A Molecularly Imprinted Polymer-Based Thermal Sensor for the Selective Detection of Melamine in Milk Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Molecularly Imprinted Polymers
2.3. Fourier Transform Infrared Spectroscopy
2.4. Batch Rebinding Experiments
2.5. Preparation and Characterization of MIP-Based Receptor Layer
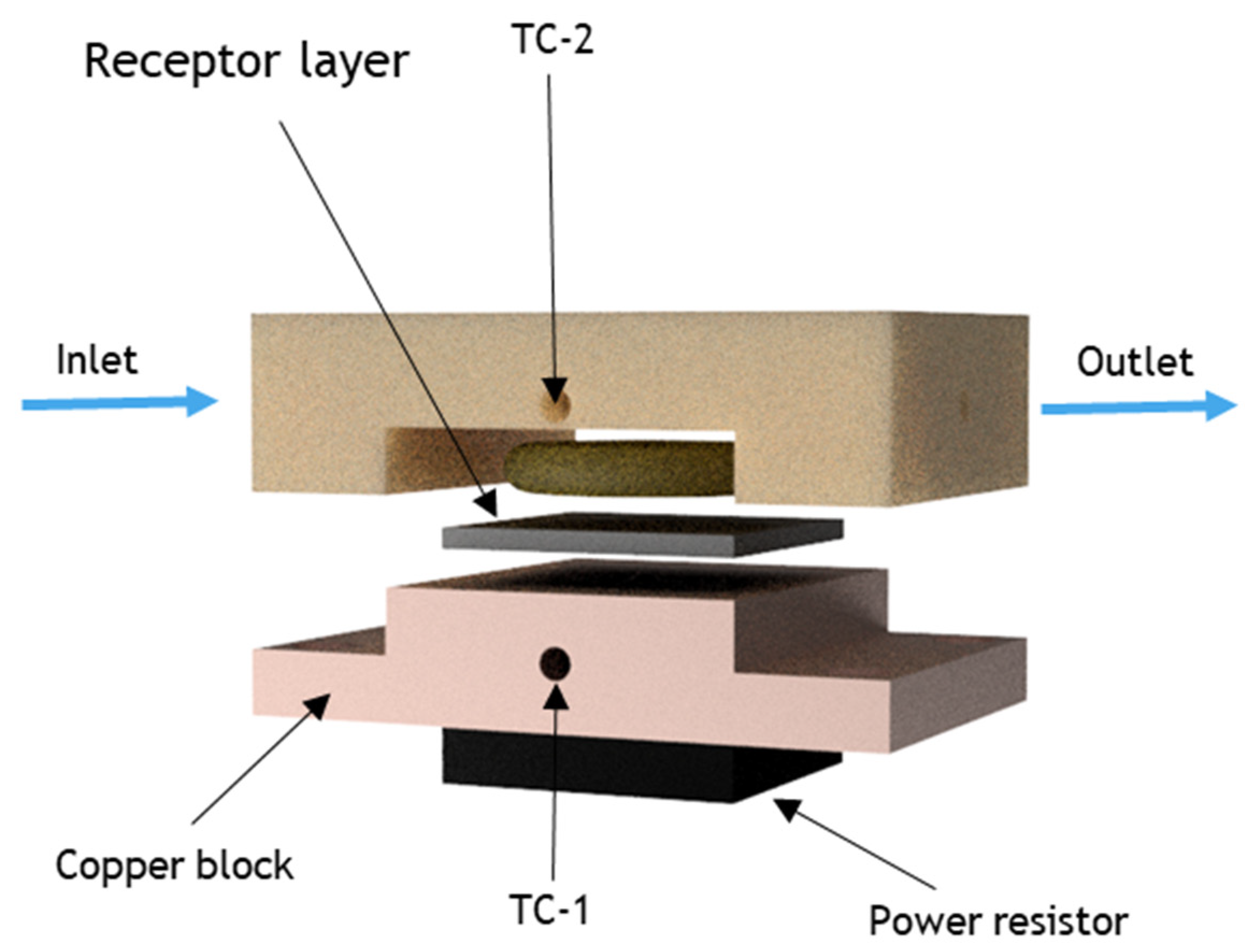
2.6. Heat-Transfer Sensing Setup
2.7. Thermal Detection of Melamine in Milk Samples
3. Results
3.1. Batch Rebinding via UV–VIS
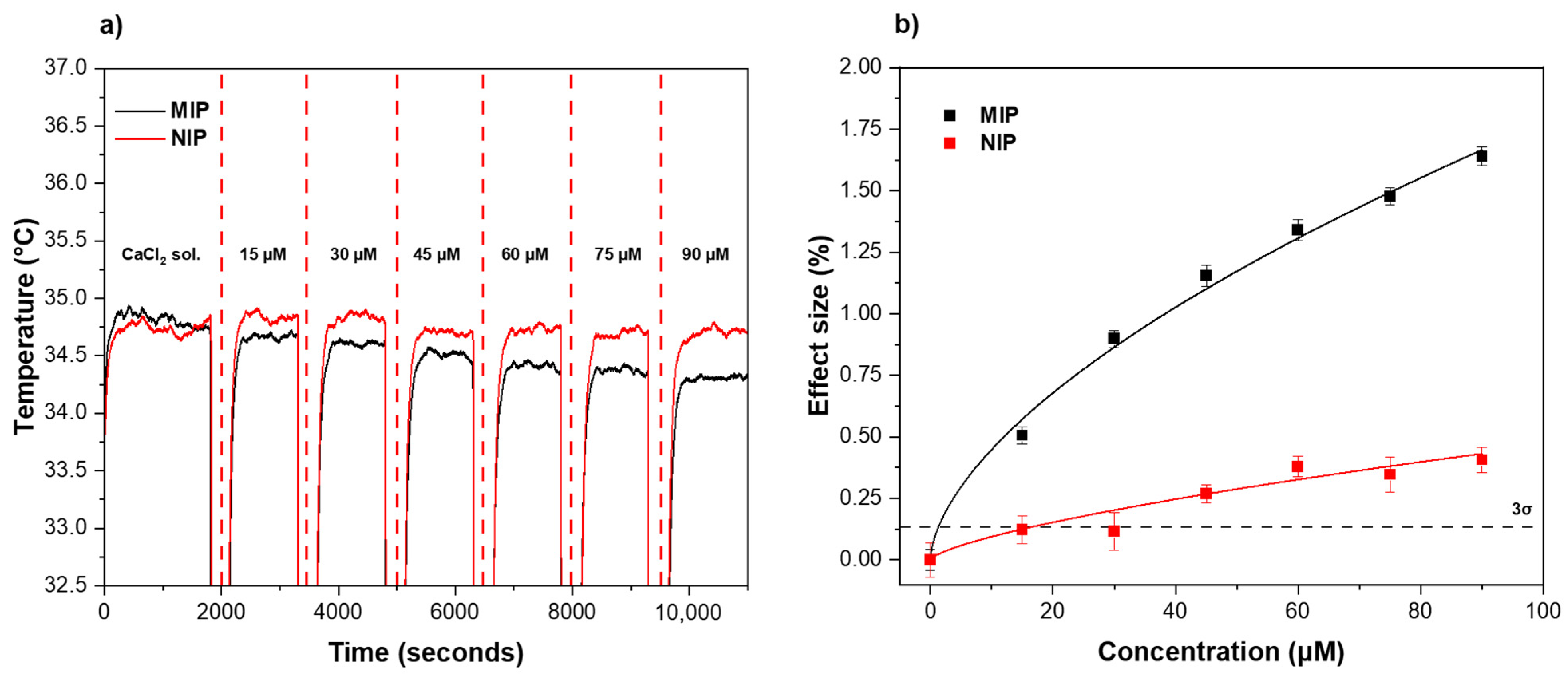
3.2. Rebinding Analysis Using HTM
3.3. Selectivity Analysis of the Receptor Layer
3.4. Real-Life Sample Analysis: Detection of Melamine in Milk Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, G.; Boor, B.E.; Schreder, E.; Salamova, A. Exposure to Melamine and Its Derivatives in Childcare Facilities. Chemosphere 2020, 244, 125505. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, J.R. Melamine and the Global Implications of Food Contamination. N. Engl. J. Med. 2008, 359, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Christa, J.; Deepa, J.R. Extraction of Melamine from Milk Using a Magnetic Molecularly Imprinted Polymer. Food Chem. 2017, 227, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Yuan, J.; Tao, G.; Wu, D.; Yang, X.; Yang, L.; Huang, H.; Zhou, L.; Xu, X.; et al. Proteome of Melamine Urinary Bladder Stones and Implication for Stone Formation. Toxicol. Lett. 2012, 212, 307–314. [Google Scholar] [CrossRef]
- Tyan, Y.-C.; Yang, M.-H.; Jong, S.-B.; Wang, C.-K.; Shiea, J. Melamine Contamination. Anal. Bioanal. Chem. 2009, 395, 729–735. [Google Scholar] [CrossRef]
- Ding, N.; Yan, N.; Ren, C.; Chen, X. Colorimetric Determination of Melamine in Dairy Products by Fe3O4 Magnetic Nanoparticles-H2O2-ABTS Detection System. ACS Anal. Chem. 2010, 82, 5897–5899. [Google Scholar] [CrossRef]
- Brown, C.A.; Jeong, K.S.; Poppenga, R.H.; Puschner, B.; Miller, D.M.; Ellis, A.E.; Kang, K.; Sum, S.; Cistola, A.M.; Brown, S.A. Outbreaks of Renal Failure Associated with Melamine and Cyanuric Acid in Dogs and Cats in 2004 and 2007. J. Vet. Diagn. Investig. 2007, 19, 525–531. [Google Scholar] [CrossRef]
- WHO International Experts Limit Melamine Levels in Food. Available online: https://www.who.int/news/item/11-12-2010-international-experts-limit-melamine-levels-in-food (accessed on 1 June 2022).
- Li, Q.; Song, P.; Wen, J. Melamine and Food Safety: A 10-Year Review. Curr. Opin. Food Sci. 2019, 30, 79–84. [Google Scholar] [CrossRef]
- Filazi, A.; Sireli, U.T.; Ekici, H.; Can, H.Y.; Karagoz, A. Determination of Melamine in Milk and Dairy Products by High Performance Liquid Chromatography. J. Dairy Sci. 2012, 95, 602–608. [Google Scholar] [CrossRef]
- Rodriguez Mondal, A.M.; Desmarchelier, A.; Konings, E.; Acheson-Shalom, R.; Delatour, T. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method Extension to Quantify Simultaneously Melamine and Cyanuric Acid in Egg Powder and Soy Protein in Addition to Milk Products. J. Agric. Food Chem. 2010, 58, 11574–11579. [Google Scholar] [CrossRef]
- Deng, X.; Guo, D.; Zhao, S.; Han, L.; Sheng, Y.; Yi, X.; Zhou, Y.; Peng, T. A Novel Mixed-Mode Solid Phase Extraction for Simultaneous Determination of Melamine and Cyanuric Acid in Food by Hydrophilic Interaction Chromatography Coupled to Tandem Mass Chromatography. J. Chromatogr. B 2010, 878, 2839–2844. [Google Scholar] [CrossRef] [PubMed]
- Nilghaz, A.; Mousavi, S.M.; Amiri, A.; Tian, J.; Cao, R.; Wang, X. Surface-Enhanced Raman Spectroscopy Substrates for Food Safety and Quality Analysis. J. Agric. Food Chem. 2022, 70, 2022. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Luo, Y.; Shi, J.; Gao, L.; Duan, G. Bowl-like Pore Array Made of Hollow Au/Ag Alloy Nanoparticles for SERS Detection of Melamine in Solid Milk Powder. Sens. Actuators B Chem. 2019, 301, 127087. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Melamine Detection in Milk and Dairy Products: Traditional Analytical Methods and Recent Developments. Food Anal. Methods 2018, 11, 128–147. [Google Scholar] [CrossRef]
- Tang, J.; Ying, Y.; Pan, X.D.; Jiang, W.; Wu, P.G. Elements Analysis of Infant Milk Formula by ICP-OES: A Comparison of Pretreatment Methods. Accredit. Qual. Assur. 2014, 19, 99–103. [Google Scholar] [CrossRef]
- Saylan, Y.; Yilmaz, F.; Özgür, E.; Derazshamshir, A.; Yavuz, H.; Denizli, A. Molecular Imprinting of Macromolecules for Sensor Applications. Sensors 2017, 17, 898. [Google Scholar] [CrossRef]
- Haghdoust, S.; Arshad, U.; Mujahid, A.; Schranzhofer, L.; Lieberzeit, P.A. Development of a MIP-Based QCM Sensor for Selective Detection of Penicillins in Aqueous Media. Chemosensors 2021, 9, 362. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Samukaite-Bubniene, U.; Ratautaite, V.; Bechelany, M.; Ramanavicius, A. Electrochemical Molecularly Imprinted Polymer Based Sensors for Pharmaceutical and Biomedical Applications (Review). J. Pharm. Biomed. Anal. 2022, 215, 114739. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Özgür, E.; Denizli, A. Recent Advances in Quartz Crystal Microbalance Biosensors Based on the Molecular Imprinting Technique for Disease-Related Biomarkers. Chemosensors 2022, 10, 106. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L.; Kong, W. Molecularly Imprinted Polymer-Based Optical Sensors for Pesticides in Foods: Recent Advances and Future Trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Turner, N.W.; Laitenberger, P. Molecularly Imprinted Polymers in Clinical Diagnostics—Future Potential and Existing Problems. Med. Eng. Phys. 2006, 28, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Ramström, O.; Skudar, K.; Haines, J.; Patel, P.; Brü, O. Food Analyses Using Molecularly Imprinted Polymers. J. Agric. Food Chem. 2001, 49, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, J.; Li, C.; Ma, M.; Yu, W.; Shen, J.; Wang, Z. Molecularly Imprinted Polymer as an Antibody Substitution in Pseudo-Immunoassays for Chemical Contaminants in Food and Environmental Samples. J. Agric. Food Chem. 2018, 66, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Pessagno, F.; Manesiotis, P.; Borrull, F.; Fontanals, N.; Maria Marcé, R. Preparation and Evaluation of Molecularly Imprinted Polymers as Selective SPE Sorbents for the Determination of Cathinones in River Water. Microchem. J. 2022, 175, 107100. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Ishikura, H.; Kvernenes, M.K.; Caldara, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K.; Diliën, H. Identifying Potential Machine Learning Algorithms for the Simulation of Binding Affinities to Molecularly Imprinted Polymers. Computation 2021, 9, 103. [Google Scholar] [CrossRef]
- Pirzada, M.; Sehit, E.; Altintas, Z. Cancer Biomarker Detection in Human Serum Samples Using Nanoparticle Decorated Epitope-Mediated Hybrid MIP. Biosens. Bioelectron. 2020, 166, 112464. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Armutcu, C.; Denizli, A. Molecularly Imprinted Polymer Film Based Plasmonic Sensors for Detection of Ochratoxin A in Dried Fig. Polym. Bull. 2022, 79, 4049–4067. [Google Scholar] [CrossRef]
- Rico-Yuste, A.; Abouhany, R.; Urraca, J.L.; Descalzo, A.B.; Orellana, G.; Moreno-Bondi, M.C. Eu(III)-Templated Molecularly Imprinted Polymer Used as a Luminescent Sensor for the Determination of Tenuazonic Acid Mycotoxin in Food Samples. Sens. Actuators B Chem. 2021, 329, 129256. [Google Scholar] [CrossRef]
- van Grinsven, B.; vanden Bon, N.; Strauven, H.; Grieten, L.; Murib, M.; Jiménez Monroy, K.L.; Janssens, S.D.; Haenen, K.; Schöning, M.J.; Vermeeren, V.; et al. Heat-Transfer Resistance at Solid-Liquid Interfaces: A Tool for the Detection of Single-Nucleotide Polymorphisms in DNA. ACS Nano 2012, 6, 2712–2721. [Google Scholar] [CrossRef]
- Eersels, K.; Diliën, H.; Lowdon, J.W.; Redeker, E.S.; Rogosic, R.; Heidt, B.; Peeters, M.; Cornelis, P.; Lux, P.; Reutelingsperger, C.P.; et al. A Novel Biomimetic Tool for Assessing Vitamin K Status Based on Molecularly Imprinted Polymers. Nutrients 2018, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Arreguin-Campos, R.; Eersels, K.; Lowdon, J.W.; Rogosic, R.; Heidt, B.; Caldara, M.; Jiménez-Monroy, K.L.; Diliën, H.; Cleij, T.J.; van Grinsven, B. Biomimetic Sensing of Escherichia Coli at the Solid-Liquid Interface: From Surface-Imprinted Polymer Synthesis toward Real Sample Sensing in Food Safety. Microchem. J. 2021, 169, 106554. [Google Scholar] [CrossRef]
- McClements, J.; Bar, L.; Singla, P.; Canfarotta, F.; Thomson, A.; Czulak, J.; Johnson, R.E.; Crapnell, R.D.; Banks, C.E.; Payne, B.; et al. Molecularly Imprinted Polymer Nanoparticles Enable Rapid, Reliable, and Robust Point-of-Care Thermal Detection of SARS-CoV-2. ACS Sens. 2022, 7, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Arreguin-Campos, R.; Eersels, K.; Rogosic, R.; Cleij, T.J.; Diliën, H.; van Grinsven, B. Imprinted Polydimethylsiloxane-Graphene Oxide Composite Receptor for the Biomimetic Thermal Sensing of Escherichia coli. ACS Sens. 2022, 7, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Givanoudi, S.; Cornelis, P.; Rasschaert, G.; Wackers, G.; Iken, H.; Rolka, D.; Yongabi, D.; Robbens, J.; Schöning, M.J.; Heyndrickx, M.; et al. Selective Campylobacter Detection and Quantification in Poultry: A Sensor Tool for Detecting the Cause of a Common Zoonosis at Its Source. Sens. Actuators B Chem. 2021, 332, 129484. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, G.; Wei, F.; Yang, J.; Hu, Q. Determination of Melamine in Powdered Milk by Molecularly Imprinted Stir Bar Sorptive Extraction Coupled with HPLC. J. Colloid Interface Sci. 2015, 454, 8–13. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Eersels, K.; Arreguin-Campos, R.; Caldara, M.; Heidt, B.; Rogosic, R.; Jimenez-Monroy, K.L.; Cleij, T.J.; Diliën, H.; van Grinsven, B. A Molecularly Imprinted Polymer-Based Dye Displacement Assay for the Rapid Visual Detection of Amphetamine in Urine. Molecules 2020, 25, 5222. [Google Scholar] [CrossRef]
- Caldara, M.; Lowdon, J.W.; Rogosic, R.; Arreguin-Campos, R.; Jimenez-Monroy, K.L.; Heidt, B.; Tschulik, K.; Cleij, T.J.; Diliën, H.; Eersels, K.; et al. Thermal Detection of Glucose in Urine Using a Molecularly Imprinted Polymer as a Recognition Element. ACS Sens. 2021, 6, 4515–4525. [Google Scholar] [CrossRef]
- Rogosic, R.; Lowdon, J.W.; Heidt, B.; Diliën, H.; Eersels, K.; van Grinsven, B.; Cleij, T.J. Studying the Effect of Adhesive Layer Composition on MIP-Based Thermal Biosensing. Phys. Status Solidi (A) Appl. Mater. Sci. 2019, 216, 1800941. [Google Scholar] [CrossRef]
- An, J.; Li, L.; Ding, Y.; Hu, W.; Duan, D.; Lu, H.; Ye, D.; Zhu, X.; Chen, H. A Novel Molecularly Imprinted Electrochemical Sensor Based on Prussian Blue Analogue Generated by Iron Metal Organic Frameworks for Highly Sensitive Detection of Melamine. Electrochim. Acta 2019, 326, 134946. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Wang, Y.; Huang, C.; Wang, Z.; Liu, L. In Situ Functionalization of Silver Nanoparticles by Gallic Acid as a Colorimetric Sensor for Simple Sensitive Determination of Melamine in Milk. ACS Omega 2021, 6, 23630–23635. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, S.; Gao, F.; Li-Chan, E.C.Y.; Grant, E.; Lu, X. Detection of Melamine in Milk Using Molecularly Imprinted Polymers–Surface Enhanced Raman Spectroscopy. Food Chem. 2015, 176, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ceylan Cömert, Ş.; Özgür, E.; Uzun, L.; Odabaşı, M. The Creation of Selective Imprinted Cavities on Quartz Crystal Microbalance Electrode for the Detection of Melamine in Milk Sample. Food Chem. 2022, 372, 131254. [Google Scholar] [CrossRef]
- Lorenzo, R.A.; Carro, A.M.; Alvarez-Lorenzo, C.; Concheiro, A. To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs). Int. J. Mol. Sci. 2011, 12, 4327–4347. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, S.; Talpur, F.N.; Afridi, H.I.; Nizamani, S.M.; Khaskheli, A.A.; Naz, S. Quick Determination of Melamine in Infant Powder and Liquid Milk by Fourier Transform Infrared Spectroscopy. Analytical Methods 2014, 6, 5269–5273. [Google Scholar] [CrossRef]



| MIP/NIP | Melamine (mg) | MAA (eq.) | EGDMA (eq.) | AIBN (mg) | DMSO (mL) | R2 | Max Sb (μmol g−1) | IF (at Cf = 0.1 mM) |
|---|---|---|---|---|---|---|---|---|
| MIP1 | 31.5 | 14 | 28 | 40 | 5 | 0.9882 | 30.00 | 2.22 |
| NIP1 | - | 14 | 28 | 40 | 5 | 0.8977 | 19.66 | |
| MIP2 | 31.5 | 3 | 20 | 40 | 5 | 0.9805 | 19.12 | 0.96 |
| NIP2 | - | 3 | 20 | 40 | 5 | 0.9529 | 22.44 | |
| MIP3 | 31.5 | 6 | 20 | 40 | 5 | 0.9848 | 27.11 | 0.82 |
| NIP3 | - | 6 | 20 | 40 | 5 | 0.9870 | 25.55 | |
| MIP4 | 31.5 | 12 | 40 | 40 | 5 | 0.9460 | 22.53 | 0.56 |
| NIP4 | - | 12 | 40 | 40 | 5 | 0.9829 | 25.94 |
| Substance | Selectivity Factor |
|---|---|
| Cyanuric acid | 3.62 |
| Bisphenol A | 12.14 |
| Lactose | 5.76 |
| Readout Technology | Limit of Detection | Real Sample Analysis | Sample Pretreatment | Reference |
|---|---|---|---|---|
| Differential pulse voltammetry (DPV) | 8.21 × 10−12 M | Liquid milk | Pretreatment needed | [41] |
| Colorimetric assay (UV–VIS) | 0.099 μM | Raw milk | Pretreatment needed | [42] |
| Surface-enhanced Raman spectroscopy (SERS) | 0.012 mmol L−1 | Whole milk | Pretreatment needed | [43] |
| Quartz crystal microbalance (QCM) | 2.3 ng mL−1 | Liquid milk | Pretreatment needed | [44] |
| Surface-enhanced Raman spectroscopy (SERS) | 0.1 ppm | Milk powder | Pretreatment needed | [14] |
| Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS) | 0.02–0.05 mg/kg | Egg powder, soy protein | Pretreatment needed | [11] |
| Heat-Transfer Method (HTM) | 6.02 μM | Whole milk | No pretreatment needed | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldara, M.; Lowdon, J.W.; Royakkers, J.; Peeters, M.; Cleij, T.J.; Diliën, H.; Eersels, K.; van Grinsven, B. A Molecularly Imprinted Polymer-Based Thermal Sensor for the Selective Detection of Melamine in Milk Samples. Foods 2022, 11, 2906. https://doi.org/10.3390/foods11182906
Caldara M, Lowdon JW, Royakkers J, Peeters M, Cleij TJ, Diliën H, Eersels K, van Grinsven B. A Molecularly Imprinted Polymer-Based Thermal Sensor for the Selective Detection of Melamine in Milk Samples. Foods. 2022; 11(18):2906. https://doi.org/10.3390/foods11182906
Chicago/Turabian StyleCaldara, Manlio, Joseph W. Lowdon, Jeroen Royakkers, Marloes Peeters, Thomas J. Cleij, Hanne Diliën, Kasper Eersels, and Bart van Grinsven. 2022. "A Molecularly Imprinted Polymer-Based Thermal Sensor for the Selective Detection of Melamine in Milk Samples" Foods 11, no. 18: 2906. https://doi.org/10.3390/foods11182906
APA StyleCaldara, M., Lowdon, J. W., Royakkers, J., Peeters, M., Cleij, T. J., Diliën, H., Eersels, K., & van Grinsven, B. (2022). A Molecularly Imprinted Polymer-Based Thermal Sensor for the Selective Detection of Melamine in Milk Samples. Foods, 11(18), 2906. https://doi.org/10.3390/foods11182906






