coffee, etc. and their decline together with that of the other pollinators, dealt with in numerous publications [9,10,11,12,13], is therefore of considerable concerns.
Strategies to combat this decline in pollinators exist [15,16], and it has been suggested that the widespread and often uncontrolled use of pesticides can be directly linked to increased insect deaths and loss of insect biodiversity. The increase in transgenic crops to repel insect pests has also been mentioned as a bee killer, because genetically altered organisms are potentially hazardous to bees. Undoubtedly some of the major reasons why we lose pollinators are deforestation, increase of pasture land for cattle and sheep and habitat loss, the latter usually a consequence of urbanisation. Air pollution, global warming and climate change are additional factors that affect insects generally and pollinators in particular. Honey bees, moreover, suffer from attacks by the Varroa mite, microsporidial parasites and a variety of diseases. Although creating pollinator-friendly habitats, improving nest sites, curbing the use of insecticides and other strategies have been suggested as counter-measures to stop the loss of pollinating insect species, it is doubtful whether all these measures are feasible and can be implemented. Since it is anybody’s guess whether the suggested measures will actually reverse the trend and how long it might take to build up pollinator populations again, I have started to focus my attention on plant products that often resemble fruits, as noticed already by Darwin see e.g., [17] but which are structures that do not arise from pollination: plant galls (Figure 2).
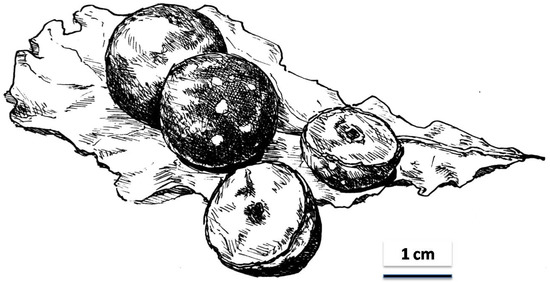
Figure 2.
Drawing of mature and bisected oak tree galls, known as oak apples, on an oak leaf.
2. Background Information
Plant galls show up as modifications of the normal development of certain plant parts [18,19,20]. They represent abnormal outgrowths that can occur on a plant’s leaves, on stems, flowers and even roots, but apparently do not significantly harm the plant. Structurally plant galls can be very diverse in texture, shape and coloration and may resemble spiky or spikeless pouches, nodules, warts, little round apples or tiny, straight and sometimes twisted chimneys. The production of galls can be a response of the plant to stimuli that stem from viruses, bacteria or fungi, but more importantly it can also be triggered by chemicals released by ovipositing gall insects, the physical damage to the plant that the latter sustains during oviposition by an insect, or salivary and other secretions released by the feeding stages of the developing insects [21,22].
Amongst the insects, major gall inducers are known from the orders of Diptera (families Cecidomyiidae, Tephritidae and Agromyzidae), Hymenoptera (Cynipidae, Chalcidoidae, Tenthredinidae), Coleoptera (Curculionidae, Buprestidae, Cerambycidae), Hemiptera (Apiomorphinae, Aphididae. Coccidae, Psyllidae), Lepidoptera (e.g., Gnorimoschema gallae solidaginis) and some Thysanoptera. Other gall-inducing invertebrates are certain mites and nematodes, the latter, in case of Fergusobia spp., in a symbiotic relationship with larvae of the fly genus Fergusonina. Plant galls may be completely closed, requiring the occupant to make a hole into the gall to get out, or they may have an opening to the outside as with the galls of many aphids and eriophyiid mites [22,23,24].
What all these gall-inducers, especially those of insect origin, have in common is that the gall provides food and shelter for the developing insect stages. A certain advantage for the plant of having the insect restricted to the gall is that the insect cannot ‘move around’ and spread to different parts of the plant: it is trapped. On the other hand, the plant invests energy and resources to produce the gall, while the plant cells’ meristematic tissue proliferates and acts as a powerful “physiologic sink”. This was shown using 14C-labelling by Inbar et al. [25], who studied galls formed on Pistacia palaestina by aphids of the family Pemphigidae. Starch, sugars and many other chemicals are being diverted from the phloem of the surrounding plant parts to the benefit of the developing insect [26]. Defensive low molecular weight phenolic substances present in the leaves of, for example, willows such as Salix spp. [27], are often substantially less prominent in galls, while condensed tannic acid levels, on the other hand, are generally elevated in the gall’s interior and especially its cortex.
It is universally accepted that the formation of a gall is the result of chemicals from the gall inducer, which in the case of the embryo can be hormones that include auxins, cytokinins, indole-3-acetic acid, etc., or are signals from the ovipositing insect and the developing brood that are encoded by transcriptionally co-regulated genes [28]. This means that the gall insect is the ‘driver’ and that the host, i.e., the plant, responds [29] in ways that are not too different from the blastema formation in, for example, the regenerating tail of lizards [30] and the abnormal growths in amphibians [31] as well as the tumours found in vertebrates, generally. Feitelson et al. [32] could show for the latter that a number of natural compounds existed (e.g., curcumin, resveratrol, indole-3-carbinol, brassinin, sulforaphane, epigallocatechin-3-gallate, genistein, ellagitannins, lycopene and quercetin) that would be able to inhibit one or more of the pathways that were known to contribute to the cell proliferation, which can be regarded as the basis for the usually rapid growth of the tumour.
A complete and exhaustive explanation of the mechanism that is involved has yet to be forthcoming [17,32], but it seems certain that with regard to plant galls, it is the gall inducer that manipulates its host to an extent that galls made by the same host varied in nutrients and appearance when different gall-inducing species of insects were involved [33]. Plant galls are therefore the products of a natural kind of genetic engineering, which according to Marx [34], postulated already in 1979, may involve “the transfer of DNA”, although to date such a transfer has not been confirmed and indeed it would be difficult to imagine a mechanism by which this could be achieved.
Numerous studies support the notion that galls are extended phenotypes of the gallers and that the latter rather than the plants determine gall morphology [35,36,37] and Nyman and Julkunen-Tiitto [27] had shown beyond doubt that not only the morphology, i.e., the physical appearance, of the induced galls can be regarded as “an extended phenotype of the galler”, but that the gallers (in their case sawflies that manipulated their willow hosts to form galls on the leaves) can also control the chemical characteristics of the galls. As a result, large volumes of dedicated plant tissue were produced, but the latter contained smaller amounts of phenolic compounds such as flavones, flavonols, salicylates, and cinnamic acid derivatives than the surrounding leaf tissue.
The rapid proliferation of undifferentiated cells that leads to the expansion of the gall, bears similarities to plant tumours [38] and evidence, presented by Schultz et al. [17] suggests that the “hijacking of the underlying genetic machinery in the host plant” by the galler has something to do with the reproductive gene ontology categories as the latter “were significantly enriched in developing galls”. Furthermore, genes involved in the floral development exhibited remarkable increases, especially in older galls. It seems that the gene expressions noted in connection with galls would be in agreement with the notion that it is the vascular cambium that gives rise to the meristematic tissue and alters leaf development towards the formation of carpels. Gene regulation in floral reversions has been studied by Wang et al. [39], who showed that plant hormone biosynthesis and signal transduction, starch and sucrose metabolism, DNA replication and modification, as well as other processes crucial for switchgrass flower reversions, were controlled by the genes earlier identified by the researchers.
In a recent publication, Korgaonkar et al. [40] suggested that the colour of galls produced by the aphid Hormaphis cornu on the leaves of Hamamelis virginiana (and maybe other aspects of the gall’s development as well) could be caused by specific secretions of the galling aphid. The researchers found that derived genetic variants in the aphid gene determinant of gall colour “are associated with strong downregulation of dgc transcription in aphid salivary glands, upregulation in galls of seven genes involved in anthocyanin synthesis, and deposition of two red anthocyanins in galls”. A small number of plant genes may, thus, be responding. The proteins secreted by the gall insect in its saliva belong to a large and diverse family, for whose members a pair of widely-spaced cysteine-tyrosine-cysteine residues is typical [40].
3. The Suggestion to Solve the Problem
Just as it is with edible insects where not all species are equally useful as a source with which to augment food supplies, it is with plant galls: not all will be equally useful when it comes to promote plant galls as a source of food or feed or as a raw material to extract pharmaceuticals from. Already as a student I was fascinated by plant galls [41] and I now believe especially the larger and fleshy kinds of galls can serve as an example of what I have in mind. There are numerous so-called “oak apples”, as these galls are commonly known by. Fully developed, these spherical galls can vary in size between one and three centimetres in diameter and may differ in colour (depending on the gall-inducing species of gall wasp involved) between shades of green and red [42,43]. Although they may appear enticing and contain juicy and spongy but usually quite bitter tissue, the galls are anything but tasty as anyone who has tried to eat them will have found out. However, a few varieties of plant galls have apparently found acceptance by human consumers: some are used as drugs [44] and some, such as the galls of Salvia pomifera according to Ion [45], quoting the Greek botanist Eleftherios Dariotis, are appreciated by local folk, because of the galls’ pungent, herbal taste.
Generally, however, galls are not appreciated as a food item although birds such as pheasants have repeatedly been reported to consume plant galls [46,47]. Nonetheless, acceptability by humans could conceivably be changed as there are a variety of means by which improvements could be achieved. The first step would have to be to prepare a detailed analysis and understanding of the chemicals that are involved in triggering the formation of the gall by the plant. This is no easy task and such chemical inducers must be expected to be specific to particular insect species. It is therefore important to focus on some common, large, and fleshy galls for which it should be possible to identify the inducing insect species and the chemical signals that the host plant receives and responds to in order to build the gall. This task completed, the next step would then have to be the determination of the suitable dose and the duration of the inducing chemical’s presence in or on the plant that can produce a large gall. The best way to identify the genes that are involved in the gall formation is the RNA-sequencing method, which shows the expression of all genes over time during gall growth. Later it should be possible to study more aspects of those genes by gene expression analysis using real time PCR [48] and gene silencing [49] to confirm the involvement of specific genes in the process.
The final step would be to transfer the relevant genes to naïve plants, which could be possible by using plasmids or modified viruses. Gene transformation and genetic engineering has indeed contributed to increases in crop productivity [50] and methods to transfer foreign genes to plants have been summarised by, for example, by Narusaka et al. [51]. The PhD thesis by Boyer [52] touches many aspects of molecular engineering within the realms of agro-ecosystems and focuses on the transition from peasant farming via Mendelian genetics to molecular transformations without ignoring unintended consequences. For plant species grown for their biomass or fuel value a report summarizing the possibilities of engineered high energy crops has already been published by the U.S. Department of Energy [53] and a focus on plant galls should be able to generate a similar report.
4. The Prospect for the Future
If I have now suggested that a molecular approach can be used to grow bio-engineered ‘designer plant galls’ of different colorations, larger than normal size and desirable content, then this need not be science fiction, but something that is in our grasp. Some plant galls have been medicinally important for centuries [43,54] and are known to be rich in anti-cancer and anti-diabetic substances [55]; others are known to be rich in resins and tannic acid and have been used for dyeing, for leather treatment, as a source for ointments and for the manufacture of permanent inks [56]. Although the controlled production of plant galls is not yet possible and has to be seen as an ambitious undertaking, it is in principle achievable, even if long times are required. Once the mechanism is fully understood by which the plant is manipulated by the gall-inducer, it could then ultimately lead to an entirely new kind of agricultural activity. In addition, once that has been achieved, galls of desired sizes, coloration and content should then be able to be grown. Of course there may well be an attitude of neophobia and, at least initially, the public’s concern of a genetically modified food item, but such concerns are not new and in western countries have been dealt with in connection with sushi and edible insects [57].
Not being the result of pollination by insects, some galls could be marketed as a kind of “vegetable” or “fruit”, while others might serve the pharmaceutical industry, for example as an alternative tannin source [58]. Methanol and acetone extracts from galls of Quercus infectoria, used traditionally to treat wound infections after childbirth by local folk in Malaysia and to alleviate toothache and gingivitis in India, were tested for their antibacterial properties. Streptococcus salivarius was found to be the most susceptible and it was therefore concluded that these galls could be considered effective phytotherapeutic agents [59]. It had even been suggested as far back as 1979 that it “may be possible to adapt the transfer process [of the genetic material] in order to introduce desirable genetic traits-such as the ability to fix nitrogen-into plants.” [34].
There is a similarity to the natural process of pollination by insects and that which is simulated by manual or artificial pollination, because the natural production of plant galls depends also, at least initially, on the inputs of live insects or some other natural agent before it can be wholly controlled by humans. However, when the process of inducing a host plant to grow a gall is fully understood and can be copied and controlled, then plants can be manipulated to produce galls without the involvement of another organism. Ultimately it should be possible to grow bio-engineered plant gall material via genetic manipulation as products generated solely by tissue culture. In view of the much publicised dependence of our crops on pollinating insects on the one hand and the general decline of the insect diversity on the other, harvestable plant growths such as galls, produced without the involvement of insects, could then become a “game changer”.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The author is grateful to Saeed M. Namin of Andong National University (Republic of Korea) for advice on bioengineering and molecular techniques and to Yoon Miro for preparing Figure 2. The constructive criticism by the reviewers was welcomed and appreciated.
Conflicts of Interest
The author declares that there has been no conflict of interest.
References
- Anonymous. Population Projected to Decline in Two-Thirds of EU Regions. 2021. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210430-2 (accessed on 12 September 2022).
- Nuttall, C.N. Population Decline to Take Emerging Europe back to the Early 20th Century. 2022. Available online: https://intellinews.com/population-decline-to-take-emerging-europe-back-to-the-early-20th-century-253068/ (accessed on 12 September 2022).
- Tsuchiya, H. East Asia’s Looming Demographic Crisis. 2020. Available online: https://www.nippon.com/en/in-depth/d00639/ (accessed on 12 September 2022).
- Roser, M.; Rodés-Guirao, L.; Future Population Growth. Published Online at OurWorldInData.org. 2013. Available online: https://ourworldindata.org/future-population-growth (accessed on 12 September 2022).
- Meyer-Rochow, V.B. Can insects help to ease the problem of world food shortage? Search 1975, 6, 261–262. [Google Scholar]
- New Scientist. Available online: https://books.google.fi/books?id=txDVQ-vzXMQC&pg=PA307&source=gbs_toc&cad=2#v=onepage&q&f=false (accessed on 12 September 2022).
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2013; pp. 1–190. [Google Scholar]
- Wagner, D.L. Insect declines in the anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Althaus, S.L.; Berenbaum, M.R.; Jordan, J.; Shalmon, D.A. No buzz for bees: Media coverage of pollinator decline. Proc. Natl. Acad. Sci. USA 2021, 118, e2002552117. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C. Pollinator decline—An ecological calamity in the making? Sci. Prog. 2018, 101, 121–160. [Google Scholar] [CrossRef] [PubMed]
- Giannini, T.C.; Costa, W.F.; Cordeiro, G.D.; Imperatriz-Fonseca, V.L.; Saraiva, A.M.; Biesmeijer, J.; Garibaldi, L.A. Projected climate change threatens pollinators and crop production in Brazil. PLoS ONE 2017, 12, e0182274. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Popescu, G.; Popesccu, C.R.G. The social, economic and environmental impact of ecological beekeeping in Romania. In Agrifood Economics and Sustainable Development in Contemporary Society; Popescu, G., Ed.; IGI Global Publ.: Hershey, PA, USA, 2019; Chapter 4; pp. 75–96. [Google Scholar] [CrossRef]
- Senapathi, D.; Biesmeijer, J.C.; Breeze, T.D.; Kleijn, D.; Potts, S.G.; Carvalheiro, L.G. Pollinator conservation—the differece between managing for pollination services and preserving pollinator diversity. Curr. Opin. Insect Sci. 2015, 12, 93–101. [Google Scholar] [CrossRef]
- Devi, S.; Devi, D.; Singh, S.J.; Kaushal, B. Strategies to combat the decline in pollinator’s population. Pharma Innov. J. 2021, SP-10, 727–737. [Google Scholar]
- Schultz, J.C.; Edger, P.P.; Body, M.J.A.; Appel, H.M. A galling insect activates plant reproductive programs during gall development. Sci. Rep. 2019, 9, 1833. [Google Scholar] [CrossRef]
- Felt, E.P. Plant Galls and Gall Makers; Constable and Co. Ltd.: London, UK, 1940. [Google Scholar]
- Meyer, J. Plant Galls and Gall Inducers; Gebrüder Bornträger Publ.: Stuttgart, Germany, 1987; ISBN 978-3-443-01023-2. [Google Scholar]
- Harris, M.A.; Pitzschke, A. Plants make galls to accommodate foreigners: Some are friends, most are foes. New Phytol. 2020, 225, 1852–1872. [Google Scholar] [CrossRef] [PubMed]
- Shorthouse, J.D.; Rohfritsch, O. Biology of Insect-Induced Galls; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Redfern, M.; Askew, R.R. Plant Galls; Richmond Publ. Co., Ltd.: Slough, UK, 1992. [Google Scholar]
- Norris, K.R. General Biology. In The Insects of Australia; Waterhouse, D.F., Ed.; Melbourne University Press: Carlton, Australia, 1970; Chapter 5; pp. 107–140. [Google Scholar]
- Borror, D.J.; Triplehorn, C.A.; Johnson, N.E. An Introduction to the Study of Insects; Saunders College Publishers: Philadelphia PA, USA, 1989. [Google Scholar]
- Inbar, M.; Amram, E.; Wool, D. Interspecific competition among phloem-feedig insects mediated by induced hots-plant sinks. Ecology 1995, 76, 1506–1515. [Google Scholar] [CrossRef]
- Ferreira, B.G.; Moreira, G.R.P.; Carneiro, R.G.S.; Isaias, R.M.S. Complex meristematic activity induced by Eucecidoses minutanus on Schinus engleri turns shoots into galls. Am. J. Bot. 2022, 109, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Nyman, T.; Julkunen-Tiitto, R. Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc. Natl. Acad. Sci. USA 2000, 97, 13184–13187. [Google Scholar] [CrossRef]
- Nabity, P.D.; Haus, M.J.; Berenbaum, M.R.; DeLucia, E.H. Leaf-galling phylloxera on grapes reprograms host metabolism morphology. Proc. Natl. Acad. Sci. USA 2013, 110, 16663–16668. [Google Scholar] [CrossRef]
- Gätjen-Boniche, O. The mechanism of plant gall induction by insects: Revealing clues, facts, and consequences in a cross-kingdom complex interaction. Rev. Biol. Trop. 2019, 67, 1359–1382. [Google Scholar] [CrossRef]
- Alibardi, L. Review: The regenerating tail blastema of lizards as a model to study organ regeneration and tumor growth regulation in amniotes. Anat. Rec. 2019, 302, 1469–1490. [Google Scholar] [CrossRef]
- Asashima, M.; Oinuma, T.; Meyer-Rochow, V.B. Tumors in amphibia. Zool. Sci. 1987, 4, 411–425. [Google Scholar]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer; mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Hartley, S.E. The chemical position of plant galls: Are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia 1998, 113, 492–501. [Google Scholar] [CrossRef]
- Marx, J.L. Crown gall disease: Nature as genetic engineer. Science 1979, 203, 254–255. [Google Scholar] [CrossRef]
- Dodson, G.N. Control of gall morphology: Tephritid gallformers (Aciurina spp.) on rabbitbrush (Chrysothamnus). Ecol. Entomol. 1991, 16, 177–181.1991. [Google Scholar] [CrossRef]
- Stern, D.L. Phylogenetic evidence that aphids, rather than plants, determine gall morphology. Proc. R. Soc. Lond. B 1995, 260, 85–89. [Google Scholar]
- Crespi, B.; Worobey, M. Comparative analysis of gall morphology in Australian gall thrips: The evolution of extended phenotypes. Evolution 2017, 52, 1686–1696. [Google Scholar]
- Ahuja, M.R. Genetic control of tumor formation in higher plants. Q. Rev. Plant Sci. 1965, 40, 329–340. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, A.; Sun, F.; Li, M.; Xu, K.; Zhang, C.; Liu, S.; Xi, Y. Transscriptome analysis to identify genes involved in switchgrass flower reversion. Front. Plant Sci. 2018, 9, 1805. [Google Scholar]
- Korgaonkar, A.; Han, C.; Lemire, A.L.; Siwanowicz, I.; Bennouna, D.; Kopec, R.E.; Andolfatto, P.; Shigenobu, S.; Stern, D.L. A novel family of secreted insect proteins linked to plant gall development. Curr. Biol. 2021, 31, 1836–1849.e12. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Pemphigus spirothecae—Erzeugerin der Spirallockengalle. Math.—Naturwiss.-Unterr. MNU 1970, 23, 292–295. [Google Scholar]
- Petrischak, H. Vielfalt der Eichengallen. Biol. Unserer Zeit 2017, 47, 223–224. [Google Scholar] [CrossRef]
- Boggs, J. Oak Apples are Growing. 2022. Available online: https://bygl.osu.edu/node/1961 (accessed on 12 September 2022).
- Gopi, M. Utility of plant galls. Indian J. Econ. Dev. 2018, 6, 1–10. [Google Scholar]
- Ion, A. What are Galls on Oak Trees. 2022. Available online: https://techhangouts.com/what-are-galls-on-oak-trees/ (accessed on 12 September 2022).
- Severin, H.C. An economic study of the food habits of the ring-necked pheasant in South Dakota. In Natural Resource Management Faculty; Publication 44; South Dakota State University: Brookings, SD, USA, 1933. [Google Scholar]
- Whiteside, M.A.; Sage, R.B.; Madden, J.R. Diet complexity in early life affects survival in released pheasants by altering foraging efficiency, food choice, handling skills and gut morphology. J. Anim. Ecol. 2015, 84, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef] [PubMed]
- Redberry, G. Gene Silencing: New Research; Nova Science Publishers: New York, NY, USA, 2006; ISBN 9781594548321. [Google Scholar]
- Sinclair, T.R.; Purcell, L.C.; Sneller, C.H. Crop transformation and the challenge to increase yield potential. Trends Plant Sci. 2004, 9, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, Y.; Narusaka, M.; Yamasaki, S.; Iwabuchi, M. Methods of transfer foreign genes to plants. In Transgenic Plants—Advances and Limitations; Çiftçi, Y.O., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 9; pp. 173–188. [Google Scholar]
- Boyer, M. Transforming Nature Relations: A Brief Hiatus in Space and Time. Ph.D. Thesis, Columbia University, New York, NY, USA, Freie Universität, Berlin, Germany, 2014. Available online: https://academiccommons.columbia.edu/doi/10.7916/D8697265 (accessed on 12 September 2022).
- Anonymous. Engineered High Energy Crop Programs Final Programmatic Environmental Impact Statement (DOE/EIS-0481). 2015. Available online: https://www.energy.gov/sites/prod/files/2015/07/f25/DOE%20EHEC%20Programs%20Final%20PEIS%20(July2015)_Body.pdf (accessed on 12 September 2022).
- Patel, S.; Rauf, R.; Khan, H. The relevance of folkloristic usage of plant galls as medicines: Finding the scientific rationale. Biomed. Pharmacother. 2018, 97, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-cancer, anti-diabetic and other pharmacologic and biological l activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Fagan, M.M. The uses of insect galls. Am. Nat. 1918, 52, 155–176. [Google Scholar] [CrossRef]
- Faccio, E.; Fovino, L.G.N. Food neophobia or distrust of novelties? Exploring consumers’ attitudes towards GMOs insects and cultured meat. Appl. Sci. 2019, 9, 4440. [Google Scholar] [CrossRef]
- Bilek, M.; Czerniakowski, Z.; Kozloska-Tylingo, K.; Gostkowski, M.; Olbrycht, T.; Cicek, C.; Staniszewski, P.; Dudek, T. Gall nuts Cynips quercusfolii (Linnaeus) and Andricus infectorius (Hartig) as tannin raw materials. Appl. Sci. 2022, 12, 4840. [Google Scholar] [CrossRef]
- Basri, D.F.; Tan, L.S.; Shafiei, Z.; Zin, N.M. In vitro antibacterial activity of galls of Quercus infectoria Olivier against oral pathogens. Evid.-Based Complement. Altern. Med. 2012, 2012, 632796. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).