Total Flavonoids from Chimonanthus nitens Oliv. Leaves Ameliorate HFD-Induced NAFLD by Regulating the Gut–Liver Axis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TFC
2.3. LC–MS and HPLC Analysis
2.4. Antioxidant Capacity of TFC In Vitro
2.5. Animals and Experiment Design
2.6. Serum and Hepatic Biochemical Analysis
2.7. Histological Analysis
2.8. Western Blotting
2.9. RT-qPCR Analysis
2.10. Gut Microbiota Analysis
2.11. Statistical Analysis
3. Results
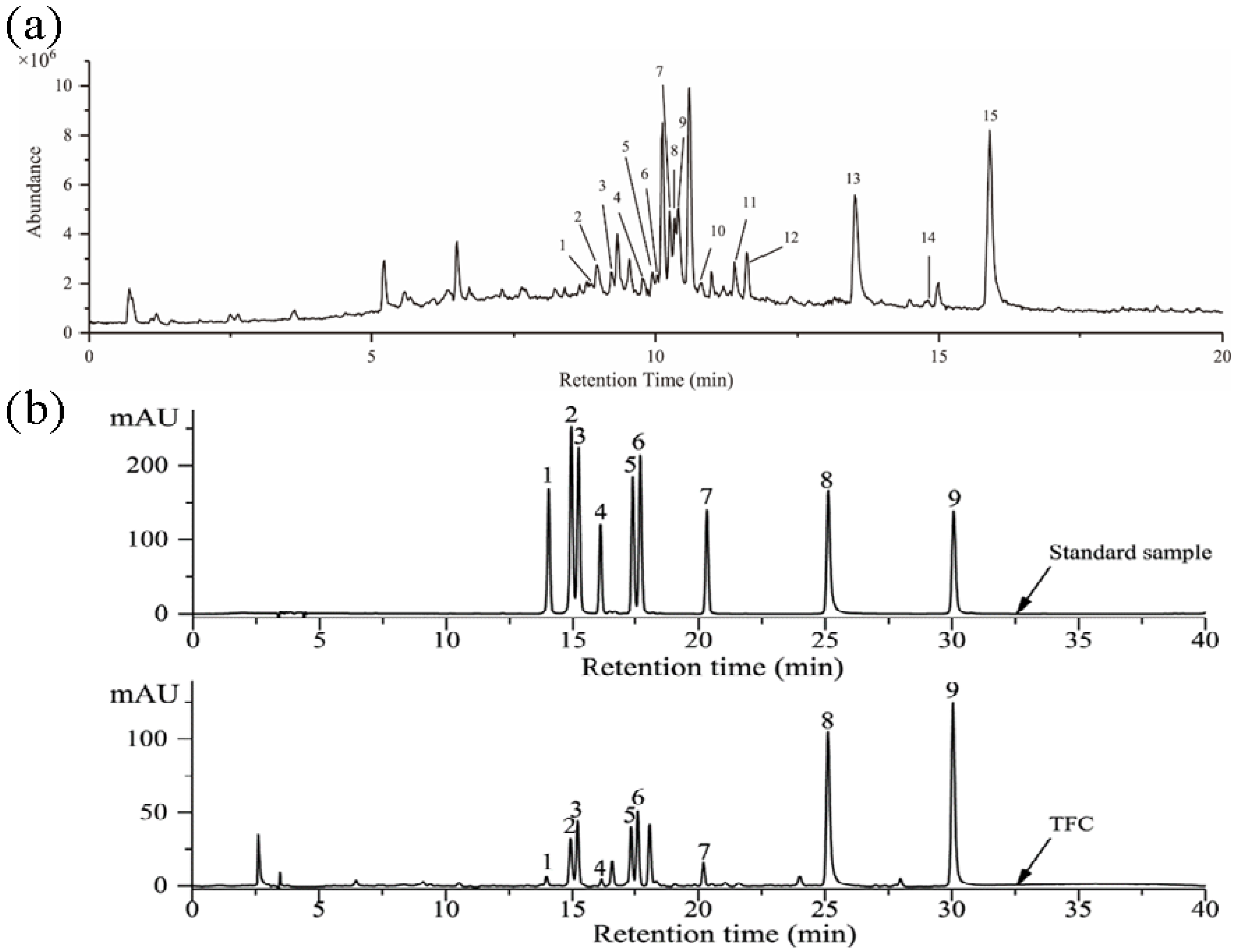
3.1. Identification of Components in TFC
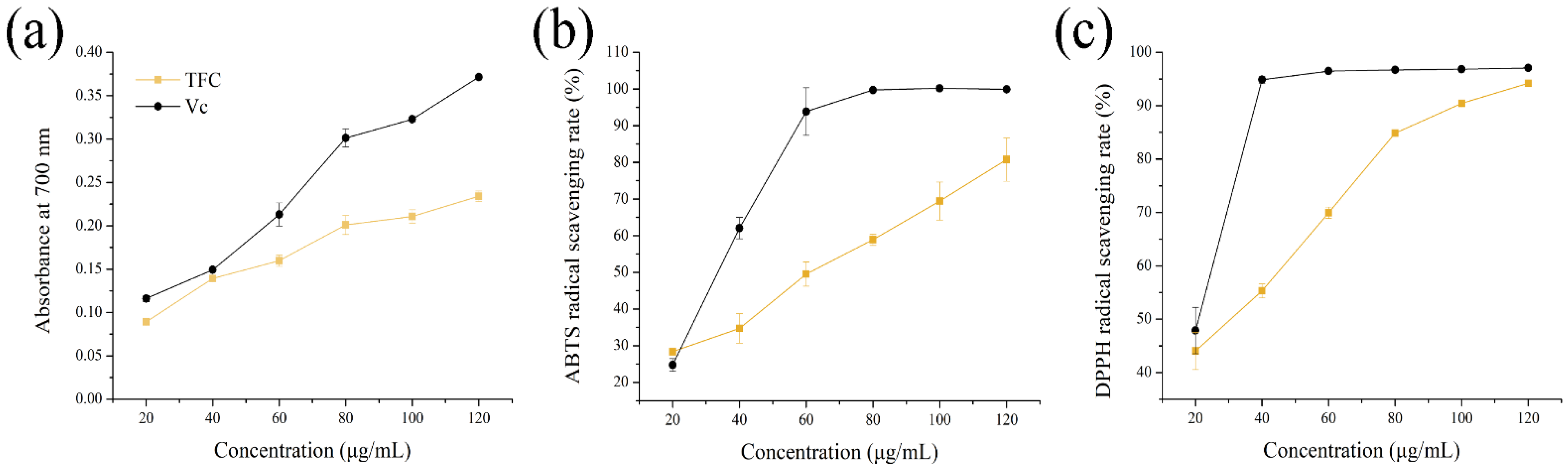
3.2. Antioxidant Activities of TFC
3.3. Effects of TFC on Physiological Indices in NAFLD Mice
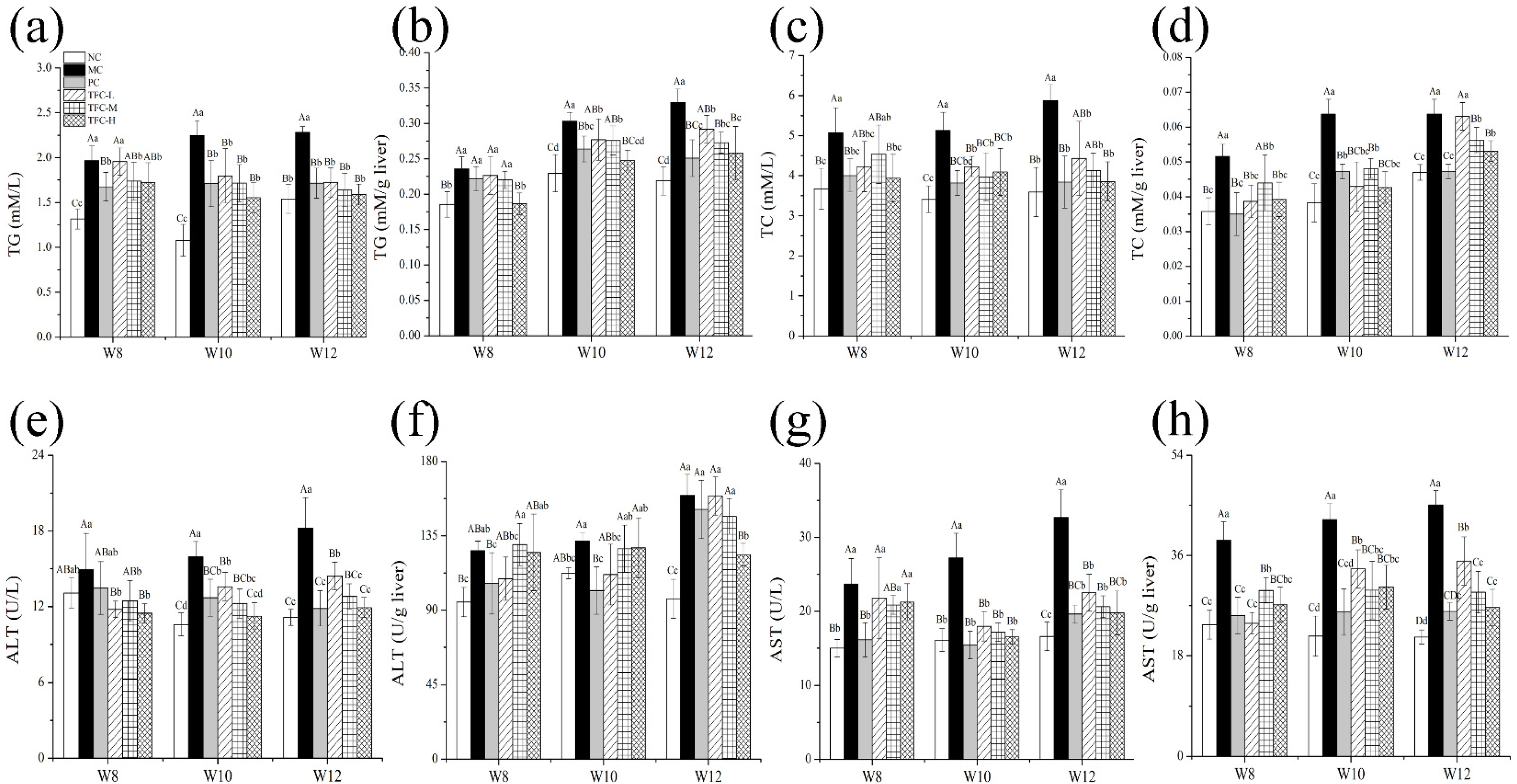
3.4. Effects of TFC on Lipid Metabolism and Liver Function in NAFLD Mice
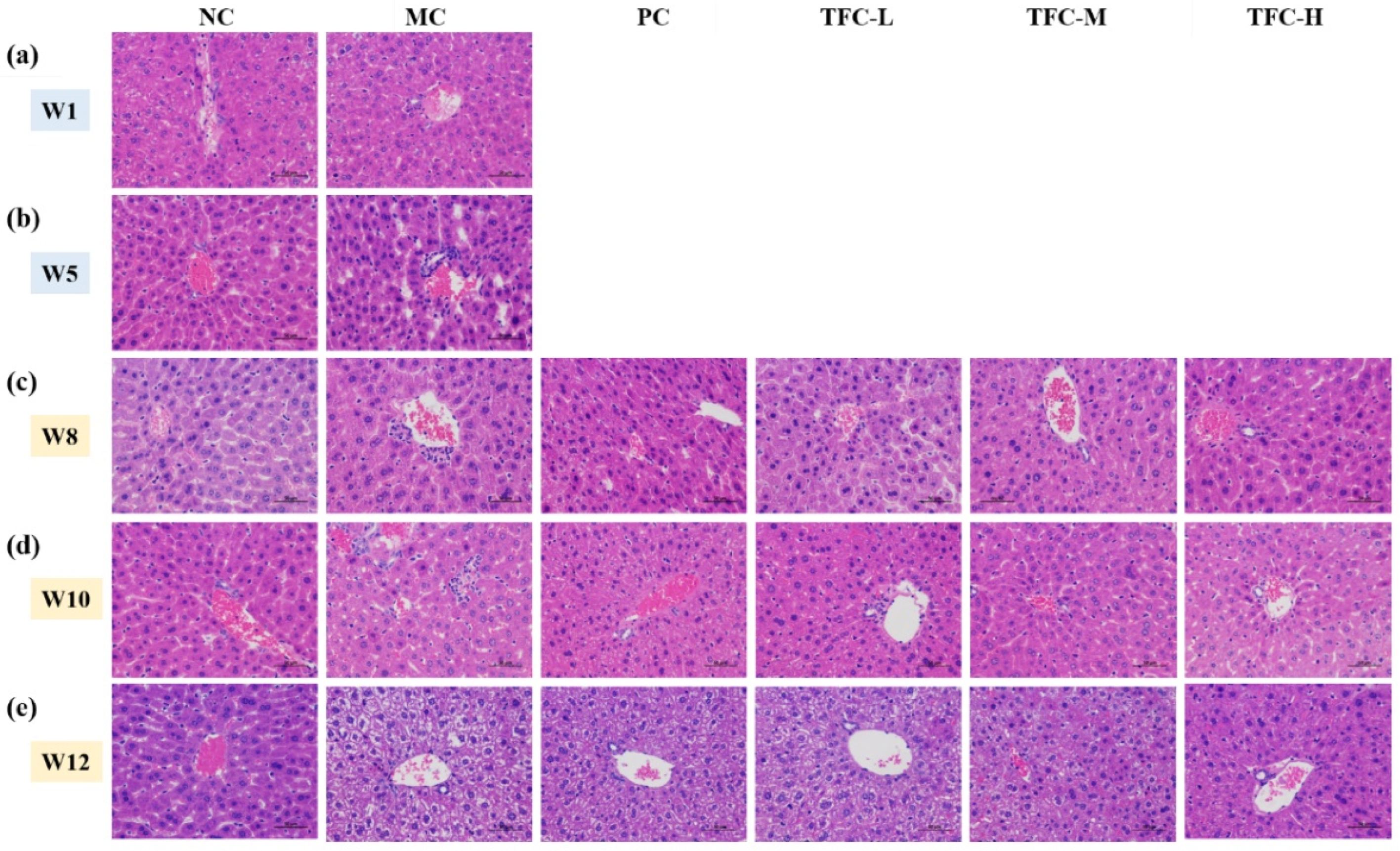
3.5. TFC Ameliorated Liver Pathological Injuries
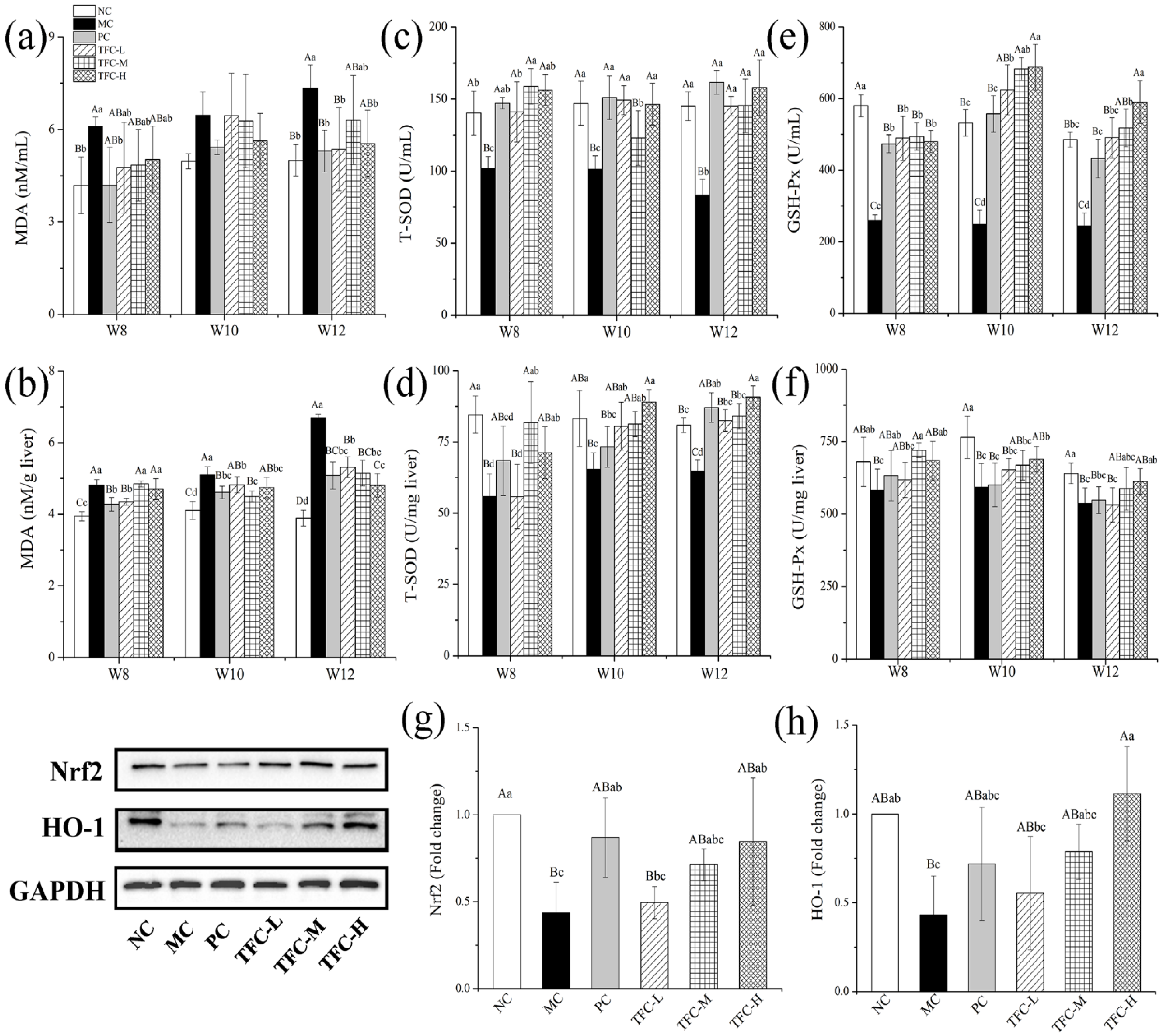
3.6. TFC Attenuated Oxidative Stress in NAFLD Mice
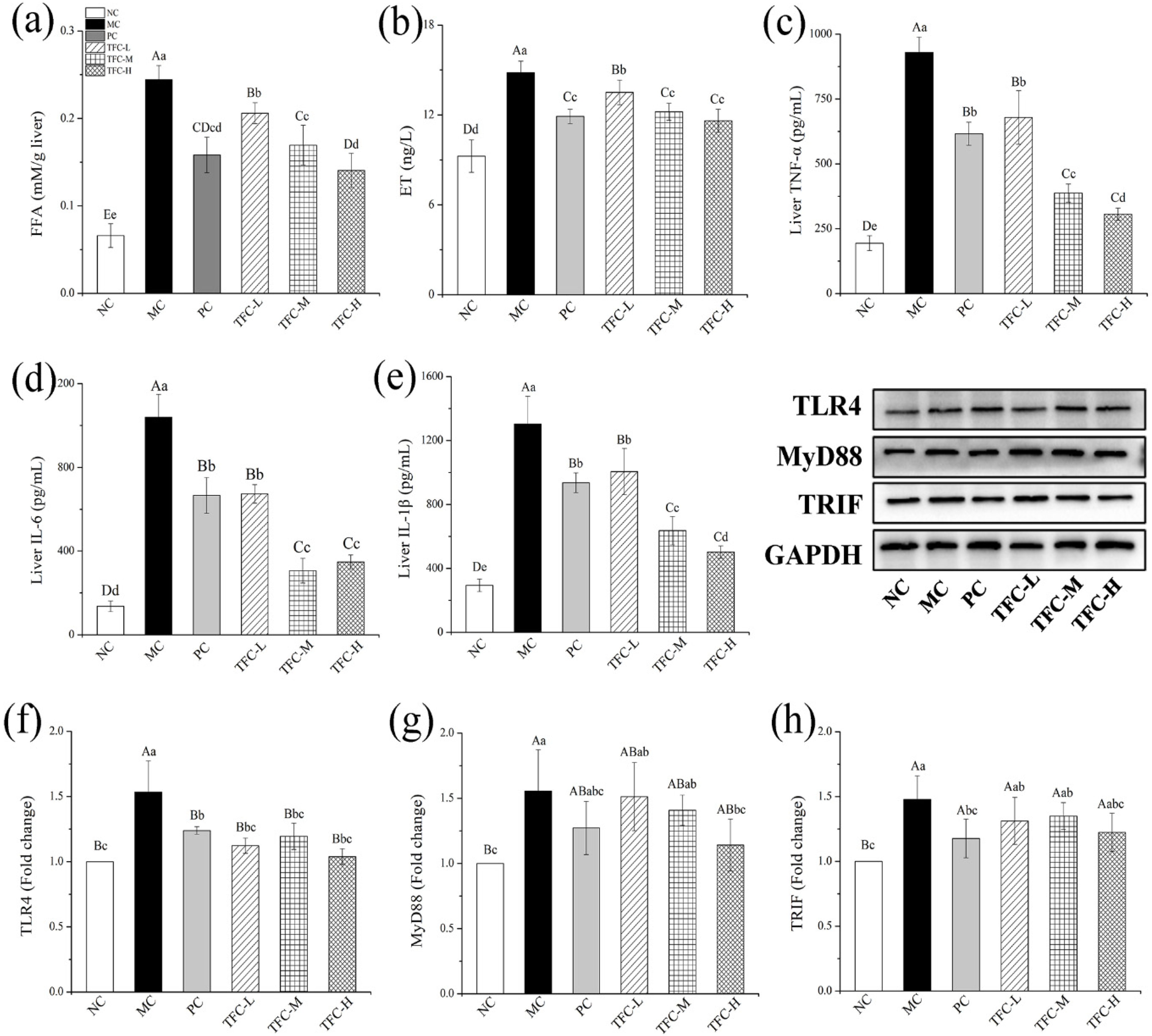
3.7. TFC Reduced FFA Level and Alleviated Liver Inflammation in NAFLD Mice
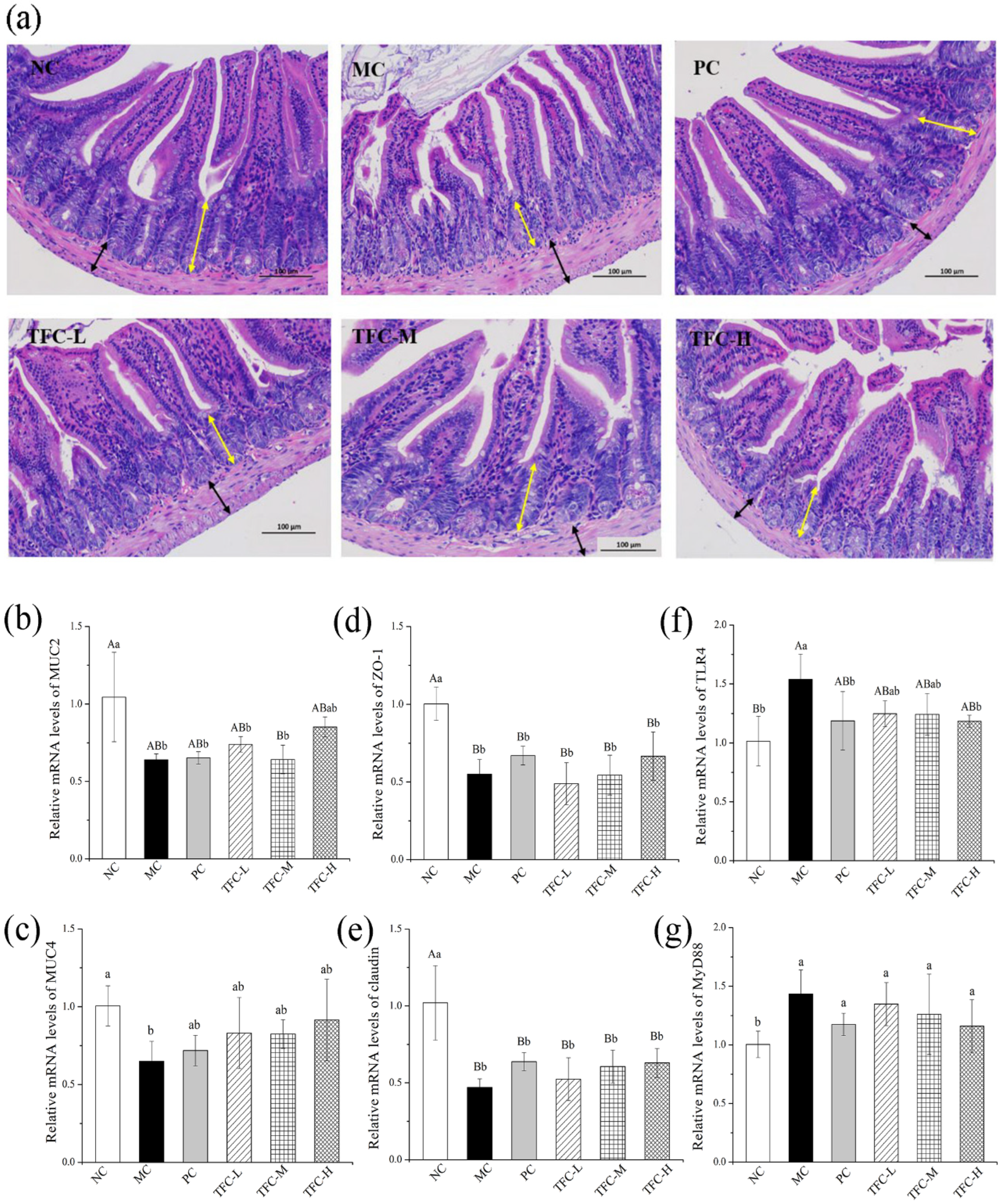
3.8. TFC Protected Intestinal Barrier Integrity in NAFLD Mice
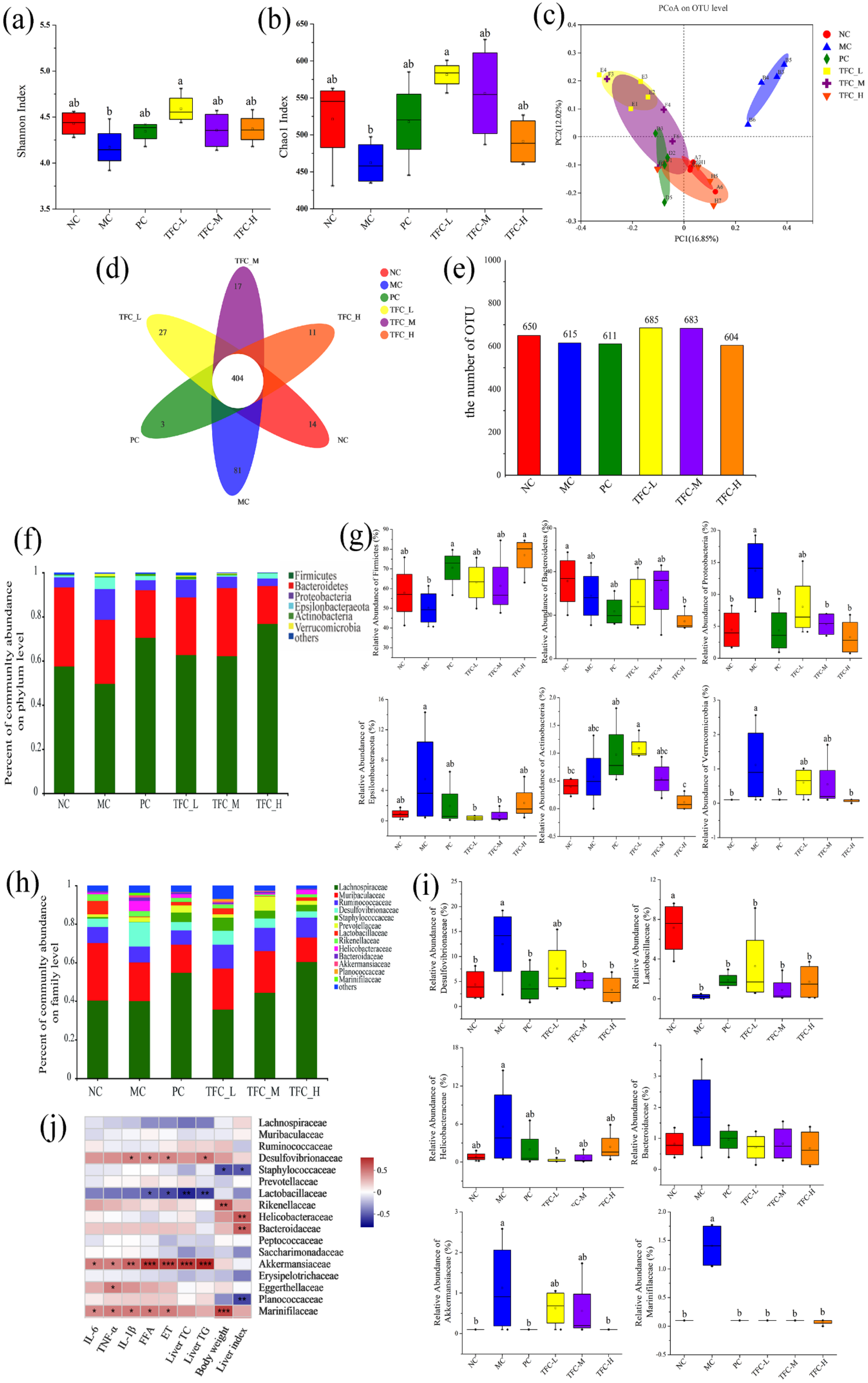
3.9. TFC Restored Intestinal Microbiome Imbalance in NAFLD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araújo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Afsharinasab, M.; Mohammad-Sadeghipour, M.; Hajizadeh, M.R.; Khoshdel, A.; Mirzaiey, V.; Mahmoodi, M. The effect of hydroalcoholic Berberis integerrima fruits extract on the lipid profile, antioxidant parameters and liver and kidney function tests in patients with nonalcoholic fatty liver disease. Saudi J. Biol. Sci. 2020, 27, 2031–2037. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, M.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180. [Google Scholar] [CrossRef]
- Arslan, N. Obesity, fatty liver disease and intestinal microbiota. World J. Gastroenterol. 2014, 20, 16452. [Google Scholar] [CrossRef]
- Randy, A.; Kim, M.; Nho, C.W. Ligularia fischeri and its constituent 3,4-dicaffeoylquinic acid improve obesity-induced nonalcoholic fatty liver disease by regulating lipid metabolism and activating AMPK. J. Funct. Foods 2016, 27, 1–16. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Nobili, V.; Svegliati-Baroni, G.; Alisi, A.; Miele, L.; Valenti, L.; Vajro, P. A 360-degree overview of paediatric NAFLD: Recent insights. J. Hepatol. 2013, 58, 1218–1229. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Deretzi, G. Nonalcoholic fatty liver disease: Multimodal treatment options for a pathogenetically multiple-hit disease. J. Clin. Gastroenterol. 2012, 46, 272–284. [Google Scholar] [CrossRef]
- Ding, J.H.; Jin, Z.; Yang, X.X.; Lou, J.; Shan, W.X.; Hu, Y.X.; Du, Q.; Liao, Q.S.; Xie, R.; Xu, J.Y. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J. Gastroenterol. 2020, 26, 6141–6162. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C. The gut as a potential trigger of exercise-induced inflammatory responses. Can. J. Pharm. 1998, 76, 479–484. [Google Scholar] [CrossRef]
- Chiu, C.C.; Ching, Y.H.; Li, Y.P.; Liu, J.Y.; Huang, Y.T.; Huang, Y.W.; Yang, S.S.; Huang, W.C.; Chuang, H.L. Nonalcoholic fatty liver disease is exacerbated in high-fat diet-fed gnotobiotic mice by colonization with the gut microbiota from patients with nonalcoholic steatohepatitis. Nutrients 2017, 9, 1220. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, J.; Sun, P.; Yi, R.; Han, X.; Zhao, X. Raw bowl tea (Tuocha) polyphenol prevention of nonalcoholic fatty liver disease by regulating intestinal function in mice. Biomolecules 2019, 9, 435. [Google Scholar] [CrossRef] [Green Version]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Federico, A.; Zulli, C.; de Sio, I.; Del, P.A.; Dallio, M.; Masarone, M.; Loguercio, C. Focus on emerging drugs for the treatment of patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 16841. [Google Scholar] [CrossRef]
- Ozturk, Z.A.; Kadayifci, A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 199. [Google Scholar] [CrossRef]
- Zhai, B.; Zhang, C.; Sheng, Y.; Zhao, C.; He, X.; Xu, W.; Huang, K.; Luo, Y. Hypoglycemic and hypolipidemic effect of S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci. Rep. 2018, 8, 3527. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Xu, W.; Huang, K.; Zhang, B.; Wang, H.; Zhang, X.; Gong, L.; Luo, Y.; He, X. Precision toxicology shows that troxerutin alleviates ochratoxin A–induced renal lipotoxicity. FASEB J. 2018, 33, 2212–2227. [Google Scholar] [CrossRef] [Green Version]
- Lemus-Conejo, A.; Grao-Cruces, E.; Toscano, R.; Varela, L.M.; Claro, C.; Pedroche, J.; Millan, F.; Millan-Linares, M.C.; Montserrat-de la Paz, S. A lupine (Lupinus angustifolious L.) peptide prevents non-alcoholic fatty liver disease in high-fat-diet-induced obese mice. Food Funct. 2020, 11, 2943–2952. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Xu, J.; Hu, Y.; Huang, K.; Luo, Y.; He, X. Broccoli ameliorate NAFLD by increasing lipolysis and promoting liver macrophages polarize toward M2-type. J. Funct. Foods 2022, 89, 104898. [Google Scholar] [CrossRef]
- Shu, R.; Wan, Y.; Wang, X. Non-volatile constituents and pharmacology of Chimonanthus: A review. Chin. J. Nat. Med. 2019, 17, 161–186. [Google Scholar] [CrossRef]
- Chen, H.; Ouyang, K.; Jiang, Y.; Yang, Z.; Hu, W.; Xiong, L.; Wang, N.; Liu, X.; Wang, W. Constituent analysis of the ethanol extracts of Chimonanthus nitens Oliv. leaves and their inhibitory effect on α-glucosidase activity. Int. J. Biol. Macromol. 2017, 98, 829–836. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; He, J.; Ouyang, K.; Zhao, M.; Cai, L.; Zhao, Z.; Meng, W.; Chen, L.; Wang, W. Digestive properties and effects of Chimonanthus nitens Oliv polysaccharides on antioxidant effects in vitro and in immunocompromised mice. Int. J. Biol. Macromol. 2021, 185, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, H.; Xiong, L.; Liu, X.; Li, X.; An, Q.; Ye, X.; Wang, W. Phytochemical profile of ethanolic extracts of Chimonanthus salicifolius S. Y. Hu. leaves and its antimicrobial and antibiotic-mediating activity. Ind. Crop. Prod. 2018, 125, 328–334. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, J.; Li, J.; Xiong, L.; Chen, H.; Liu, X.; Wang, N.; Ouyang, K.; Wang, W. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohyd. Polym. 2018, 18, 11–20. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Li, J.; Lin, L.; Zheng, G. A flavonoid-rich Smilax china L. extract prevents obesity by upregulating the adiponectin-receptor/AMPK signalling pathway and modulating the gut microbiota in mice. Food Funct. 2021, 12, 5862–5875. [Google Scholar] [CrossRef]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Zhonghua, L. Fuzhuan brick tea attenuates high-fat diet-induced obesity and associated metabolic disorders by shaping gut microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef]
- Yilmaz, M. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Ind. Crop. Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Tian, C.; Wang, H.; Guo, Y.; Qiu, P.; Cui, C.; Liu, M. Abutilon theophrasti medic. Episperms as a total flavonoids fraction for pharmaceutical applications: In vitro antioxidant, antibacterial, anti-inflammatory activities, extraction technology and HPLC-MS profiles. Ind. Crop. Prod. 2019, 134, 100–106. [Google Scholar] [CrossRef]
- Ersoy, E.; Eroglu, O.; Boga, M.; Yilmaz, M.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC–MS/MS. Ind. Crop. Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Delourdesmatabilbao, M.; Andreslacueva, C.; Jauregui, O.; Lamuela Raventos, R. Determination of flavonoids in a Citrus fruit extract by LC–DAD and LC–MS. Food Chem. 2007, 101, 1742–1747. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, W.; Jiao, L.; Yang, P.; Guo, M. Analysis of flavonoids in Lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [Green Version]
- Mari, A.; Lyon, D.; Fragner, L.; Montoro, P.; Piacente, S.; Wienkoop, S.; Egelhofer, V.; Weckwerth, W. Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC-MS and LC-MS metabolomics platform. Metabolomics 2013, 9, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Yao, T.; Jin, X.; Mao, X.; Sun, R.; Peng, Y. Simultaneous determination of seven flavonoids in cherry leaves by HPLC-MS/MS. J. Shen. Pharm. Univ. 2017, 34, 221–228. (In Chinese) [Google Scholar] [CrossRef]
- Neugart, S.; Rohn, S.; Schreiner, M. Identification of complex, naturally occurring flavonoid glycosides in Vicia faba and Pisum sativum leaves by HPLC-DAD-ESI-MSn and the genotypic effect on their flavonoid profile. Food Res. Int. 2015, 76, 114–121. [Google Scholar] [CrossRef]
- Abu-Reidah, I.; Contreras, M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolic profiling of vegetables: Lactuca sativa as an example of its application. J. Chromatogr. A 2013, 1313, 212–227. [Google Scholar] [CrossRef]
- Feng, X.; Yu, W.; Li, X.; Zhou, F.; Zhang, W.; Shen, Q.; Li, J.; Zhang, C.; Shen, P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017, 136, 136–149. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, M.; Gao, X.; Hu, P.; Li, C.; Yang, X. Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz. J. Med. Biol. Res. 2013, 46, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, G.; Wu, S.; Song, M.; Wang, J.; Cai, W.; Mi, S.; Liu, C. Schaftoside alleviates HFD-induced hepatic lipid accumulation in mice via upregulating farnesoid X receptor. J. Ethnopharmacol. 2020, 255, 112776. [Google Scholar] [CrossRef] [PubMed]
- Amirinejad, A.; Totmaj, A.S.; Mardali, F.; Hekmatdoost, A.; Emamat, H.; Safa, M.; Shidfar, F. Administration of hydro-alcoholic extract of spinach improves oxidative stress and inflammation in high-fat diet-induced NAFLD rats. BMC Complement. Med. Ther. 2021, 21, 221. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Shu, F.; Gong, J.; Ding, P.; Cheng, R.; Li, J.; Tong, R.; Ding, L.; Sun, H.; Huang, Z.; et al. Sweroside ameliorates NAFLD in high-fat diet induced obese mice through the regulation of lipid metabolism and inflammatory response. J. Ethnopharmacol. 2020, 255, 112556. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Yu, H.; Li, J.; Wang, S.; Zhang, Y.; Qiu, F.; Wang, T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J. Nat. Med. 2018, 72, 655–666. [Google Scholar] [CrossRef]
- Zhu, X.; Xiong, T.; Liu, P.; Guo, X.; Xiao, L.; Zhou, F.; Yang, Y.; Yao, P. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem. Toxicol. 2018, 114, 52–60. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. BBA-Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Drygalski, K.; Konstantynowicz-Nowicka, K.; Berk, K.; Chabowski, A. Alternative treatment methods attenuate the development of NAFLD: A review of resveratrol molecular mechanisms and clinical trials. Nutrition 2017, 34, 108–117. [Google Scholar] [CrossRef]
- Sotiropoulou, M.; Katsaros, I.; Vailas, M.; Lidoriki, I.; Papatheodoridis, G.; Kostomitsopoulos, N.; Valsami, G.; Tsaroucha, A.; Schizas, D. Nonalcoholic fatty liver disease: The role of quercetin and its therapeutic implications. Saudi J. Gastroenterol. 2021, 27, 319. [Google Scholar] [CrossRef]
- Ying, H.; Liu, Y.; Yu, B.; Wang, Z.; Zang, J.; Yu, C. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 2013, 52, 53–60. [Google Scholar] [CrossRef]
- Sun, B.; Jia, Y.; Hong, J.; Sun, Q.; Gao, S.; Hu, Y.; Zhao, N.; Zhao, R. Sodium butyrate ameliorates high-fat-diet-induced non-alcoholic fatty liver disease through peroxisome proliferator-activated receptor α-mediated activation of β oxidation and suppression of inflammation. J. Agric. Food Chem. 2018, 66, 7633–7642. [Google Scholar] [CrossRef]
- Pu, X.; Fan, W.; Yu, S.; Li, Y.; Ma, X.; Liu, L.; Ren, J.; Zhang, W. Polysaccharides from Angelica and Astragalus exert hepatoprotective effects against carbon-tetrachloride-induced intoxication in mice. Can. J. Physiol. Pharm. 2015, 93, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, H.X.; Guo, J.Y.; Huang, Y.Q.; Wang, H.; Talukder, M.; Li, J.L. Di (2-ethyl hexyl) phthalate (DEHP)-induced spleen toxicity in quail (Coturnix japonica) via disturbing Nrf2-mediated defense response. Environ. Pollut. 2019, 251, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wei, Y.; Xu, W.; Lu, F.; Ma, H. Corn peptides improved obesity-induced non-alcoholic fatty liver disease through relieving lipid metabolism, insulin resistance and oxidative stress. Food Funct. 2022, 13, 5782–5793. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Wang, M.; Zhang, X.; Zhang, S.; Gao, Y.; Chen, L.; Wu, M.; Zhou, L.; Zhou, Y.; et al. Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 5105–5119. [Google Scholar] [CrossRef]
- Li, X.; Chu, L.; Liu, S.; Zhang, W.; Lin, L.; Zheng, G. Smilax China L. flavonoid alleviates HFHS-induced inflammation by regulating the gut-liver axis in mice. Phytomedicine 2022, 95, 153728. [Google Scholar] [CrossRef]
- Liu, P.; Wu, P.; Yang, B.; Wang, T.; Li, J.; Song, X.; Sun, W. Kaempferol prevents the progression from simple steatosis to non-alcoholic steatohepatitis by inhibiting the NF-κB pathway in oleic acid-induced HepG2 cells and high-fat diet-induced rats. J. Funct. Food. 2021, 85, 104655. [Google Scholar] [CrossRef]
- Boursier, J.; Mueller, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Matute, P.; Pérez-Martínez, L.; Aguilera-Lizarraga, J.; Blanco, J.R.; Oteo, J.A. Maraviroc modifies gut microbiota composition in a mouse model of obesity: A plausible therapeutic option to prevent metabolic disorders in HIV-infected patients. Rev. Esp. Quim. 2015, 28, 200–206. [Google Scholar]
- Xiao, S.; Fei, N.; Pang, X.; Shen, J.; Wang, L.; Zhang, B.; Zhang, M.; Zhang, X.; Zhang, C.; Li, M.; et al. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol. Ecol. 2014, 87, 357–367. [Google Scholar] [CrossRef]
- Xia, M.; Chen, H.; Liu, S. The synergy of resveratrol and alcohol against Helicobacter pylori and underlying anti-Helicobacter pylori mechanism of resveratrol. J. Appl. Microbiol. 2020, 128, 1179–1190. [Google Scholar] [CrossRef]
- Sumida, Y.; Kanemasa, K.; Imai, S.; Mori, K.; Tanaka, S.; Shimokobe, H.; Kitamura, Y.; Fukumoto, K.; Kakutani, A.; Ohno, T.; et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Shin, M.J.; Youn, G.S.; Yoon, S.J.; Choi, Y.R.; Kim, H.S.; Gupta, H.; Han, S.H.; Kim, B.K.; Lee, D.Y.; et al. Lactobacillus attenuates progression of nonalcoholic fatty liver disease by lowering cholesterol and steatosis. Clin. Mol. Hepatol. 2021, 27, 110–124. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Forward | Reverse |
|---|---|---|
| ZO-1 | 5′-CCAGAGCCTCAGAAACCTCA-3′ | 5′-GCAGGAAGATGTGCAGAAGG-3′ |
| Claudin | 5′-ACGGTCTTTGCACTTTGGTC-3′ | 5′-GGGAGAGGAGAAGCACAGTT-3′ |
| MUC-2 | 5′-TGTGGTCTGTGTGGGAACTT-3′ | 5′-GCTTACATCTGGGCAAGTGG-3′ |
| MUC-4 | 5′-AACTCCTTCAGCCTCCTCAC-3′ | 5′-GCTGTTGTGTGTCCTGAGTC-3′ |
| TLR4 | 5′-TAGCCATTGCTGCCAACATC-3′ | 5′-CCTCAGCAGGGACTTCTCAA-3′ |
| MyD88 | 5′-GCATGGTGGTGGTTGTTTCT-3′ | 5′-AACCGCAGGATACTGGGAAA-3′ |
| β-actin | 5′-CCAGCCTTCCTTCTTGGGTA-3′ | 5′-CAATGCCTGGGTACATGGTG-3′ |
| No. | RT (min) | Molecular ion (m/z) | Fragment ions (m/z) | Formula | Proposed Compounds |
|---|---|---|---|---|---|
| 1 | 8.888 | 609 | 300 | C27H30O16 | Rutin |
| 2 | 8.986 | 463 | 301; 151 | C21H20O12 | Hyperin |
| 3 | 9.218 | 463 | 301/300; 271 | C21H20O12 | Isoquercitrin |
| 4 | 9.788 | 593 | 285 | C27H30O15 | Kaempferol-3-O-rutinoside |
| 5 | 9.935 | 447 | 285/284; 151 | C21H20O11 | Luteolin-5-O-glucoside |
| 6 | 10.049 | 433 | 301/300 | C20H18O11 | Quercetin-3-O-xyloside or Quercetin pentoside |
| 7 | 10.239 | 447 | 285/284 | C21H20O11 | Kaempferol-3-O-galactoside |
| 8 | 10.350 | 447 | 285/284 | C21H20O11 | Astragalin |
| 9 | 10.414 | 447 | 301/300; 151 | C21H20O11 | Quercetin-3-O-α-L-rhamnoside |
| 10 | 10.812 | 417 | 285 | C20H18O10 | Kaempferol-3-O-arabinoside |
| 11 | 11.407 | 489 | 285/284 | C23H22O12 | Kaempferol-3-O-acetyl-galactoside |
| 12 | 11.618 | 431 | 285 | C21H20O10 | Afzelin |
| 13 | 13.499 | 301 | 151 | C15H10O7 | Quercetin |
| 14 | 14.801 | 271 | 151 | C15H12O5 | Naringenin |
| 15 | 15.917 | 285 | 227 | C15H10O6 | Kaempferol |
| Peak | Compounds | RT (min) | Calibration Curve | R2 | TFC (μg/mg) | LOD | LOQ |
|---|---|---|---|---|---|---|---|
| 1 | Rutin | 13.970 | Y = 10.714x − 2.5708 | 0.9986 | 2.612 ± 0.275 | 0.070 | 0.235 |
| 2 | Hyperin | 14.919 | Y = 26.761x − 1.4147 | 0.9986 | 5.308 ± 0.093 | 0.032 | 0.105 |
| 3 | Isoquercitrin | 15.194 | Y = 13.457x − 9.2557 | 0.9985 | 13.301 ± 0.072 | 0.063 | 0.209 |
| 4 | Kaempferol-3-O-rutinoside | 16.192 | Y = 9.640x + 7.5889 | 0.9999 | 1.321 ± 0.097 | 0.094 | 0.314 |
| 5 | Luteolin-5-O-glucoside | 17.360 | Y = 11.221x + 2.8667 | 0.9982 | 13.479 ± 0.041 | 0.080 | 0.265 |
| 6 | Astragalin | 17.621 | Y = 11.88x + 55.128 | 0.9936 | 13.959 ± 0.025 | 0.084 | 0.279 |
| 7 | Afzelin | 20.211 | Y = 24.013x − 352.22 | 0.9909 | 9.781 ± 0.033 | 0.044 | 0.145 |
| 8 | Quercetin | 25.117 | Y = 13.745x − 21.717 | 0.9999 | 45.489 ± 1.206 | 0.080 | 0.267 |
| 9 | Kaempferol | 30.047 | Y = 13.554x – 3.8523 | 0.9993 | 51.293 ± 0.715 | 0.088 | 0.294 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, W.; Zhao, Z.; Chen, L.; Lin, S.; Zhang, Y.; He, J.; Ouyang, K.; Wang, W. Total Flavonoids from Chimonanthus nitens Oliv. Leaves Ameliorate HFD-Induced NAFLD by Regulating the Gut–Liver Axis in Mice. Foods 2022, 11, 2169. https://doi.org/10.3390/foods11142169
Meng W, Zhao Z, Chen L, Lin S, Zhang Y, He J, Ouyang K, Wang W. Total Flavonoids from Chimonanthus nitens Oliv. Leaves Ameliorate HFD-Induced NAFLD by Regulating the Gut–Liver Axis in Mice. Foods. 2022; 11(14):2169. https://doi.org/10.3390/foods11142169
Chicago/Turabian StyleMeng, Wenya, Zitong Zhao, Lingli Chen, Suyun Lin, Yang Zhang, Jing He, Kehui Ouyang, and Wenjun Wang. 2022. "Total Flavonoids from Chimonanthus nitens Oliv. Leaves Ameliorate HFD-Induced NAFLD by Regulating the Gut–Liver Axis in Mice" Foods 11, no. 14: 2169. https://doi.org/10.3390/foods11142169
APA StyleMeng, W., Zhao, Z., Chen, L., Lin, S., Zhang, Y., He, J., Ouyang, K., & Wang, W. (2022). Total Flavonoids from Chimonanthus nitens Oliv. Leaves Ameliorate HFD-Induced NAFLD by Regulating the Gut–Liver Axis in Mice. Foods, 11(14), 2169. https://doi.org/10.3390/foods11142169







