Classification and Feature Extraction Using Supervised and Unsupervised Machine Learning Approach for Broiler Woody Breast Myopathy Detection
Abstract
1. Introduction
2. Related Works
3. Materials and Methods
3.1. K-Means Clustering (k-Means)
- Input: Data set (D) and initial cluster values depending on the prior knowledge, i.e., based on experience or exploratory data analysis. Output: Set a cluster representative P in the vector space.
- Repeat: Assign the data points to the closest cluster mean and update the cluster ID of jth point in the data set.
- Relocation of means: Update P so that Pj is the mean of the jth cluster until the whole algorithm converges in Equation (1) with minimum local optima.
3.2. Fuzzy C Means (FCM)
3.3. K-Nearest Neighbor (k-NN)
3.4. Support Vector Machines (SVM) Algorithms
3.5. Bioelectrical Impedance Analysis
4. Data Analysis
5. Results and Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Chicken Council. Per Capita Consumption of Poultry and Livestock, 1965 to Estimated 2021. 2020. Available online: https://www.nationalchickencouncil.org/about-theindustry/statistics/percapita-consumption-of-poultry-and-livestock-1965-to-estimated2021-in-pounds/ (accessed on 30 July 2021).
- Petracci, M.; Bianchi, M.; Mudalal, S.; Cavani, C. Functional ingredients for poultry meat products. Trends Food Sci. Technol. 2013, 33, 27–39. [Google Scholar] [CrossRef]
- Petracci, M.; Cavani, C. Muscle growth and poultry meat quality issues. Nutrients 2012, 4, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Puolanne, E.; Schwartzkopf, M.; Arner, A. Altered sarcomeric structure and function in woody breast myopathy of avian pectoralis major muscle. Front. Physiol. 2020, 11, 287. [Google Scholar] [CrossRef]
- Elmasry, G.; Barbin, D.F.; Sun, D.W.; Allen, P. Meat quality evaluation by hyperspectral imaging technique: An overview. Crit. Rev. Food Sci. Nutr. 2012, 52, 689–711. [Google Scholar] [CrossRef]
- Fletcher, D.L. Poultry meat quality. World’s Poult. Sci. J. 2002, 58, 131–145. [Google Scholar] [CrossRef]
- Morey, A.; Smith, A.E.; Garner, L.J.; Cox, M.K. Application of bioelectrical impedance analysis to detect broiler breast filets affected with woody breast myopathy. Front. Physiol. 2020, 11, 808. [Google Scholar] [CrossRef]
- Hu, M.H.; Dong, Q.L.; Liu, B.L.; Malakar, P.K. The potential of double K-means clustering for banana image segmentation. J. Food Process Eng. 2014, 37, 10–18. [Google Scholar] [CrossRef]
- Mallmann, B. Prediction and Evaluation of Breast Myopathy. 2019. Available online: https://core.ac.uk/download/pdf/250604484.pdf (accessed on 7 July 2022).
- Barbut, S. Understanding the woody breast syndrome and other myopathies in modern broiler chickens. In Proceedings of the Australian Poultry Science Symposium, Sydney, Australia, 16–19 February 2020; pp. 99–102. [Google Scholar]
- Kuttappan, V.A.; Hargis, B.M.; Owens, C.M. White striping and woody breast myopathies in the modern poultry industry: A review. Poult. Sci. 2016, 95, 2724–2733. [Google Scholar] [CrossRef]
- Chatterjee, D.; Zhuang, H.; Bowker, B.C.; Rincon, A.M.; Sanchez-Brambila, G. Instrumental texture characteristics of broiler Pectoralis major with the wooden breast condition. Poult. Sci. 2016, 95, 2449–2454. [Google Scholar] [CrossRef]
- Tijare, V.V.; Yang, F.L.; Kuttappan, V.A.; Alvarado, C.Z.; Coon, C.N.; Owens, C.M. Meat quality of broiler chicken breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. J. 2016, 95, 2167–2173. [Google Scholar] [CrossRef]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estévez, M. Wooden-breast, white striping, and spaghetti meat: Causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Geronimo, B.C.; Mastelini, S.M.; Carvalho, R.H.; Júnior, S.B.; Barbin, D.F.; Shimokomaki, M.; Ida, E.I. Computer vision system and near-infrared spectroscopy for identification and classification of chicken with wooden breast, and physicochemical and technological characterization. Infrared Phys. Technol. 2019, 96, 303–310. [Google Scholar] [CrossRef]
- Wold, J.P.; Måge, I.; Løvland, A.; Sanden, K.W.; Ofstad, R. Near-infrared spectroscopy detects woody breast syndrome in chicken fillets by the markers protein content and degree of water binding. Poult. Sci. 2019, 98, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Wold, J.P.; Veiseth-Kent, E.; Høst, V.; Løvland, A. Rapid on-line detection and grading of wooden breast myopathy in chicken fillets by near-infrared spectroscopy. PLoS ONE 2017, 12, e0173384. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.C.; Bowker, B.C.; Zhuang, H. Toward a fusion of optical coherence tomography and hyperspectral imaging for poultry meat quality assessment. Electron. Imaging 2016, 14, 1–5. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Composition of the ESPEN Working Group. Bioelectrical impedance analysis—part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical impedance methods for noninvasive health monitoring: A review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef]
- Abasht, B.; Zhou, N.; Lee, W.R.; Zhuo, Z.; Peripolli, E. The metabolic characteristics of susceptibility to wooden breast disease in chickens with high feed efficiency. Poult. Sci. 2019, 98, 3246–3256. [Google Scholar] [CrossRef]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative stress and metabolic perturbations in wooden breast disorder in chickens. PLoS ONE 2016, 11, e0153750. [Google Scholar] [CrossRef]
- Greene, E.; Flees, J.; Dadgar, S.; Mallmann, B.; Orlowski, S.; Dhamad, A.; Dridi, S. Quantum blue reduces the severity of woody breast myopathy via modulation of oxygen homeostasis-related genes in broiler chickens. Front. Physiol. 2019, 10, 1251. [Google Scholar] [CrossRef]
- Lee, W.R.; Brannick, E.M.; Mutryn, M.F.; Fu, W.; Abasht, B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. BMC Genom. 2015, 16, 399. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Pan, D.; He, J.; Cao, J.; Wang, H.; Ertbjerg, P. Metabolite profile based on 1H NMR of broiler chicken breasts affected by wooden breast myodegeneration. Food Chem. 2020, 310, 125852. [Google Scholar] [CrossRef] [PubMed]
- Nyboer, J.; Kreider, M.M.; Hannapel, L. Electrical impedance plethysmography: A physical and physiologic approach to peripheral vascular study. Circulation 1950, 2, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, E.C.; Meador, C.K.; Simpson, D.C. Correlation of whole-body impedance with total body water volume. J. Appl. Physiol. 1969, 27, 531–534. [Google Scholar] [CrossRef]
- Cox, M.K.; Heintz, R.; Hartman, K. Measurements of resistance and reactance in fish with the use of bioelectrical impedance analysis: Sources of error. Fish. Bull. 2011, 109, 34–47. [Google Scholar]
- Hartman, K.J.; Margraf, F.J.; Hafs, A.W.; Cox, M.K. Bioelectrical impedance analysis: A new tool for assessing fish condition. Fisheries 2015, 40, 590–600. [Google Scholar] [CrossRef]
- Siddique, A.; Shirzaei, S.; Smith, A.E.; Valenta, J.; Garner, L.J.; Morey, A. Acceptability of Artificial Intelligence in Poultry Processing and Classification Efficiencies of Different Classification Models in the Categorisation of Breast Fillet Myopathies. Front. Physiol. 2021, 1472. [Google Scholar] [CrossRef]
- Dehariya, V.K.; Shrivastava, S.K.; Jain, R.C. Clustering of image data set using k-means and fuzzy k-means algorithms. In Proceedings of the 2010 International Conference on Computational Intelligence and Communication Networks, Bhopal, India, 26–28 November 2010; pp. 386–391. [Google Scholar]
- DeSarbo, W.S.; Cron, W.L. A maximum likelihood methodology for cluster wise linear regression. J. Classif. 1988, 5, 249–282. [Google Scholar] [CrossRef]
- Van Hattum, P.; Hoijtink, H. Market segmentation using brand strategy research: Bayesian inference with respect to mixtures of log-linear models. J. Classif. 2009, 26, 297–328. [Google Scholar] [CrossRef]
- Wang, Q.; Fleury, E. Uncovering overlapping community structure. In Complex Networks; Springer: Berlin/Heidelberg, Germany, 2011; pp. 176–186. [Google Scholar]
- Pérez-Suárez, A.; Martínez-Trinidad, J.F.; Carrasco-Ochoa, J.A.; Medina-Pagola, J.E. OClustR: A new graph-based algorithm for overlapping clustering. Neurocomputing 2013, 121, 234–247. [Google Scholar] [CrossRef]
- Aliguliyev, R.M. A new sentence similarity measure and sentence based extractive technique for automatic text summarization. Expert Syst. Appl. 2009, 36, 7764–7772. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, C.; Liu, M. Research of fast SOM clustering for text information. Expert Syst. Appl. 2011, 38, 9325–9333. [Google Scholar] [CrossRef]
- Baker, R.S.J.D. Data mining for education. Int. Encycl. Educ. 2010, 7, 112–118. [Google Scholar]
- McCallum, A.; Nigam, K.; Ungar, L.H. Efficient clustering of high dimensional data sets with application to reference matching. In Proceedings of the Sixth ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Boston, MA, USA, 20–23 August 2000; pp. 169–178. [Google Scholar]
- Khanmohammadi, S.; Adibeig, N.; Shanehbandy, S. An improved overlapping k means clustering method for medical applications. Expert Syst. Appl. 2017, 67, 12–18. [Google Scholar] [CrossRef]
- Jain, A.K. Data clustering: 50 years beyond K-means. Pattern recognition letters. Corrected Proof 2010, 31, 651–666. [Google Scholar] [CrossRef]
- Jain, A.K.; Murty, M.N.; Flynn, P.J. Data clustering: A review. ACM Comput. Surv. CSUR 1999, 31, 264–323. [Google Scholar] [CrossRef]
- Celebi, M.E.; Kingravi, H.A.; Vela, P.A. A comparative study of efficient initialization methods for the k-means clustering algorithm. Expert Syst. Appl. 2013, 40, 200–210. [Google Scholar] [CrossRef]
- Wu, X.; Kumar, V. (Eds.) The Top Ten Algorithms in Data Mining; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Singh, A.K.; Purohit, N.; Varma, S. Fuzzy logic-based clustering in wireless sensor networks: A survey. Int. J. Electron. 2013, 100, 126–141. [Google Scholar] [CrossRef]
- Mustafi, D.; Sahoo, G. A hybrid approach using genetic algorithm and the differential evolution heuristic for enhanced initialization of the k-means algorithm with applications in text clustering. Soft Comput. 2019, 23, 6361–6378. [Google Scholar] [CrossRef]
- Jiang, D.; Tang, C.; Zhang, A. Cluster analysis for gene expression data: A survey. IEEE Trans. Knowl. Data Eng. 2004, 16, 1370–1386. [Google Scholar] [CrossRef]
- Swanson, M.D.; Kobayashi, M.; Tewfik, A.H. Multimedia data-embedding and watermarking technologies. Proc. IEEE 1998, 86, 1064–1087. [Google Scholar] [CrossRef]
- Yong, Y.; Chongxun, Z.; Pan, L. A Novel Fuzzy C-Means Clustering Algorithm for Image Thresholding. Meas. Sci. Rev. 2004, 4, 11–19. [Google Scholar]
- Rao, V.S.; Vidyavathi, D.S. Comparative investigations and performance analysis of FCM and MFPCM algorithms on iris data. Indian J. Comput. Sci. Eng. 2010, 1, 145–151. [Google Scholar]
- Fix, E.; Hodges, J.L. Discriminatory analysis, nonparametric discrimination: Consistency properties. Int. Stat. Rev./Rev. Int. Stat. Tech. 1989, 57, 238–247. [Google Scholar] [CrossRef]
- Dai, M.; Dong, F.; Hirota, K. Fuzzy Three-Dimensional Voronoi Diagram and its Application to Geographical Data Analysis. J. Adv. Comput. Intell. Intell. Inform. 2012, 16, 191–198. [Google Scholar] [CrossRef]
- Sun, S.; Huang, R. An adaptive k-nearest neighbor algorithm. In Proceedings of the 2010 Seventh International Conference on Fuzzy Systems and Knowledge Discovery, Yantai, China, 10–12 August 2010; Volume 1, pp. 91–94. [Google Scholar] [CrossRef]
- Romeo, L.; Loncarski, J.; Paolanti, M.; Bocchini, G.; Mancini, A.; Frontoni, E. Machine learning-based design support system for the prediction of heterogeneous machine parameters in industry 4.0. Expert Syst. Appl. 2020, 140, 112869. [Google Scholar] [CrossRef]
- Lopez-Bernal, D.; Balderas, D.; Ponce, P.; Molina, A. Education 4.0: Teaching the Basics of KNN, LDA and Simple Perceptron Algorithms for Binary Classification Problems. Future Internet 2021, 13, 193. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Shi, N. IgA Nephropathy Prediction in Children with Machine Learning Algorithms. Future Internet 2020, 12, 230. [Google Scholar] [CrossRef]
- Hu, L.Y.; Huang, M.W.; Ke, S.W.; Tsai, C.F. The distance function effect on k nearest neighbor classification for medical datasets. Springer Plus 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Vapnik, V.; Izmailov, R. Rethinking statistical learning theory: Learning using statistical invariants. Mach. Learn. 2019, 108, 381–423. [Google Scholar] [CrossRef]
- Maji, S.; Berg, A.C.; Malik, J. Classification using intersection kernel support vector machines is efficient. In Proceedings of the 2008 IEEE Conference on Computer Vision and Pattern Recognition, Anchorage, AK, USA, 23–28 June 2008; pp. 1–8. [Google Scholar] [CrossRef]
- Murphy, R.J.; Monteiro, S.T. Mapping the distribution of ferric iron minerals on a vertical mine face using derivative analysis of hyperspectral imagery (430–970 nm). ISPRS J. Photogramm. Remote Sens. 2013, 75, 29–39. [Google Scholar] [CrossRef]
- Lukaski, H.C. Methods for the assessment of human body composition: Traditional and new. Am. J. Clin. Nutr. 1987, 46, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Soglia, F.; Mudalal, S.; Babini, E.; Di Nunzio, M.; Mazzoni, M.; Sirri, F.; Petracci, M. Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult. Sci. 2016, 95, 651–659. [Google Scholar] [PubMed]
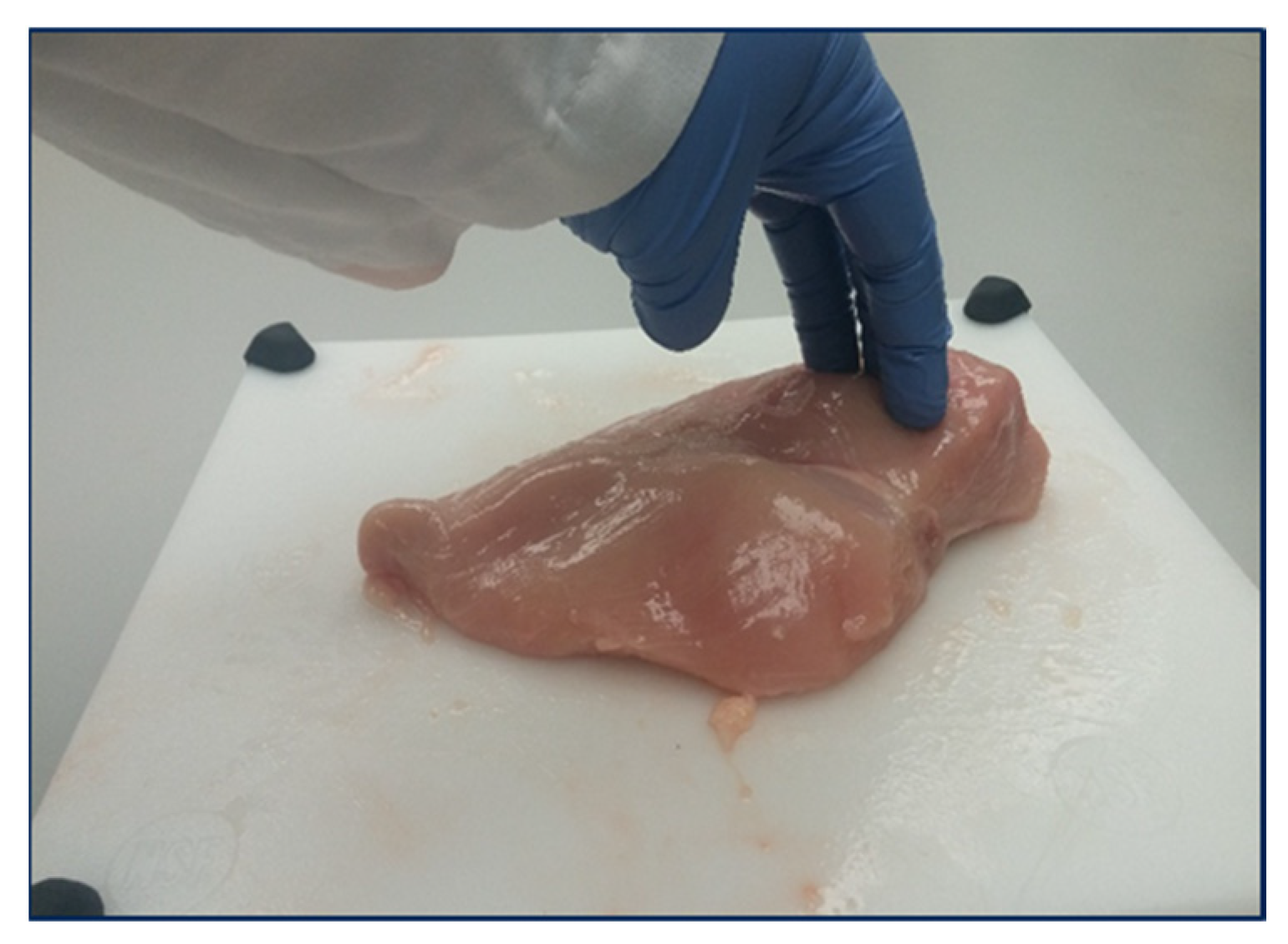

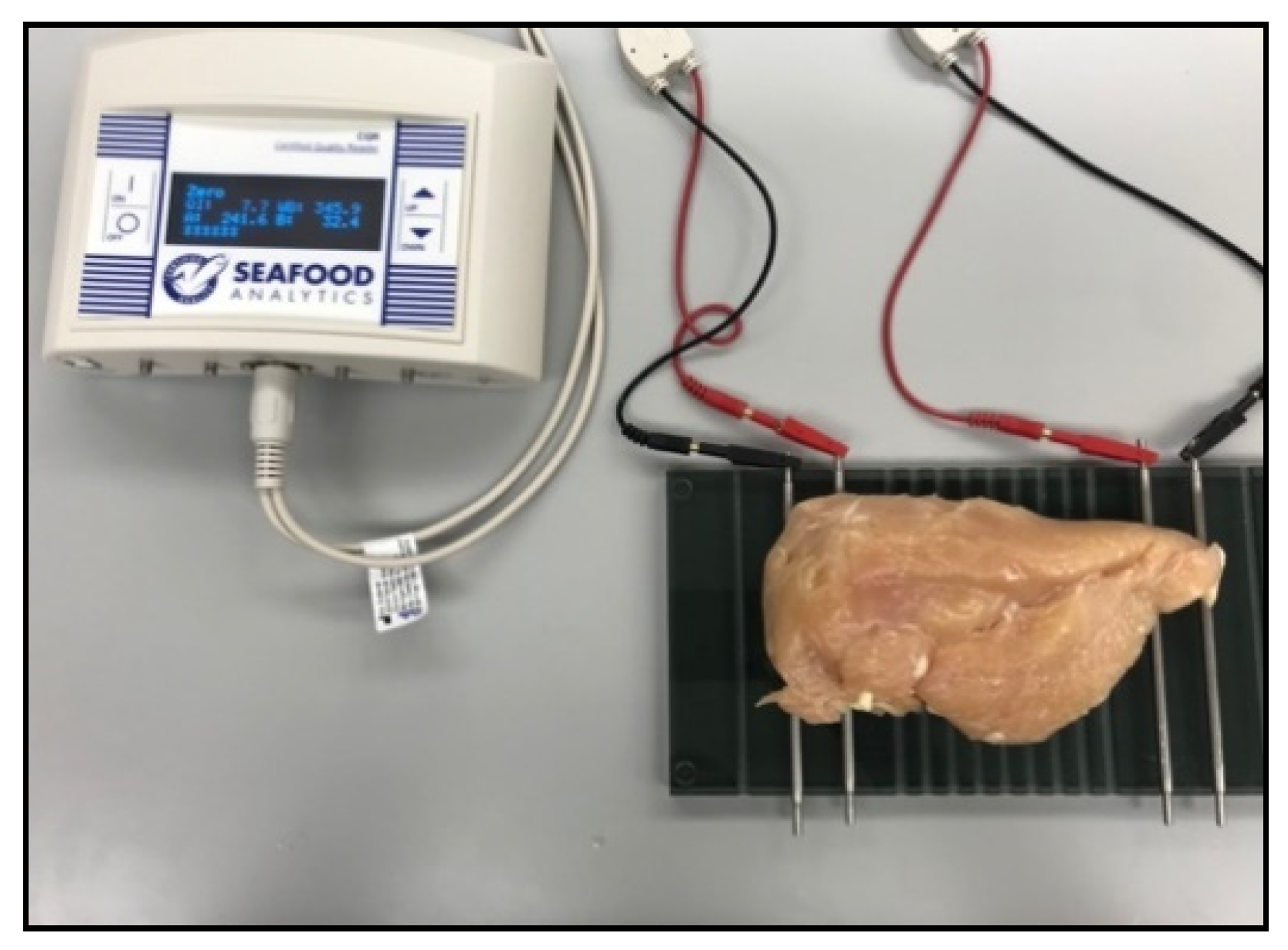
| Hand-Held BIA | Plate BIA | ||||
|---|---|---|---|---|---|
| WB Type | Fillet Weight (g) | Resistance (R; Ω) | Reactance (Xc, Ω) | Resistance (R; Ω) | Reactance (Xc, Ω) |
| Normal | 473.54 ± 54.56 b | 72.89 ± 6.25 a | 25.76 ± 5.50 a | 103.34 ± 15.89 ab | 31.02 ± 8.91 a |
| Moderate | 510.10 ± 57.26 ab | 67.88 ± 6.19 b | 22.14 ± 5.51 b | 101.61 ± 14.50 b | 28.78 ± 9.02 a |
| Severe | 514.96 ± 69.33 a | 70.60 ± 8.24 ab | 21.76 ± 6.48 b | 112.02 ± 16.68 a | 33.98 ± 10.47 a |
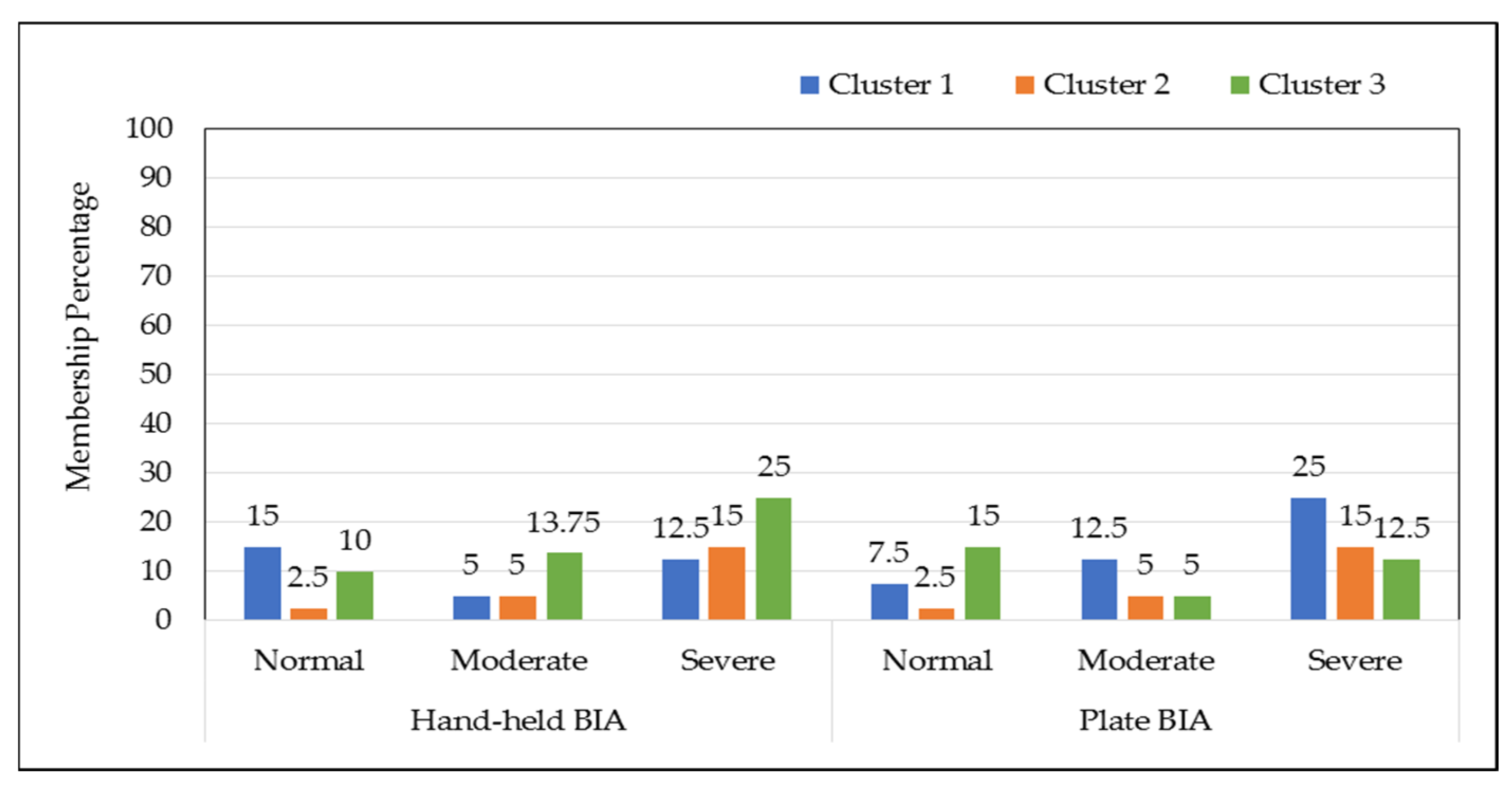
| Fillets Type | Cluster Probability Percentage for Hand-Held BIA | Cluster Probability Percentage for Plate BIA | ||||
|---|---|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 1 | Cluster 2 | Cluster 3 | |
| Normal | 54.5 | 9.09 | 36.3 | 30 | 10 | 60 |
| Moderate | 21.0 | 21.0 | 57.8 | 55.5 | 22.2 | 22.2 |
| Severe | 23.8 | 28.5 | 47.6 | 47.6 | 28.5 | 23.8 |
| Hand-Held BIA | Plate BIA | ||||
|---|---|---|---|---|---|
| WB Classification | No. of Fillets | Training (%) | Testing (%) | Training (%) | Testing (%) |
| Normal | 21 | 38.50 | 50.00 | 31.30 | 40.00 |
| Moderate | 17 | 10.0 | 42.90 | 7.70 | 25.00 |
| Severe | 42 | 57.70 | 87.50 | 57.10 | 78.60 |
| Hand-Held BIA | Plate BIA | ||||
|---|---|---|---|---|---|
| WB Classification | No. of Fillets | Testing (%) | No. of Classified Fillets/Total fillets | Testing (%) | No. of Classified Fillets/Total Fillets |
| Normal | 21 | 77.78 | 7/9 | 80.00 | 4/5 |
| Moderate | 17 | 85.71 | 6/7 | 66.67 | 6/9 |
| Severe | 42 | 88.89 | 16/18 | 85.00 | 17/20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, A.; Herron, C.B.; Valenta, J.; Garner, L.J.; Gupta, A.; Sawyer, J.T.; Morey, A. Classification and Feature Extraction Using Supervised and Unsupervised Machine Learning Approach for Broiler Woody Breast Myopathy Detection. Foods 2022, 11, 3270. https://doi.org/10.3390/foods11203270
Siddique A, Herron CB, Valenta J, Garner LJ, Gupta A, Sawyer JT, Morey A. Classification and Feature Extraction Using Supervised and Unsupervised Machine Learning Approach for Broiler Woody Breast Myopathy Detection. Foods. 2022; 11(20):3270. https://doi.org/10.3390/foods11203270
Chicago/Turabian StyleSiddique, Aftab, Charles B. Herron, Jaroslav Valenta, Laura J. Garner, Ashish Gupta, Jason T. Sawyer, and Amit Morey. 2022. "Classification and Feature Extraction Using Supervised and Unsupervised Machine Learning Approach for Broiler Woody Breast Myopathy Detection" Foods 11, no. 20: 3270. https://doi.org/10.3390/foods11203270
APA StyleSiddique, A., Herron, C. B., Valenta, J., Garner, L. J., Gupta, A., Sawyer, J. T., & Morey, A. (2022). Classification and Feature Extraction Using Supervised and Unsupervised Machine Learning Approach for Broiler Woody Breast Myopathy Detection. Foods, 11(20), 3270. https://doi.org/10.3390/foods11203270









