Production of Protein Concentrates from Macauba (Acrocomia aculeata and Acrocomia totai) Kernels by Sieve Fractionation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Macauba Kernels
2.3. Influence of Oil Extraction and Macauba Species on the Quality and Sieve Fractionation of Macauba Kernel Meals
2.3.1. Oil Extraction and Preparation of Macauba Kernel Meals
2.3.2. Sieve Fractionation
2.3.3. Determination of Yields and Process Efficiency
2.4. Analytics
2.4.1. Composition of the MKM and the Sieved Fractions
2.4.2. Functional Properties of the MKM and Sieved Fractions
Soluble Protein Content (SPC) and Protein Solubility (PS)
Water- and Oil-Binding Capacities (WBC and OBC)
Emulsifying Activity Index (EAI) and Emulsion Stability (ES)
Least Gelling Concentration (LGC)
2.5. Statistical Evaluation of Data
3. Results
3.1. Influence of Oil Extraction Conditions and Macauba Species on the Functionality of MKM
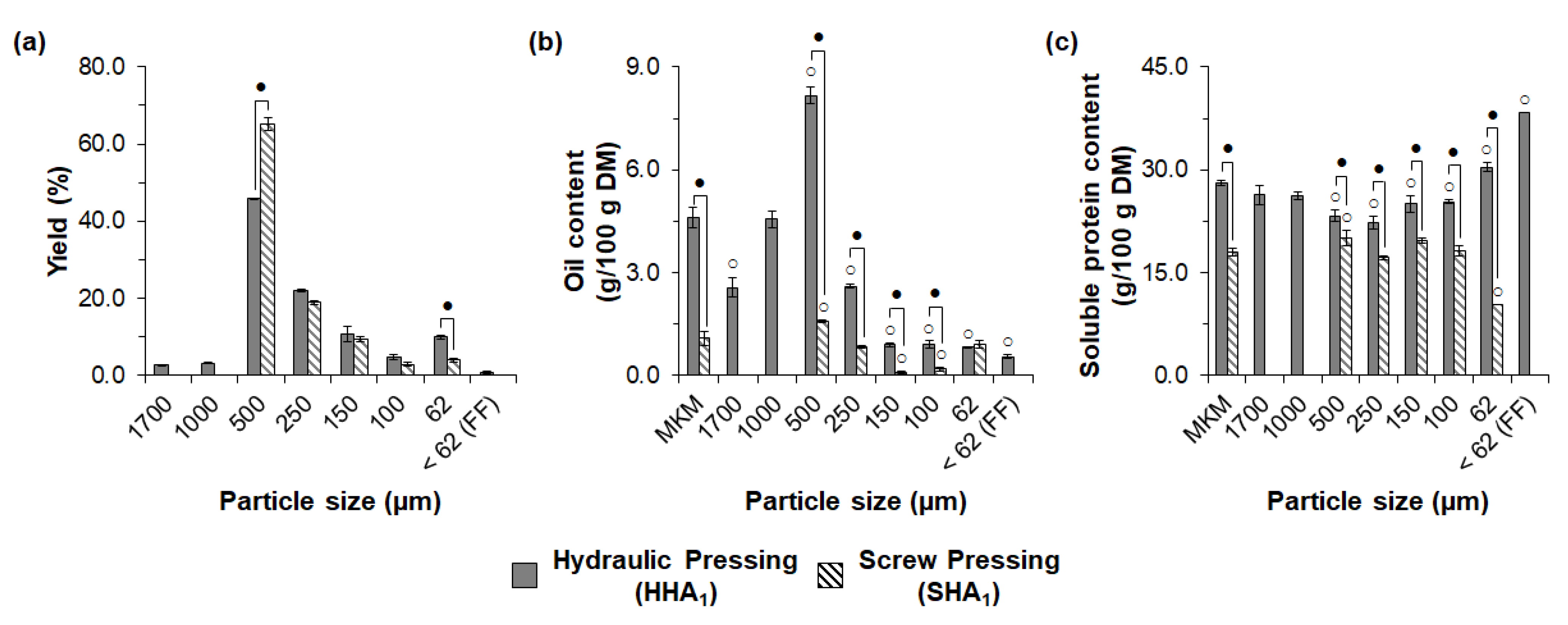
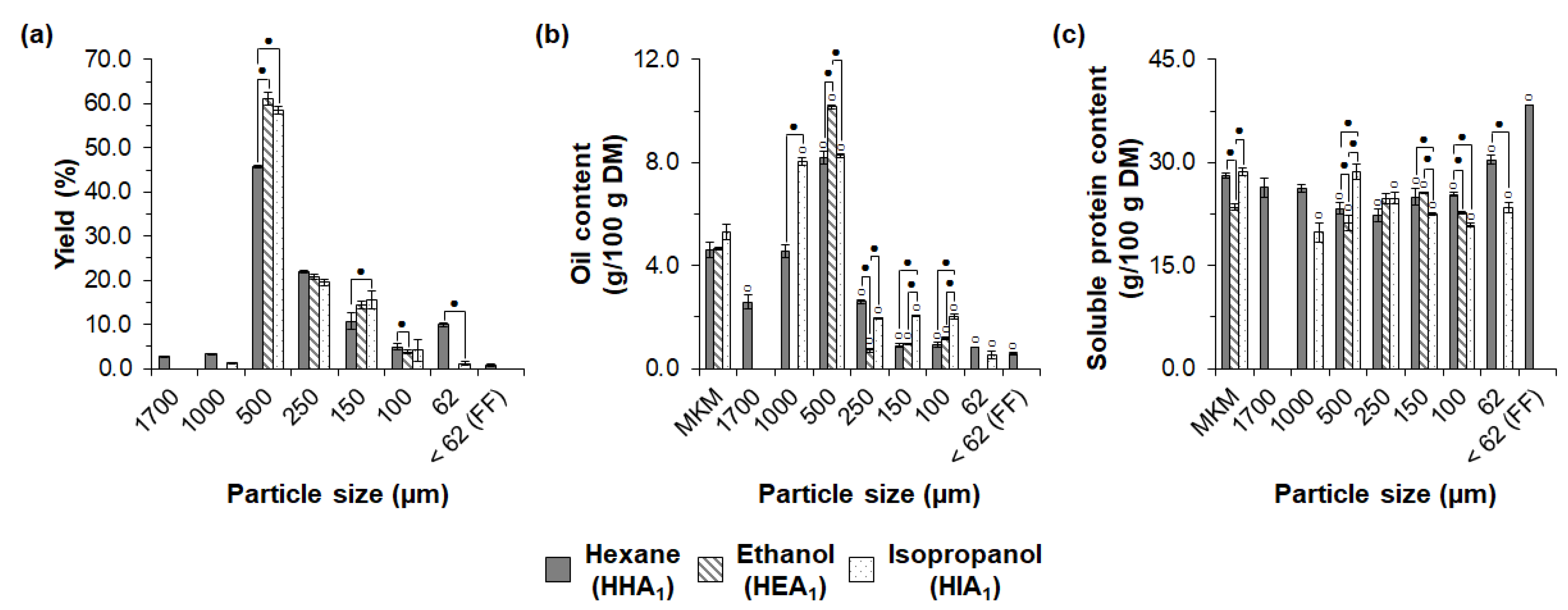
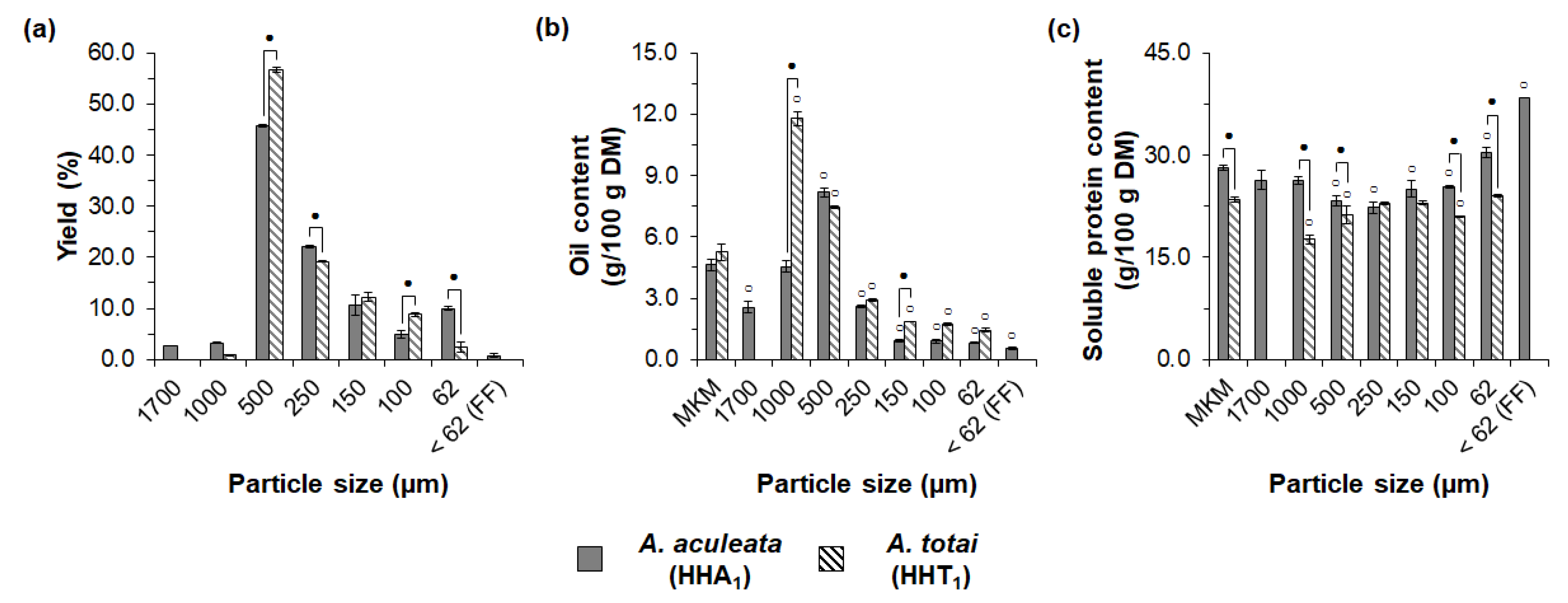
3.2. Influence of Oil Extraction Conditions and Macauba Species on Exploratory Sieve Fractionation of MKM Proteins
3.3. Representative Sieve Fractionation of Macauba Kernel Proteins
3.3.1. Composition of the Fractions and Sieving Performance after the Representative Sieve Fractionation
3.3.2. Functionality of the Sieved Fractions after the Representative Sieve Fractionation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Colombo, C.A.; Berton, L.H.C.; Diaz, B.G.; Ferrari, R.A. Macauba: A promising tropical palm for the production of vegetable oil. OCL 2018, 25, D108. [Google Scholar] [CrossRef]
- César, A.S.; Almeida, F.d.A.; Souza, R.P.; Silva, G.C.; Atabani, A.E. The prospects of using Acrocomia aculeata (macaúba) a non-edible biodiesel feedstock in brazil. Renew. Sustain. Energy Rev. 2015, 49, 1213–1220. [Google Scholar] [CrossRef]
- Cardoso, A.; Laviola, B.G.; Santos, G.S.; de Sousa, H.U.; de Oliveira, H.B.; Veras, L.C.; Ciannella, R.; Favaro, S.P. Opportunities and challenges for sustainable production of A. aculeata through agroforestry systems. Ind. Crop. Prod. 2017, 107, 573–580. [Google Scholar] [CrossRef]
- Vargas-Carpintero, R.; Hilger, T.; Mössinger, J.; Souza, R.F.; Barroso Armas, J.C.; Tiede, K.; Lewandowski, I. Acrocomia spp.: Neglected crop, ballyhooed multipurpose palm or fit for the bioeconomy? A review. Agron. Sustain. Dev. 2021, 41, 75. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ibañez, E.; Block, J.M. Emerging lipids from arecaceae palm fruits in brazil. Molecules 2022, 27, 4188. [Google Scholar] [CrossRef]
- Díaz, B.; Zucchi, M.; Alves-Pereira, A.; Azevedo-Filho, J.; Sanitá, M.; Colombo, C. Species delimitation and hybrid identification of Acrocomia aculeata and A. totai by genetic population approach. Authorea 2021. under review. [Google Scholar]
- Díaz, B.G.; Zucchi, M.I.; Alves-Pereira, A.; de Almeida, C.P.; Moraes, A.C.L.; Vianna, S.A.; Azevedo-Filho, J.; Colombo, C.A. Genome-wide snp analysis to assess the genetic population structure and diversity of acrocomia species. PLoS ONE 2021, 16, e0241025. [Google Scholar] [CrossRef]
- de Lima, N.E.; Carvalho, A.A.; Meerow, A.W.; Manfrin, M.H. A review of the palm genus Acrocomia: Neotropical green gold. Org. Divers. Evol. 2018, 18, 151–161. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Proximate composition of guariroba (Syagrus oleracea), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata) palm fruits. Food Res. Int. 2011, 44, 2139–2142. [Google Scholar] [CrossRef]
- Hiane, P.A.; Baldasso, P.A.; Marangoni, S.; Macedo, M.L.R. Chemical and nutritional evaluation of kernels of bocaiuva, Acrocomia aculeata (Jacq.) Lodd. Food Sci. Technol. 2006, 26, 683–689. [Google Scholar] [CrossRef]
- Lescano, C.H.; Oliveira, I.P.; Silva, L.R.; Baldivia, D.S.; Sanjinez-Argandoña, E.J.; Arruda, E.J.; Moraes, I.C.F.; Lima, F.F. Nutrients content, characterization and oil extraction from Acrocomia aculeata (Jacq.) Lodd. Fruits. Afr. J. Food Sci. 2015, 9, 113–119. [Google Scholar] [CrossRef]
- Ciconini, G.; Favaro, S.P.; Roscoe, R.; Miranda, C.H.B.; Tapeti, C.F.; Miyahira, M.A.M.; Bearari, L.; Galvani, F.; Borsato, A.V.; Colnago, L.A.; et al. Biometry and oil contents of Acrocomia aculeata fruits from the cerrados and pantanal biomes in mato grosso do sul, brazil. Ind. Crop. Prod. 2013, 45, 208–214. [Google Scholar] [CrossRef]
- Belén-Camacho, D.R.; López, I.; García, D.; González, M.; Moreno-Álvarez, M.J.; Medina, C. Physicochemical evaluation of seed and sed oil of corozo (Acrocomia aculeata jacq). Grasas Aceites 2005, 56, 311–316. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Characterization of the pulp and kernel oils from Syagrus oleracea, Syagrus romanzoffiana, and Acrocomia aculeata. J. Food Sci. 2011, 76, C1156–C1161. [Google Scholar] [CrossRef]
- Toledo e Silva, S.H.; Bader-Mittermaier, S.; Silva, L.B.; Doer, G.; Eisner, P. Electrophoretic characterization, amino acid composition and solubility properties of macauba (Acrocomia aculeata L.) kernel globulins. Food Biosci. 2021, 40, 100908. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.-L.; Vorobiev, E. Mechanical continuous oil expression from oilseeds: A review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- O’brien, R.D. Fats and Oils: Formulating and Processing for Applications; CRC Press: London, UK, 2008. [Google Scholar]
- Johnson, L.A.; Lusas, E.W. Comparison of alternative solvents for oils extraction. J. Am. Oil Chem. Soc. 1983, 60, 229–242. [Google Scholar] [CrossRef]
- Trentini, C.P.; Cuco, R.P.; Cardozo-Filho, L.; Silva, C.d. Extraction of macauba kernel oil using supercritical carbon dioxide and compressed propane. Can. J. Chem. Eng. 2019, 97, 785–792. [Google Scholar] [CrossRef]
- Rosa, A.C.S.; Stevanato, N.; Iwassa, I.; Garcia, V.A.d.S.; Silva, C. Obtaining oil from macauba kernels by ultrasound-assisted extraction using ethyl acetate as the solvent. Braz. J. Food Technol. 2019, 22, e2018195. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Savoire, R.; Subra-Paternault, P.; Harscoat-Schiavo, C. Oil and protein recovery from corn germ: Extraction yield, composition and protein functionality. Food Bioprod. Process. 2020, 120, 131–142. [Google Scholar] [CrossRef]
- Lopes Lessa, V.; Harumi Omura, M.; Pacheco, S.; Basílio de Oliveira, E.; Ribeiro de Barros, F.A. Obtention and evaluation of physico-chemical and techno-functional properties of macauba (Acrocomia aculeata) kernel protein isolate. Food Res. Int. 2022, 161, 111848. [Google Scholar] [CrossRef] [PubMed]
- Moura, E.F.; Ventrella, M.C.; Motoike, S.Y. Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeata (Arecaceae). Sci. Agric. 2010, 67, 399–407. [Google Scholar] [CrossRef]
- Ibl, V.; Stoger, E. The formation, function and fate of protein storage compartments in seeds. Protoplasma 2012, 249, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Neumann, D. Protein bodies, storage organelles in plant seeds. Biochem. Physiol. Pflanz. 1980, 175, 279–306. [Google Scholar] [CrossRef]
- Laguna, O.; Barakat, A.; Alhamada, H.; Durand, E.; Baréa, B.; Fine, F.; Villeneuve, P.; Citeau, M.; Dauguet, S.; Lecomte, J. Production of proteins and phenolic compounds enriched fractions from rapeseed and sunflower meals by dry fractionation processes. Ind. Crop. Prod. 2018, 118, 160–172. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Berghout, J.A.M.; van der Goot, A.J.; Boom, R.M.; Schutyser, M.A.I. Preparation of functional lupine protein fractions by dry separation. LWT-Food Sci. Technol. 2014, 59, 680–688. [Google Scholar] [CrossRef]
- Xing, Q.; de Wit, M.; Kyriakopoulou, K.; Boom, R.M.; Schutyser, M.A.I. Protein enrichment of defatted soybean flour by fine milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Vissers, A.M.; Boom, R.M.; Schutyser, M.A.I. Dry fractionation for production of functional pea protein concentrates. Food Res. Int. 2013, 53, 232–239. [Google Scholar] [CrossRef]
- Assatory, A.; Vitelli, M.; Rajabzadeh, A.R.; Legge, R.L. Dry fractionation methods for plant protein, starch and fiber enrichment: A review. Trends Food Sci. Technol. 2019, 86, 340–351. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; van der Goot, A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Technol. 2011, 22, 154–164. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; van der Goot, A.J.; Boom, R.M. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Liu, K.; Barrows, F.T.; Obert, D. Dry fractionation methods to produce barley meals varying in protein, beta-glucan, and starch contents. J. Food Sci. 2009, 74, C487–C499. [Google Scholar] [CrossRef]
- Maaroufi, C.; Melcion, J.P.; de Monredon, F.; Giboulot, B.; Guibert, D.; Le Guen, M.-P. Fractionation of pea flour with pilot scale sieving. I. Physical and chemical characteristics of pea seed fractions. Anim. Feed. Sci. Technol. 2000, 85, 61–78. [Google Scholar] [CrossRef]
- Sundberg, B.; Åman, P. Fractionation of different types of barley by roller milling and sieving. J. Cereal Sci. 1994, 19, 179–184. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Villanueva, M.; Vela, A.J.; Ronda, F. Protein and lipid enrichment of quinoa (cv.Titicaca) by dry fractionation. Techno-functional, thermal and rheological properties of milling fractions. Food Hydrocoll. 2020, 105, 105770. [Google Scholar] [CrossRef]
- Vázquez-Ovando, A.; Betancur-Ancona, D.; Chel-Guerrero, L. Physicochemical and functional properties of a protein-rich fraction produced by dry fractionation of chia seeds (Salvia hispanica L.). CyTA-J. Food 2013, 11, 75–80. [Google Scholar] [CrossRef]
- Mejicanos, G.A.; Rogiewicz, A.; Nyachoti, C.M.; Slominski, B.A. Fractionation of canola meal using sieving technology. Can. J. Anim. Sci. 2017, 97, 613–621. [Google Scholar] [CrossRef]
- Jia, W.; Rodriguez-Alonso, E.; Bianeis, M.; Keppler, J.K.; van der Goot, A.J. Assessing functional properties of rapeseed protein concentrate versus isolate for food applications. Innov. Food Sci. Emerg. Technol. 2021, 68, 102636. [Google Scholar] [CrossRef]
- Challa, R.; Srinivasan, R.; To, F. Fractionation of soybean meal, cottonseed meal and wheat middlings using combination of sieving and air classification. Anim. Feed Sci. Technol. 2010, 159, 72–78. [Google Scholar] [CrossRef][Green Version]
- German Food Act. Bvl Bundesamt Fuer Verbraucherschutz und Lebensmittelsicherheit (ed.), Amtliche Sammlung von Untersuchungsverfahren nach § 64 lfgb, § 35 Vorlaeufiges tabakgesetz, § 28b Gentg-i-Lebensmittel-Band i (l) Verfahren zur Probenahme und Untersuchung von Lebensmitteln; Beuth Verlag GmbH: Berlin, Germany, 1980. [Google Scholar]
- AOAC International. Method 920.39 Fat (Crude) or Ether Extract, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- AOAC International. Protein (Crude) Determination in Animal Feed: Copper Catalyst Kjeldahl Method 984.13, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- AOAC International. Method 991.43 Total Dietary Fiber. Enzymatic-Gravimetric Method, 17th ed.; Association of Official Analytical Chemists: Gaitherburg, MD, USA, 2000. [Google Scholar]
- Zayas, J.F. Functionality of Proteins in Food, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Sathe, S.K. Protein solubility and functionality. In Food Proteins and Peptides: Chemistry, Functionality, Interactions, and Commercialization; Taylor & Francis Group: London, UK, 2012; pp. 95–124. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the AACC, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Muranyi, I.S.; Otto, C.; Pickardt, C.; Osen, R.; Koehler, P.; Schweiggert-Weisz, U. Influence of the isolation method on the technofunctional properties of protein isolates from Lupinus angustifolius L. J. Food Sci. 2016, 81, C2656–C2663. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Silventoinen, P.; Sipponen, M.H.; Holopainen-Mantila, U.; Poutanen, K.; Sozer, N. Use of air classification technology to produce protein-enriched barley ingredients. J. Food Eng. 2018, 222, 169–177. [Google Scholar] [CrossRef]
- Labuckas, D.; Maestri, D.; Lamarque, A. Effect of different oil extraction methods on proximate composition and protein characteristics of walnut (Juglans regia L.) flour. LWT-Food Sci. Technol. 2014, 59, 794–799. [Google Scholar] [CrossRef]
- Rabadán, A.; Álvarez-Ortí, M.; Gómez, R.; Alvarruiz, A.; Pardo, J.E. Optimization of pistachio oil extraction regarding processing parameters of screw and hydraulic presses. LWT-Food Sci. Technol. 2017, 83, 79–85. [Google Scholar] [CrossRef]
- Fetzer, A.; Herfellner, T.; Stäbler, A.; Menner, M.; Eisner, P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind. Crop. Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Östbring, K.; Malmqvist, E.; Nilsson, K.; Rosenlind, I.; Rayner, M. The effects of oil extraction methods on recovery yield and emulsifying properties of proteins from rapeseed meal and press cake. Foods 2020, 9, 19. [Google Scholar] [CrossRef]
- Riaz, M.N.; Cheewapramong, P. Characterization of partially defatted peanut flour using dry extruder and screw pressing. Int. J. Food Prop. 2009, 12, 427–437. [Google Scholar] [CrossRef]
- Hojilla-Evangelista, M.P.; Evangelista, R.L.; Isbell, T.A.; Selling, G.W. Effects of cold-pressing and seed cooking on functional properties of protein in pennycress (Thlaspi arvense L.) seed and press cakes. Ind. Crop. Prod. 2013, 45, 223–229. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Melachouris, N. Functional properties of proteins in foods: A survey. CRC Crit. Rev. Food Technol. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Hu, S.; Liu, X.; Duan, X. Consequences of ball-milling treatment on the physicochemical, rheological and emulsifying properties of egg phosvitin. Food Hydrocoll. 2019, 95, 418–425. [Google Scholar] [CrossRef]
- Wu, Y.V.; Inglett, G.E. Denaturation of plant proteins related to functionality and food applications. A review. J. Food Sci. 1974, 39, 218–225. [Google Scholar] [CrossRef]
- Kauzmann, W. Some factors in the interpretation of protein denaturation11the preparation of this article has been assisted by a grant from the national science foundation. In Advances in Protein Chemistry; Anfinsen, C.B., Anson, M.L., Bailey, K., Edsall, J.T., Eds.; Academic Press: London, UK, 1959; Volume 14, pp. 1–63. [Google Scholar]
- Navarro, S.L.B.; Capellini, M.C.; Aracava, K.K.; Rodrigues, C.E.C. Corn germ-bran oils extracted with alcoholic solvents: Extraction yield, oil composition and evaluation of protein solubility of defatted meal. Food Bioprod. Process. 2016, 100, 185–194. [Google Scholar] [CrossRef]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of different alcoholic extraction conditions on soybean oil yield, fatty acid composition and protein solubility of defatted meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Capellini, M.C.; Giacomini, V.; Cuevas, M.S.; Rodrigues, C.E.C. Rice bran oil extraction using alcoholic solvents: Physicochemical characterization of oil and protein fraction functionality. Ind. Crop. Prod. 2017, 104, 133–143. [Google Scholar] [CrossRef]
- Bader, S.; Oviedo, J.P.; Pickardt, C.; Eisner, P. Influence of different organic solvents on the functional and sensory properties of lupin (Lupinus angustifolius L.) proteins. LWT-Food Sci. Technol. 2011, 44, 1396–1404. [Google Scholar] [CrossRef]
- L’hocine, L.; Boye, J.I.; Arcand, Y. Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J. Food Sci. 2006, 71, C137–C145. [Google Scholar] [CrossRef]
- Capellini, M.C.; Chiavoloni, L.; Giacomini, V.; Rodrigues, C.E.C. Alcoholic extraction of sesame seed cake oil: Influence of the process conditions on the physicochemical characteristics of the oil and defatted meal proteins. J. Food Eng. 2019, 240, 145–152. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carrín, M.E.; Carelli, A.A. Extraction of sunflower oil using ethanol as solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Magsumov, T.; Ziying, L.; Sedov, I. Comparative study of the protein denaturing ability of different organic cosolvents. Int. J. Biol. Macromol. 2020, 160, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, O.; Tatsuno, M. Thermodynamic analysis of alcohol effect on thermal stability of proteins. J. Biosci. Bioeng. 2011, 111, 198–203. [Google Scholar] [CrossRef]
- Khatib, K.A.; Herald, T.J.; Aramouni, F.M.; MacRitchie, F.; Schapaugh, W.T. Characterization and functional properties of soy β-conglycinin and glycinin of selected genotypes. J. Food Sci. 2002, 67, 2923–2929. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, P.; Rommi, K.; Holopainen-Mantila, U.; Poutanen, K.; Nordlund, E. Biochemical and techno-functional properties of protein- and fibre-rich hybrid ingredients produced by dry fractionation from rice bran. Food Bioprocess Technol. 2019, 12, 1487–1499. [Google Scholar] [CrossRef]
- Murru, M.; Calvo, C.L. Sunflower protein enrichment. Methods and potential applications. OCL 2020, 27, 17. [Google Scholar] [CrossRef]
- Toda, T.A.; Sawada, M.M.; Rodrigues, C.E.C. Kinetics of soybean oil extraction using ethanol as solvent: Experimental data and modeling. Food Bioprod. Process. 2016, 98, 1–10. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Wang, J.; Boom, R.M.; Schutyser, M.A.I. Pre- and post-treatment enhance the protein enrichment from milling and air classification of legumes. J. Food Eng. 2015, 155, 53–61. [Google Scholar] [CrossRef]
- Oyedeji, F.O.; Oderinde, R.A. Characterization of isopropanol extracted vegetable oils. J. Appl. Sci. 2006, 6, 2510–2513. [Google Scholar]
- Opazo-Navarrete, M.; Tagle Freire, D.; Boom, R.M.; Janssen, A.E.M.; Schutyser, M.A.I. Dry fractionation of quinoa sweet varieties atlas and riobamba for sustainable production of protein and starch fractions. J. Food Compos. Anal. 2018, 74, 95–101. [Google Scholar] [CrossRef]
- Wu, Y.; Stringfellow, A.C.; Inglett, G.E. Protein-and beta-glucan enriched fractions from high-protein, high beta-glucan barleys by sieving and air classification. Cereal Chem. 1994, 71, 220–223. [Google Scholar]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Method development to increase protein enrichment during dry fractionation of starch-rich legumes. Food Bioprocess Technol. 2015, 8, 1495–1502. [Google Scholar] [CrossRef]
- Anjum, F.M.; Walker, C.E. Review on the significance of starch and protein to wheat kernel hardness. J. Sci. Food Agric. 1991, 56, 1–13. [Google Scholar] [CrossRef]
- Loveday, S.M. Plant protein ingredients with food functionality potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar] [CrossRef]
- Vose, J.R. Separating grain components by air classification. Sep. Purif. Methods 1978, 7, 1–29. [Google Scholar] [CrossRef]
- Rempel, C.; Geng, X.; Zhang, Y. Industrial scale preparation of pea flour fractions with enhanced nutritive composition by dry fractionation. Food Chem. 2019, 276, 119–128. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food gels: Gelling process and new applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef]
- Saldanha do Carmo, C.; Silventoinen, P.; Nordgård, C.T.; Poudroux, C.; Dessev, T.; Zobel, H.; Holtekjølen, A.K.; Draget, K.I.; Holopainen-Mantila, U.; Knutsen, S.H.; et al. Is dehulling of peas and faba beans necessary prior to dry fractionation for the production of protein- and starch-rich fractions? Impact on physical properties, chemical composition and techno-functional properties. J. Food Eng. 2020, 278, 109937. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]


| Meal Code | Processing Parameters | |||
|---|---|---|---|---|
| Pressing Method | Solvent | Macauba Species | Milling | |
| SHA1 | Screw Pressing | Hexane | A. aculeata | 1 mm |
| HHA1 | Hydraulic Pressing | Hexane | A. aculeata | 1 mm |
| HHA0.5 | Hydraulic Pressing | Hexane | A. aculeata | 0.5 mm |
| HHA2 | Hydraulic Pressing | Hexane | A. aculeata | 2 mm |
| HEA1 | Hydraulic Pressing | Ethanol | A. aculeata | 1 mm |
| HIA1 | Hydraulic Pressing | Isopropanol | A. aculeata | 1 mm |
| HHT1 | Hydraulic Pressing | Hexane | A. totai | 1 mm |
| Meal Code | Processing Parameters (Pressing Method, Milling, Solvent, Macauba Species) | OC (% DM) | Functional Properties | ||||
|---|---|---|---|---|---|---|---|
| PS (%) | WBC (mL/g DM) | OBC (mL/g DM) | EAI (m2/g protein) | ES (min) | |||
| SHA1 | Screw pressing, 1 mm, hexane, A. aculeata | 1.1 ± 0.2 b | 47.6 ± 1.2 c | 4.7 ± 0.1 b | 1.4 ± 0.0 e | 109.0 ± 2.0 c | 84.7 ± 13.5 c |
| HHA1 | Hydraulic pressing, 1 mm, hexane, A. aculeata | 4.6 ± 0.3 a | 77.1 ± 0.7 a | 4.1 ± 0.1 cd | 2.2 ± 0.0 d | 181.0 ± 4.8 a | 149.3 ± 18.2 a |
| HHA0.5 | Hydraulic pressing, 0.5 mm, hexane, A. aculeata | 4.2 ± 0.5 a | 78.1 ± 0.5 a | 3.8 ± 0.2 d | 2.2 ± 0.1 d | 179.7 ±3.0 a | 74.0 ± 8.9 cd |
| HHA2 | Hydraulic pressing, 2 mm, hexane, A. aculeata | 4.6 ± 0.1 a | 74.9 ± 1.3 a | 4.1 ± 0.1 cd | 2.7 ± 0.1 b | 168.4 ± 2.7 a | 45.8 ± 0.7 de |
| HEA1 | Hydraulic pressing, 1 mm, ethanol, A. aculeata | 4.7 ± 0.1 a | 64.4 ± 0.9 b | 4.3 ± 0.1 bc | 3.1 ± 0.0 a | 107.8 ± 1.3 c | 59.3 ± 4.1 cde |
| HIA1 | Hydraulic pressing, 1 mm, isopropanol, A. aculeata | 5.3 ± 0.3 a | 79.3 ± 1.2 a | 4.3 ± 0.0 bc | 3.1 ± 0.0 a | 135.8 ± 7.8 b | 138.9 ± 0.9 b |
| HHT1 | Hydraulic pressing, 1 mm, hexane, A. totai | 5.3 ± 0.4 a | 63.1 ± 0.7 b | 5.2 ± 0.1 a | 2.9 ± 0.0 b | 102.5 ± 2.0 c | 31.5 ± 0.7 e |
| Fraction | Oil Content (% DM) | Total Protein Content (% DM) | Yield (%) | Protein Separation Efficiency (%) | Protein Enrichment (%) |
|---|---|---|---|---|---|
| MKM | 5.2 ± 0.2 b | 37.7 ± 0.2 b | NA | NA | NA |
| 500 µm | 8.0 ± 0.0 a | 34.4 ± 0.1 d | 51.9 ± 1.4 a | 47.4 | −8.7 |
| 250 µm | 3.0 ± 0.3 c | 33.6 ± 0.0 e | 18.9 ± 0.9 b | 16.8 | −10.9 |
| 150 µm | 2.0 ± 0.0 d | 33.8 ± 0.2 e | 9.2 ± 0.5 c | 8.3 | −10.2 |
| 100 µm | 1.4 ± 0.1 de | 33.9 ± 0.1 e | 4.8 ± 0.2 d | 4.3 | −10.1 |
| 62 µm | 1.6 ± 0.0 d | 37.3 ± 0.1 c | 5.8 ± 0.3 d | 5.7 | −1.0 |
| FF (<62 µm) | 0.7 ± 0.1 e | 65.6 ± 0.1 a | 9.4 ± 0.7 c | 16.4 | 74.1 |
| Fraction | PS (%) | WBC (mL/g DM) | OBC (mL/g DM) | EAI (m2/g Protein) | ES (min) | LGC (%) |
|---|---|---|---|---|---|---|
| MKM | 77.86 ± 2.46 b | 3.53 ± 0.11 c | 2.70 ± 0.01 d | 183.81 ± 8.26 a | 147.29 ± 4.79 c | 8.0 ± 0.0 b |
| 500 µm | 87.62 ± 1.03 a | 3.43 ± 0.08 c | 2.52 ± 0.06 e | 180.22 ± 4.19 ab | 269.09 ± 6.40 b | 8.0 ± 0.0 b |
| 250 µm | 71.22 ± 2.45 c | 3.84 ± 0.07 c | 2.97 ± 0.04 c | 175.71 ± 2.39 abc | 313.15 ± 2.83 ab | 10.0 ± 0.0 a |
| 150 µm | 65.77 ± 0.70 d | 7.22 ± 0.23 a | 6.15 ± 0.03 a | 191.33 ± 3.18 a | 128.64 ± 0.48 cd | 8.0 ± 0.0 b |
| 100 µm | 64.10 ± 0.31 d | 7.41 ± 0.19 a | 6.14 ± 0.04 a | 180.62 ± 3.18 a | 95.56 ± 0.1.05 d | 6.0 ± 0.0 c |
| 62 µm | 64.61 ± 0.52 d | 5.11 ± 0.05 b | 4.45 ± 0.01 b | 164.12 ± 3.07 bc | 157.49 ± 1.22 c | 8.0 ± 0.0 b |
| FF (<62 µm) | 60.90 ± 0.60 e | 1.41 ± 0.03 d | 1.55 ± 0.07 f | 163.34 ± 1.80 c | 345.22 ± 6.88 a | 6.0 ± 0.0 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo e Silva, S.H.; Silva, L.B.; Eisner, P.; Bader-Mittermaier, S. Production of Protein Concentrates from Macauba (Acrocomia aculeata and Acrocomia totai) Kernels by Sieve Fractionation. Foods 2022, 11, 3608. https://doi.org/10.3390/foods11223608
Toledo e Silva SH, Silva LB, Eisner P, Bader-Mittermaier S. Production of Protein Concentrates from Macauba (Acrocomia aculeata and Acrocomia totai) Kernels by Sieve Fractionation. Foods. 2022; 11(22):3608. https://doi.org/10.3390/foods11223608
Chicago/Turabian StyleToledo e Silva, Sérgio Henrique, Lidiane Bataglia Silva, Peter Eisner, and Stephanie Bader-Mittermaier. 2022. "Production of Protein Concentrates from Macauba (Acrocomia aculeata and Acrocomia totai) Kernels by Sieve Fractionation" Foods 11, no. 22: 3608. https://doi.org/10.3390/foods11223608
APA StyleToledo e Silva, S. H., Silva, L. B., Eisner, P., & Bader-Mittermaier, S. (2022). Production of Protein Concentrates from Macauba (Acrocomia aculeata and Acrocomia totai) Kernels by Sieve Fractionation. Foods, 11(22), 3608. https://doi.org/10.3390/foods11223608






