Evaluation of the Influence of Flavor Characteristics of Cooked Bacon with Different Sterilization Methods by GC-IMS Combined with HS-SPME-GC-MS and Electronic Nose
Abstract
1. Introduction
2. Material and Methods
2.1. Bacon Preparation and Storage Conditions
2.2. Radiation Process
2.3. Electronic Nose Measurement
2.4. Analysis of Volatile Compounds
2.5. Odor Threshold
2.6. GC-IMS Analysis of Volatile Compounds
2.7. Sensory Evaluation
2.8. Data Analysis
3. Results and Discussion
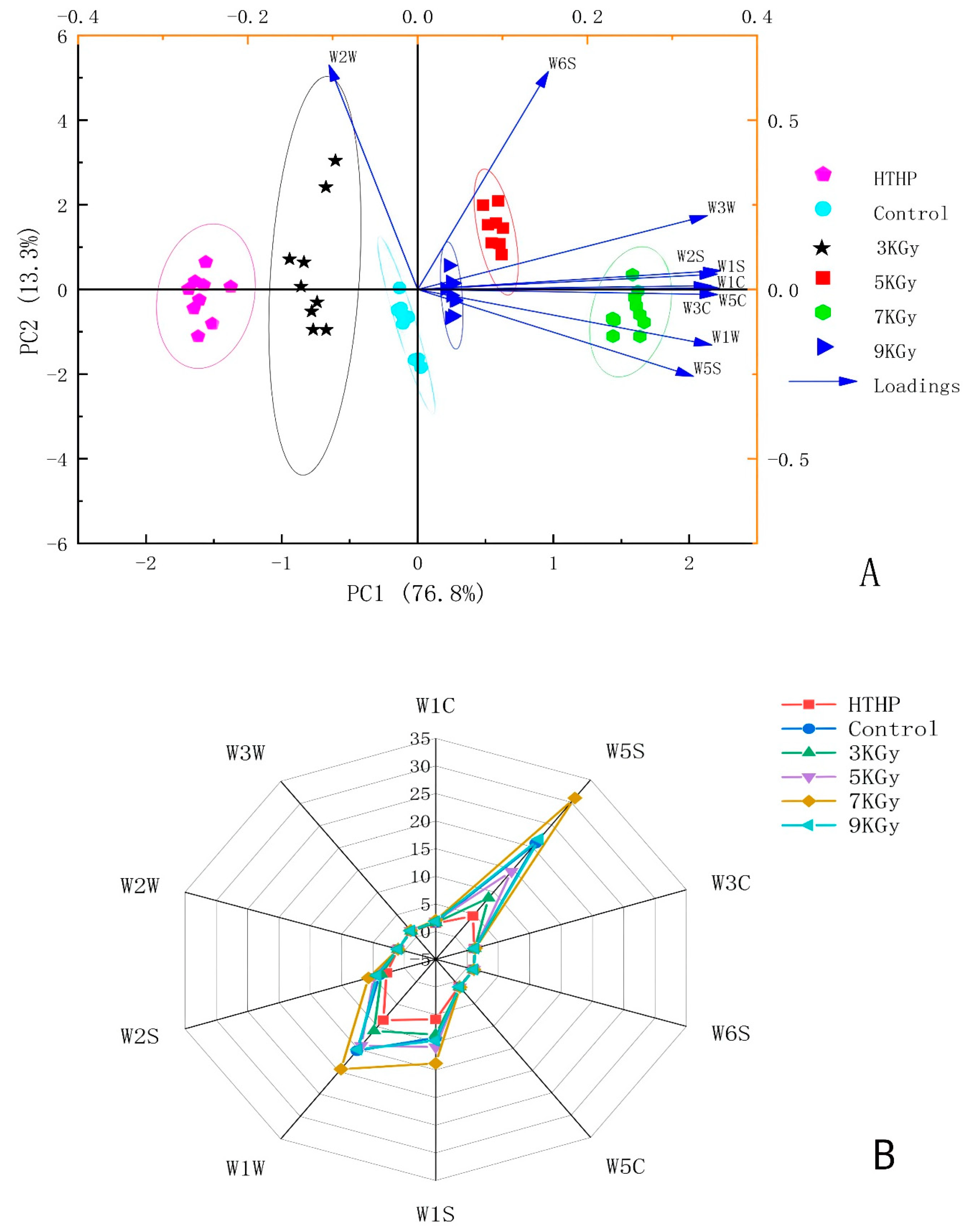
3.1. E-Nose Analysis
3.2. Volatile Compounds and OAV Analyses
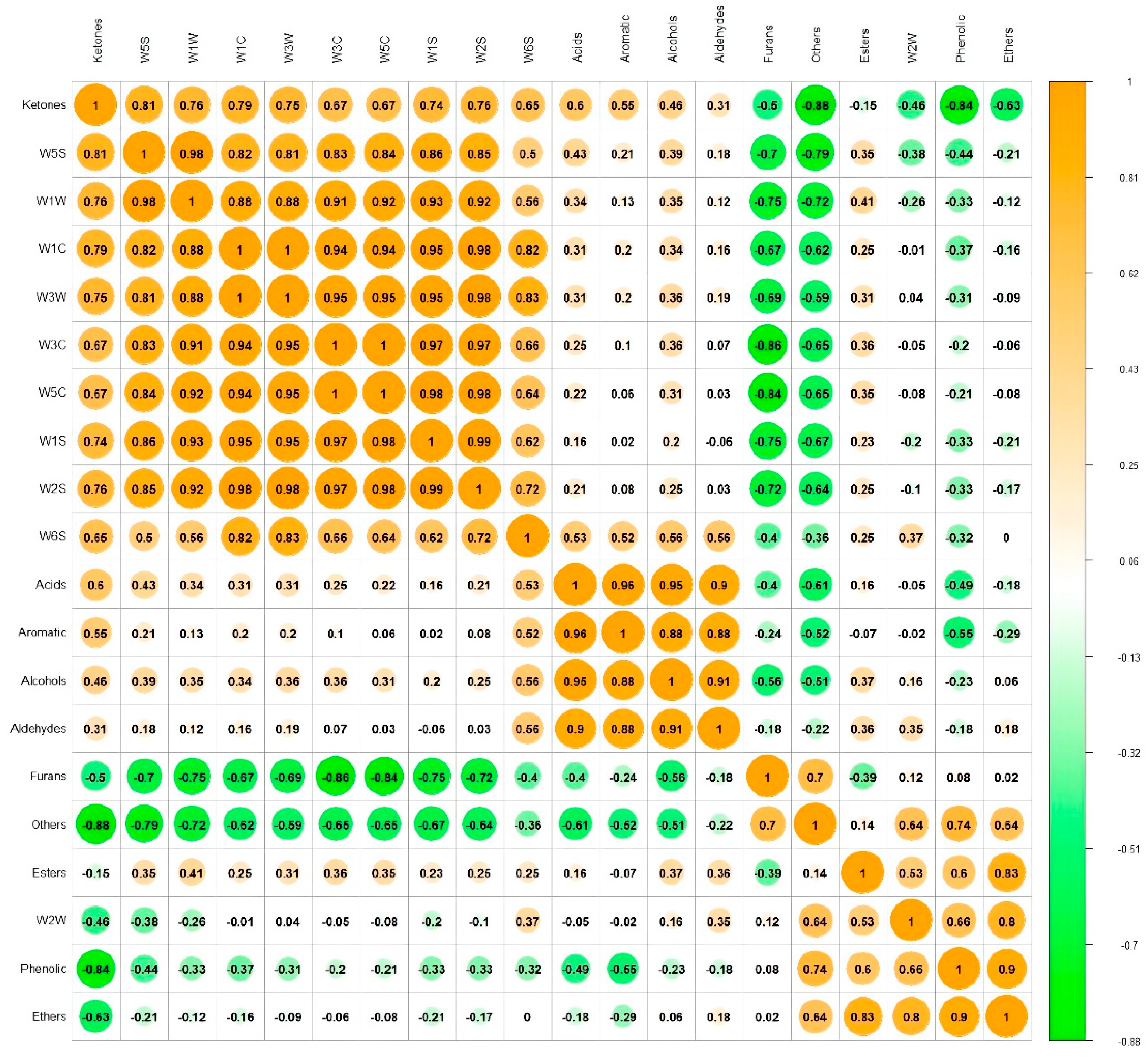
3.3. Correlation between E-Nose and GC-MS
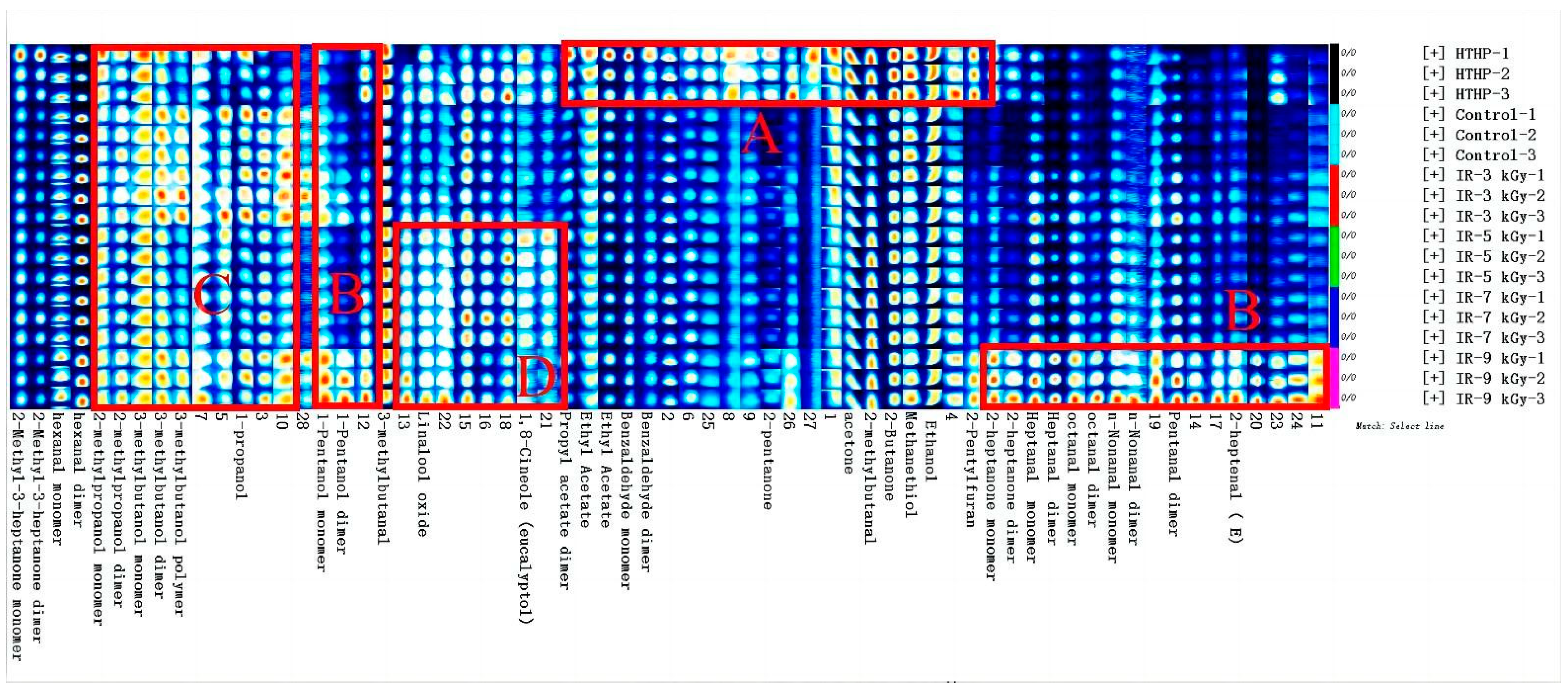
3.4. Volatile Aroma Compound Profiling with GC-IMS
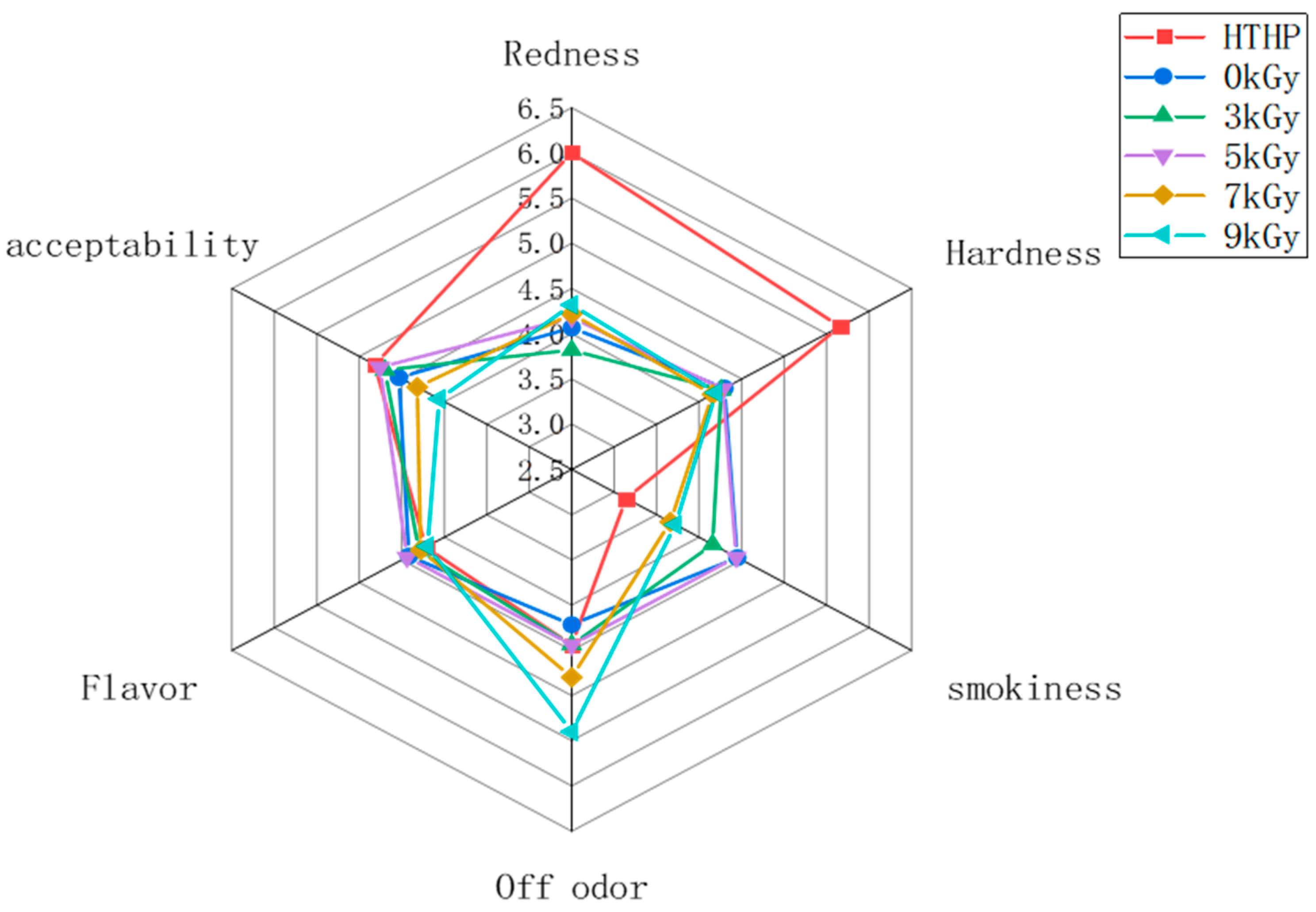
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sikorski, Z.E. Smoking|Traditional. Encycl. Meat Sci. 2004, 1265–1272. [Google Scholar] [CrossRef]
- Deng, S.Y.; Liu, Y.H.; Huang, F.; Liu, J.Q.; Han, D.; Zhang, C.H.; Blecker, C. Evaluation of volatile flavor compounds in bacon made by different pig breeds during storage time. Food Chem. 2021, 357, 129765. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.J.; Jayathilakan, K.; Pandey, M.C. Effect of ionizing radiation on the protein and lipid quality characteristics of mutton kheema treated with rice bran oil and sunflower oil. Radiat. Phys. Chem. 2015, 117, 217–224. [Google Scholar] [CrossRef]
- Patterson, R.L.S.; Stevenson, M.H. Irradiation-induced off-odour in chicken and its possible control. Br. Poult. Sci. 1995, 36, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ahn, D.U. Volatile profile, lipid oxidation and protein oxidation of irradiated ready-to-eat cured turkey meat products. Radiat. Phys. Chem. 2016, 127, 27–33. [Google Scholar] [CrossRef]
- Feng, X.; Moon, S.H.; Lee, H.; Ahn, D.U. Effect of irradiation on themparameters that influence quality characteristics of raw turkey breast meat. Radiat. Phys. Chem. 2017, 130, 40–46. [Google Scholar] [CrossRef]
- Feng, X.; Moon, S.H.; Lee, H.; Ahn, D.U. Effect of irradiation on the parameters that influence quality characteristics of uncured and cured cooked turkey meat products. Poult. Sci. 2016, 95, 2986–2992. [Google Scholar] [CrossRef]
- Yin, X.; Lv, Y.; Wen, R.; Wang, Y.; Kong, B. Characterization of selected Harbin red sausages on the basis of their flavour profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108345. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Ropartz, D.; García-Campaa, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion Mobility Spectrometry in food analysis: Principles, current applications and future trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef]
- Chen, C.; Tabrizchi, M.; Li, H.Y. Ion Gating in Ion Mobility Spectrometry: Principles and advances. TrAC Trends Anal. Chem. 2020, 133, 116100. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—Gas chromatography–ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, N.; Birkenmeier, M.; Sanders, D.; Rohn, S.; Weller, P. Resolution-optimized headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) for non-targeted olive oil profiling. Anal. Bioanal. Chem. 2017, 409, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Y.; Wen, R.X.; Sun, F.D.; Wang, Y.; Kong, B.H.; Chen, Q. Collaborative analysis on differences in volatile compounds of Harbin red sausages smoked with different types of woodchips based on gas chromatography–mass spectrometry combined with electronic nose. LWT Food Sci. Technol. 2021, 143, 111144. [Google Scholar] [CrossRef]
- Xing, T.; Li, Z.; Chao, Y.; Wu, Z.; Zhou, M.; Xiao, S.; Zeng, J.; Zhe, J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Res. Int. 2020, 137, 109456. [Google Scholar] [CrossRef]
- Du, H.Z.; Chen, Q.; Liu, Q.; Wang, Y.; Kong, B.H. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci. 2021, 182, 108626. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Cordeiro, A.A.R.R.; de Medeiros, L.L.; Bezerra, T.K.A.; Pacheco, M.T.B.; de Sousa Galvão, M.; Madruga, M.S. Effects of thermal processing on the flavor molecules of goat by-product hydrolysates. Food Res. Int. 2020, 138, 109758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.Y.; Wang, Y.; Kong, B.H.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT Food Sci. Technol. 2020, 140, 110764. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Brewer, M.S. Irradiation effects on meat flavor: A review. Meat Sci. 2009, 81, 1–14. [Google Scholar] [CrossRef]
- Ahn, D.U.; Lee, E.J.; Feng, X.; Zhang, W.G.; Lee, J.H.; Jo, C.; Nam, K. Mechanisms of volatile production from sulfur-containing amino acids by irradiation. Radiat. Phys. Chem. 2016, 119, 80–84. [Google Scholar] [CrossRef]
- Kong, Q.; Yan, W.; Yue, L.; Chen, Z.; He, X. Volatile compounds and odor traits of dry-cured ham (Prosciutto crudo) irradiated by electron beam and gamma rays. Radiat. Phys. Chem. 2017, 130, 265–272. [Google Scholar] [CrossRef]
- Yang, K.M.; Chiang, P.Y. Effects of smoking process on the aroma characteristics and sensory qualities of dried longan. Food Chem. 2019, 287, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jang, H.W.; Lee, K.G. Sensory and instrumental volatile flavor analysis of commercial orange juices prepared by different processing methods. Food Chem. 2018, 267, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B.H. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef]
- Song, S.; Tang, Q.; Fan, L.; Xu, X.; Song, Z.; Hayat, K.; Feng, T.; Wang, Y. Identification of pork flavour precursors from enzyme-treated lard using maillard model system assessed by GC–MS and partial least squares regression. Meat Sci. 2017, 124, 15–24. [Google Scholar] [CrossRef]
- Wu, H.Z.; Zhuang, H.; Zhang, Y.Y.; Tang, J.; Yu, X.; Long, M.; Wang, J.M.; Zhang, J.H. Influence of partial replacement of NaCl with KCl on profiles of volatile compounds in dry-cured bacon during processing. Food Chem. 2015, 172, 391–399. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Gallardo, E.; León-Camacho, M. Analysis of volatile compounds from Iberian hams: A review. Grasas Y Aceites 2012, 63, 432–454. [Google Scholar] [CrossRef]
- Purriños, L.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lacón”. Meat Sci. 2012, 92, 627–634. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.L.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T. Relationship between sensory attributes and volatile compounds qualifying dry-cured hams. Meat Sci. 2008, 80, 315–325. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Zhang, H.; Wang, H.; Kong, B. Influence of glycated nitrosohaemoglobin prepared from porcine blood cell on physicochemical properties, microbial growth and flavour formation of Harbin dry sausages. Meat Sci. 2019, 148, 96–104. [Google Scholar] [CrossRef]
- Gabler, F.M.; Mercier, J.; Jiménez, J.I.; Smilanick, J.L. Integration of continuous biofumigation with Muscodor albus with pre-cooling fumigation with ozone or sulfur dioxide to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2010, 55, 78–84. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Xie, J.; Ai, L.Z.; Tian, H.X. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019, 293, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.H.; Kang, D.C.; Liu, R.; Qi, J.; Zhou, G.H.; Zhang, W.G. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochem. 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Saldaña, E.; Saldarriaga, L.; Cabrera, J.; Siche, R.; Behrens, J.H.; Selani, M.M.; de Almeida, M.A.; Silva, L.D.; Pinto, J.S.S.; Contreras-Castillo, C.J. Relationship between volatile compounds and consumer-based sensory characteristics of bacon smoked with different Brazilian woods. Food Res. Int. 2019, 119, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Q.; Chen, C.G.; Yu, H.; Xu, B.C. Effects of different smoking methods on sensory properties, free amino acids and volatile compounds in bacon. J. Sci. Food Agric. 2021, 101, 2984–2993. [Google Scholar] [CrossRef]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.M.; Marino, R.; Malva, A.D.; Centoducati, P.; De Palo, P. Foal meat volatile compounds: Effect of vacuum ageing on semimembranosus muscle. J. Sci. Food Agric. 2018, 99, 1660–1667. [Google Scholar] [CrossRef]
- Cha, Y.J.; Baek, H.-H.; Hsieh, T.C.-Y. Volatile components in flavour concentrates from crayfish processing waste. J. Sci. Food Agric. 1992, 58, 239–248. [Google Scholar] [CrossRef]
- Ahn, D.U.; Lee, E.J.; Feng, X.; Zhang, W.; Lee, J.H.; Jo, C.; Nam, K. Mechanisms of volatile production from non-sulfur amino acids by irradiation. Radiat. Phys. Chem. 2016, 119, 64–73. [Google Scholar] [CrossRef]
- Madruga, M.S.; Elmore, J.S.; Oruna-Concha, M.J.; Balagiannis, D.; Mottram, D.S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010, 123, 513–520. [Google Scholar] [CrossRef]
- Scalone, G.L.L.; Lamichhane, P.; Cucu, T.; De Kimpe, N.; De Meulenaer, B. Impact of different enzymatic hydrolysates of whey protein on the formation of pyrazines in maillard model systems. Food Chem. 2019, 278, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Sandra, P.; Marušić, R.N.; Katarina, L.; Eddy, L.; Helga, M. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; D’Souza, L.A.; Teranishi, R.; Buttery, R.G. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Crit. Rev. Food Sci. Nutr. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Keeney, M.; Stahl, W.H. The role of methional as a flavor compound. J. Food Sci. 1958, 23, 130–132. [Google Scholar] [CrossRef]
| Volatile Compounds | R.T. (min) | LRI | Content (μg/kg−1) (Mean ± SE R) | |||||
|---|---|---|---|---|---|---|---|---|
| HTHP | Control | 3 kGy | 5 kGy | 7 kGy | 9 kGy | |||
| Alcohols | ||||||||
| Ethanol | 8.69 | 813 | 5.09 ± 0.70 a | 12.48 ± 1.76 b | 13.22 ± 0.14 b | 13.12 ± 0.46 b | 18.22 ± 1.72 c | 41.25 ± 1.33 d |
| Isobutyl alcohol | 13.5 | 1096 | 0.38 ± 0.03 a | 1.44 ± 0.01 ab | 1.11 ± 0.34 ab | 1.81 ± 0.07 ac | 4.29 ± 0.59 b | 9.57 ± 1.35 c |
| 2-Cyclopentyl Cyclopentanol | 16.69 | 1117 | ND | 4.14 ± 0.00 a | ND | ND | ND | ND |
| Isoamyl alcohol | 16.89 | 1131 | 10.35 ± 1.03 a | 33.52 ± 0.23 bc | 30.24 ± 0.41 bc | 43.60 ± 2.15 d | 56.04 ± 4.19 e | 84.85 ± 2.60 f |
| Cineole | 17.05 | 1144 | 21.67 ± 2.42 a | 34.54 ± 4.26 bc | 25.09 ± 1.69 bc | 40.60 ± 2.15 bd | 23.33 ± 2.99 a | 81.34 ± 3.54 e |
| 2-Undecanethiol,2-methyl- | 20.20 | 1228 | 0.59 ± 0.00 a | ND | ND | ND | ND | ND |
| Hexyl alcohol | 21.48 | 1292 | 1.74 ± 0.24 a | 2.56 ± 0.06 bc | 2.40 ± 0.28 bc | 3.35 ± 0.03 d | 1.52 ± 0.18 a | 1.68 ± 0.00 a |
| 1-Octen-3-ol | 24.34 | 1356 | 1.96 ± 0.29 a | 2.35 ± 0.22 a | 1.65 ± 0.21 ab | 2.41 ± 0.30 a | 0.50 ± 0.00 b | ND |
| Linalool | 27.10 | 1454 | 23.36 ± 2.62 a | 41.27 ± 0.50 b | 16.46 ± 4.35 a | 32.72 ± 1.53 bc | 3.13 ± 0.38 d | 6.95 ± 0.07 de |
| 1-Octanol” | 27.37 | 1487 | 0.97 ± 0.09 a | ND | 0.66 ± 0.00 b | 1.44 ± 0.00 c | ND | ND |
| Terpinen-4-ol | 28.89 | 1620 | 5.99 ± 0.26 a | 9.01 ± 0.71 ab | 4.66 ± 0.34 a | 6.55 ± 0.65 ad | ND | 18.58 ± 0.00 c |
| Furfuryl alcohol | 30.33 | 1658 | 4.25 ± 0.15 a | 5.00 ± 0.38 a | 6.97 ± 1.02 a | 5.20 ± 0.03 a | 3.33 ± 0.41 a | 4.26 ± 0.60 a |
| (S)-(-)-α-terpineol | 31.19 | 1716 | 2.97 ± 0.39 a | 5.46 ± 0.32 b | 1.52 ± 0.42 a | 3.43 ± 0.37 a | ND | ND |
| Total | 79.36 ± 8.21 d | 151.75 ±8.45 b | 110.60 ± 10.47 c | 154.22 ± 7.75 b | 110.35 ± 10.47 c | 248.47 ± 9.49 a | ||
| Aldehydes | ||||||||
| 2-Methylbutyraldehyde | 8.14 | 659 | ND | ND | ND | ND | 1.12 ± 0.00 a | 5.60 ± 0.60 b |
| Isovaleraldehyde | 8.24 | 672 | ND | ND | ND | 1.26 ± 0.00 a | 3.34 ± 0.00 a | 25.54 ± 0.91 b |
| Hexanal | 12.91 | 1085 | ND | 5.28 ± 0.00 a | ND | ND | ND | ND |
| Heptaldehyde | 16.33 | 1196 | 1.77 ± 0.00 a | 1.51 ± 0.00 a | ND | 2.50 ± 0.33 ab | 0.87 ± 0.10 a | ND |
| Octanal | 19.68 | 1202 | 2.73 ± 0.10 a | 1.99 ± 0.08 a | 2.77 ± 0.34 a | 3.92 ± 0.04 ac | 1.28 ± 0.00 b | ND |
| 1-Nonanal | 22.92 | 1307 | 7.72 ± 1.00 a | 7.89 ± 0.46 a | 4.13 ± 0.02 b | 9.82 ± 0.41 a | 2.07 ± 0.00 bc | 2.14 ± 0.38 bd |
| Decanal | 25.98 | 1414 | 1.51 ± 0.10 a | 1.00 ± 0.10 b | ND | 2.45 ± 0.03 c | ND | ND |
| Total | 13.73 ± 1.21 c | 17.67 ± 0.64 b | 6.90 ± 0.37 d | 19.90 ± 0.81 b | 8.68 ± 0.44 d | 33.27 ± 1.88 a | ||
| Acids | ||||||||
| Acetic acid | 24.59 | 1365 | 2.84 ± 0.02 a | 4.00 ± 0.07 a | 3.78 ± 0.47 a | 3.69 ± 0.14 a | 5.63 ± 0.46 a | 14.62 ± 1.33 b |
| Isovaleric acid | 30.40 | 1678 | ND | 2.40 ± 0.10 a | ND | 2.02 ± 0.10 a | ND | ND |
| Benzoic acid, 2-acetyl-2-phenylhydrazide | 30.60 | 1686 | 1.11 ± 0.00 a | ND | ND | ND | ND | ND |
| Total | 3.95 ± 0.02 cd | 6.39 ± 0.18 b | 3.78 ± 0.47 d | 5.71 ± 0.24 b | 5.63 ± 0.46 bc | 14.62 ± 1.33 a | ||
| Esters | ||||||||
| Ethyl acetate | 7.51 | 789 | ND | ND | ND | ND | 6.68 ± 0.96 a | 8.16 ± 1.15 a |
| Octadecanoic Acid, ethenyl ester | 25.82 | 1408 | 0.67 ± 0.00 a | ND | ND | ND | ND | ND |
| 3,7-Dimethyl-1,6-octadien-3-yl 2-aminobenzoate | 27.46 | 1468 | 2.31 ± 0.00 a | 22.31 ± 0.00 b | 3.58 ± 0.10 a | 17.53 ± 0.95 c | 1.05 ± 0.13 ad | 1.67 ± 0.21 a |
| Ethyl caprate | 29.60 | 1648 | 0.38 ± 0.10 a | ND | ND | ND | ND | ND |
| Nerol acetate | 31.66 | 1728 | 0.44 ± 0.10 a | 0.89 ± 0.10 a | ND | ND | ND | ND |
| Total | 3.78 ± 0.20 d | 23.20 ± 0.10 a | 3.58 ± 0.10 d | 17.53 ± 0.95 b | 7.73 ± 1.09 c | 9.83 ± 1.36 c | ||
| Phenols | ||||||||
| Guaiacol | 35.30 | 1724 | 9.01 ± 1.14 a | 13.52 ± 1.93 a | 11.62 ± 1.69 a | 14.73 ± 0.35 b | 3.09 ± 0.28 c | 2.93 ± 0.39 d |
| 2-Methoxy-6-methylphenol | 35.63 | 1760 | 0.61 ± 0.00 a | 6.92 ± 0.00 a | ND | 1.16 ± 0.14 a | ND | ND |
| O-Cresol | 37.95 | 1905 | 4.13 ± 0.17 a | 5.40 ± 0.81 a | 3.97 ± 0.27 a | 6.17 ± 0.18 a | 0.90 ± 0.11 b | ND |
| Phenol | 38.38 | 2027 | 7.00 ± 0.35 a | 9.20 ± 0.36 a | 9.98 ± 1.17 a | 10.66 ± 0.03 a | 2.49 ± 0.06 b | 4.21 ± 0.00 b |
| 4-Ethyl-2-methoxyphenol | 38.42 | 2029 | 3.39 ± 0.10 a | 4.95 ± 0.00 a | 2.96 ± 0.10 a | 4.99 ± 0.00 a | 0.49 ± 0.00 b | ND |
| 2,3-Dimethylphenol | 39.78 | 2098 | 0.68 ± 0.00 a | ND | ND | ND | ND | ND |
| p-Cresol | 39.79 | 2099 | ND | 1.19 ± 0.00 a | ND | ND | ND | ND |
| Total | 21.43 ±1.76 c | 41.17 ±3.09 a | 28.52 ±3.23 b | 41.78 ±0.70 a | 6.97 ± 0.45 d | 7.14 ± 0.39 d | ||
| Furans | ||||||||
| 2-Methylfuran | 7.20 | 770 | 2.10 ± 0.00 a | ND | ND | ND | ND | ND |
| 2-Ethylfuran | 9.08 | 836 | 1.58 ± 0.00 a | ND | ND | ND | ND | ND |
| 2-Pentylfuran | 17.72 | 1193 | 14.77 ± 0.00 a | ND | ND | 0.86 ± 0.00 b | ND | ND |
| 2-Acetylfuran | 26.48 | 1432 | 1.00 ± 0.14 a | 1.73 ± 0.00 a | 1.73 ± 0.24 a | 1.474 ± 0.00 a | ND | ND |
| Total | 19.45 ± 0.14 a | 1.73 ± 0.00 c | 1.73 ± 0.24 c | 2.34 ± 0.00 b | ND | ND | ||
| Ketones | ||||||||
| Acetone | 6.38 | 720 | ND | ND | ND | ND | ND | 2.16 ± 0.10 a |
| 2-Pentanone | 9.84 | 881 | ND | ND | 1.61 ± 0.00 a | 1.68 ± 0.00 b | 1.62 ± 0.02 ab | 2.09 ± 0.03 c |
| 3-Eicosanone | 14.83 | 1150 | 1.01 ± 0.10 a | 1.20 ± 0.10 b | 0.94 ± 0.10 c | 1.33 ± 0.10 d | ND | ND |
| 2-Heptanone | 16.22 | 1193 | 2.16 ± 0.15 a | 0.73 ± 0.10 b | 0.66 ± 0.10 b | 0.98 ± 0.10 b | 0.52 ± 0.10 bc | ND |
| 2-Octanone | 19.57 | 1196 | 0.64 ± 0.00 a | ND | ND | ND | ND | ND |
| 3-Hydroxy-2-butanone | 19.81 | 1208 | 5.28 ± 0.38 a | 8.35 ± 1.05 a | 10.62 ± 1.01 a | 13.01 ± 0.02 ab | 45.00 ± 6.35 b | 34.523 ± 4.208 b |
| Hydroxyacetone | 20.40 | 1238 | 1.13 ± 0.14 a | ND | ND | ND | 2.08 ± 0.09 a | 4.08 ± 0.50 a |
| Methylheptenone | 21.23 | 1280 | 2.90 ± 0.27 a | 2.48 ± 0.35 a | 1.14 ± 0.15 a | 2.43 ± 0.30 a | 0.66 ± 0.00 a | 1.06 ± 0.15 a |
| 2-Cyclopenten-1-one,2-methyl- | 22.55 | 1385 | 1.11 ± 0.20 a | ND | ND | ND | ND | ND |
| 2-Cyclopenten-1-one,2,3-dimethyl- | 27.65 | 1474 | 1.66 ± 0.16 a | 2.46 ± 0.07 a | 2.48 ± 0.49 a | 2.74 ± 0.02 a | 0.53 ± 0.21 a | ND |
| Acetophenone | 30.61 | 1686 | ND | 1.70 ± 0.00 a | ND | 0.98 ± 0.00 a | ND | ND |
| Total | 15.89 ±1.41 b | 16.92 ± 2.78 b | 17.45 ±4.95 b | 23.13 ± 0.55 b | 48.33 ± 6.76 a | 43.91 ± 4.99 a | ||
| Aromatic hydrocarbons | ||||||||
| Toluene | 11.62 | 1021 | ND | ND | 1.34 ± 0.20 a | 2.82 ± 0.02 a | 2.58 ± 0.37 a | 4.00 ± 0.47 a |
| 1-Methyl-4-isopropylbenzene | 19.09 | 1170 | ND | ND | ND | 4.78 ± 0.00 a | ND | ND |
| m-isopropyltoluene | 19.16 | 1174 | 2.58 ± 0.10 a | ND | ND | ND | ND | 48.29 ± 0.00 a |
| Benzene,1,3-bis(1,1-dimethylethyl) | 23.82 | 1338. | ND | ND | ND | 0.49 ± 0.00 a | ND | ND |
| 3,4-Dimethoxytoluene | 34.06 | 1626 | 0.75 ± 0.00 a | ND | ND | ND | ND | ND |
| 3-Hydroxy-4-methoxytoluene | 35.77 | 1670 | 0.90 ± 0.10 a | ND | ND | ND | ND | ND |
| Total | 4.23 ± 0.20 a | ND | 1.34 ± 0.20 b | 8.09 ± 0.02 c | 2.58 ± 0.37 d | 52.29 ± 0.47 e | ||
| Ethers | ||||||||
| 4-Allylanisole | 30.75 | 1692 | 0.99 ± 0.02 a | 2.81 ± 0.41 a | ND | 2.77 ± 0.36 a | ND | ND |
| Cis-Anethol | 33.93 | 1721 | 31.02 ± 0.90 a | 79.40 ± 0.63 b | 20.92 ± 0.40 c | 74.61 ± 1.38 d | ND | 15.68 ± 1.86 e |
| Total | 32.01 ± 0.92 a | 82.21 ± 1.56 b | 20.92 ± 0.40 c | 77.38 ± 1.74 d | ND | 15.68 ± 1.86 e | ||
| Terpenes | ||||||||
| α-Pinene | 10.86 | 968 | 1.59 ± 0.04 b | 2.53 ± 0.26 b | 0.97 ± 0.01 b | 12.21 ± 0.75 a | 12.19 ± 0.62 a | 11.62 ± 0.70 a |
| g-Terpinene | 18.2 | 1121 | 1.62 ± 0.14 a | 5.88 ± 0.11 b | 0.72 ± 0.01 c | 3.54 ± 0.18 d | ND | ND |
| Myrcene | 15.39 | 1167 | 2.60 ± 0.16 a | 5.68 ± 0.23 b | ND | 5.05 ± 0.04 c | ND | ND |
| (4R)-1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene | 16.64 | 1114 | 9.07 ± 0.14 c | 20.89 ± 0.29 a | 5.43 ± 0.27 d | 18.25 ± 0.49 b | 4.28 ± 0.01 e | 5.84 ± 0.01 d |
| 3-Carene | 14.95 | 1153 | ND | 6.7 ± 0.19 a | ND | 5.4 ± 0.01 b | 4.74 ± 0.24 c | 3.43 ± 0.18 d |
| Total | 14.88 ± 0.48 c | 41.68 ± 1.08 a | 7.12 ± 0.29 d | 44.45 ± 0.48 a | 21.21 ± 0.87 b | 20.89 ± 0.89 b | ||
| Others | ||||||||
| 2,6-Dimethylpyrazine | 21.13 | 1275 | 0.36 ± 0.00 a | ND | ND | ND | ND | ND |
| 2,3,5,6-Tetramethylpyrazine | 25.46 | 1395 | 0.25 ± 0.00 a | 0.39 ± 0.04 b | ND | 1.16 ± 0.11 c | ND | ND |
| 2-Acetyl pyrrole | 28.72 | 1614 | 0.89 ± 0.05 a | 1.11 ± 0.00 a | 1.16 ± 0.00 a | 0.81 ± 0.00 a | ND | ND |
| Naphthalene | 33.06 | 1785 | 0.92 ± 0.10 a | ND | ND | ND | ND | ND |
| Total | 2.41 ± 0.15 a | 1.50 ± 0.04 b | 1.16 ± 0.00 c | 1.97 ± 0.11 d | ND | ND | ||
| Name | OT (μg L−1 of Water) | HTHP | Control | 3 kGy | 5 kGy | 7 kGy | 9 kGy |
|---|---|---|---|---|---|---|---|
| Isobutyl alcohol | 0.500 | 0.764 | 2.882 | 2.222 | 3.610 | 8.576 | 19.136 |
| Isoamyl alcohol | 1.000 | 10.351 | 33.520 | 30.238 | 43.602 | 56.037 | 84.850 |
| Cineole | 5.000 | 4.339 | 8.907 | 8.120 | 8.720 | 4.666 | 16.269 |
| Hexyl alcohol | 1.600 | 1.086 | 1.598 | 1.501 | 2.092 | 0.952 | 1.048 |
| 1-Octen-3-ol | 0.010 | 196.200 | 234.700 | 164.700 | 241.200 | 50.100 | – |
| Linalool | 0.005 | 4672.000 | 8253.000 | 3292.400 | 6544.400 | 625.200 | 1389.600 |
| 1-Octanol | 0.190 | 5.121 | – | 3.489 | 7.574 | – | – |
| Furfuryl alcohol | 2.000 | 2.125 | 2.498 | 3.486 | 2.599 | 1.663 | 2.130 |
| (S)-(-)-α-terpineol | 0.28 | 10.611 | 19.511 | 5.425 | 12.254 | – | – |
| Isovaleraldehyde | 0.002 | – | – | – | 525.000 | 1390.000 | 10,639.583 |
| Octanal | 0.080 | 34.100 | 24.863 | 34.675 | 48.950 | 15.988 | – |
| 1-Nonanal | 0.004 | 1754.773 | 1793.636 | 937.500 | 2231.136 | 470.455 | 485.909 |
| Guaiacol | 0.020 | 450.450 | 675.950 | 580.900 | 736.250 | 154.550 | 146.350 |
| o-Cresol | 0.650 | 6.355 | 8.303 | 6.109 | 9.486 | 1.389 | – |
| Phenol | 4.000 | 1.750 | 2.301 | 2.494 | 2.666 | 0.623 | 1.053 |
| 4-Ethyl-2-methoxyphenol | 0.044 | 77.091 | 112.386 | – | 113.318 | 11.045 | – |
| 2-Pentanone | 0.050 | – | – | 32.180 | 33.620 | 32.480 | 41.840 |
| 2-Heptanone | 0.140 | 15.436 | 5.236 | 4.686 | 6.993 | 3.700 | – |
| 3-Hydroxy-2-butanone | 8.000 | 0.660 | – | 1.327 | 1.626 | 5.625 | 4.315 |
| Toluene | 0.527 | – | – | 2.537 | 5.355 | 4.899 | 7.594 |
| 4-Allylanisole | 0.035 | 28.314 | 80.400 | – | 79.171 | – | – |
| 2,3,5,6-Tetramethylpyrazine | 1.000 | 0.254 | 0.387 | – | 1.155 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Yang, C.; Xi, L.; Wang, T.; Zhang, J.; Kou, L.; Ding, W. Evaluation of the Influence of Flavor Characteristics of Cooked Bacon with Different Sterilization Methods by GC-IMS Combined with HS-SPME-GC-MS and Electronic Nose. Foods 2022, 11, 3547. https://doi.org/10.3390/foods11223547
Wu R, Yang C, Xi L, Wang T, Zhang J, Kou L, Ding W. Evaluation of the Influence of Flavor Characteristics of Cooked Bacon with Different Sterilization Methods by GC-IMS Combined with HS-SPME-GC-MS and Electronic Nose. Foods. 2022; 11(22):3547. https://doi.org/10.3390/foods11223547
Chicago/Turabian StyleWu, Ruixiao, Chunjie Yang, Linjie Xi, Tian Wang, Ju Zhang, Liping Kou, and Wu Ding. 2022. "Evaluation of the Influence of Flavor Characteristics of Cooked Bacon with Different Sterilization Methods by GC-IMS Combined with HS-SPME-GC-MS and Electronic Nose" Foods 11, no. 22: 3547. https://doi.org/10.3390/foods11223547
APA StyleWu, R., Yang, C., Xi, L., Wang, T., Zhang, J., Kou, L., & Ding, W. (2022). Evaluation of the Influence of Flavor Characteristics of Cooked Bacon with Different Sterilization Methods by GC-IMS Combined with HS-SPME-GC-MS and Electronic Nose. Foods, 11(22), 3547. https://doi.org/10.3390/foods11223547




