Abstract
Perilla (Perilla frutescens) oil reduces high-fat-diet-induced colon inflammation by suppressing the NF-κB pathway. In the current study, we compared the effect of endogenously produced and externally supplemented omega-3 fatty acids on high-fat-diet-induced colon inflammation. The fat-1 transgenic mice that endogenously synthesize omega-3 fatty acids were backcrossed with C57BL/6J wild-type mice to obtain transgenic (TR) and wild-type (WT) littermates. Five-week-old male littermates were divided into five groups: two groups fed 10% normal diet (WTLD, TRLD) and three groups fed with a 60% fat high-fat diet (WTHD, TRHD, and WTPO). In the WTPO group, 8% (w/w) of perilla oil was added. Perilla oil supplemented WT mice and fat-1 transgenic mice suppressed high-fat-diet-induced body weight and improved serum lipid levels. Furthermore, the WTPO and TRHD groups exhibited increased colon length, lower macroscopic scores, and reduced levels of pro-inflammatory markers and improved epithelial integrity barrier markers. The expression of GPR120 was increased in the WTPO group. Altogether, our results indicated that perilla oil could improve the symptoms of colon inflammation as an alternate omega-3 fatty acid supplement.
1. Introduction
Prolonged consumption of a high-fat diet (HFD) results in the accumulation of fat in the body, increasing health risks and causing several health complications such as cardiovascular disease, hypertension, fatty liver, blood pressure, type 2 diabetes, and colitis that contribute to the increasing economic burden in society [1]. Inflammatory reactions resulting from chronic HFD consumption contribute significantly to the initiation of obesity-associated complications. Shifting of the intestinal microbiota toward an increased ratio of Enterobacteriaceae owing to a HFD could be the first step toward chronic systemic inflammation. Changes in intestinal microbiota stimulate the Toll-like receptor pathway, that affects the intestinal permeability to endotoxins, increasing its penetration into the circulation [2]. Furthermore, intestinal cells may also be affected by the increased free fatty acid (FFA) levels in the HFD. These elevated levels of endotoxins and FFAs result in the enhanced generation of pro-inflammatory cytokines including tumor necrosis factor (TNF-α), interleukin (IL)-1β, and IL-6 in the gut. Persistence of such condition leads to a continuous flow of lipopolysaccharides (LPS), pro-inflammatory cytokines, and FFA into the systemic circulation, triggering low-grade systemic inflammation [3].
Inflammatory bowel disease (IBD) indicates a set of chronic inflammatory diseases, such as ulcerative colitis (UC) and Crohn’s disease (CD), affecting the gastrointestinal tract. Previously known to be localized only in Western countries, IBD incidence is now increasing in countries of Asia and Africa because of industrialization, changes in dietary patterns, and lifestyle [4]. As the global burden of IBD is anticipated to rise in the years ahead, given the current trend, research and practical interventions to manage or prevent these diseases are needed. Dietary modification involving the supplementation of anti-inflammatory compounds is an effective approach for managing IBD. Particularly, omega-3 fatty acids have been receiving much attention over the past several years because of their improving effects on conditions such as cardiovascular disease, rheumatoid arthritis, and neurodegenerative disorders [5,6,7]. Because of their anti-inflammatory effects, omega-3 fatty acids are also being examined for attenuating IBD. Several studies in experimental animal models have reported that omega-3 fatty acids present in fish oil ameliorate colitis [8,9]. Furthermore, dietary intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) has been reported to lower the risk of UC in humans [10]. Surprisingly, the possible effects of plant-derived omega-3 fatty acid-rich oils on IBD have not been as extensively studied as those of fish oil (FO). Perilla oil (PO), extracted from the seeds of Perilla frutescens, has a high content of omega-3 fatty acids. It is reported to have anti-inflammatory, anti-atherosclerotic, and lipid-lowering properties in animal models [11,12,13]. Toshiaki et al. reported that PO with various concentrations of vitamin E reduced the levels of leukotriene B4 (LTB4) in a UC mice model [14]. A study conducted on an experimental model of CD showed that PO significantly suppressed the levels of plasma LTB4, and the effect was better than the fish-oil-supplemented group [15]. Considering the regular use of PO in Korean cuisine, we aimed to investigate the possible effects of PO on colon inflammation in different animal models.
The fat-1 transgenic mice contain the fat-1 gene, that encodes a desaturase enzyme that can produce omega-3 fatty acids from omega-6 fatty acids within the body. The generation of fat-1 transgenic mice has facilitated the investigation of the effects of omega-3 fatty acids in different disease conditions by completely avoiding interference from other dietary factors. Some previous studies with fat-1 transgenic mice have shown that the endogenous omega-3 fatty acids protect against UC and CD [16,17]. In a previous study, we observed that PO exerts protective effects against HFD-induced colon inflammation. The effects were similar to those of FO, which was used as a positive control [18]. In the present study, we evaluated the comparative effect of endogenously produced omega-3 fatty acids in fat-1 transgenic mice and diet-supplemented omega-3 fatty acids in wild-type (WT) mice against HFD-induced colon inflammation.
2. Materials and Methods
2.1. Animals and Diet
All experimental procedures carried out were permitted by the Animal Use and Care Committee of Chungnam National University (CNU-01038). Mice were sustained in a temperature-regulated housing, 23 °C ± 1 °C, with light and dark cycles for 12 h each. A set of fat-1 transgenic male mice were kindly provided by Dr. Byung Hyun Park (Jeonbuk National University Medical School, Jeonju, Korea) [19]. The mice were crossed with C57BL/6J female mice to obtain fat-1 transgenic and WT littermates. Five-week-old WT littermates were divided into three groups (n = 8, total 24): WTLD, WTHD, and WTPO; while transgenic littermates were divided into two groups (n = 8, total 16): TRLD and TRHD. A total of 40 mice were used in the study. The animals were divided randomly so that the groups did not have any significant differences in their body weight before starting the experimental diet. The experimental diet given to each group is as follows: WTLD and TRLD—10% fat diet (low-fat diet, LD); WTHD, TRHD, and WTPO—60% fat diet (high-fat diet, HFD).
For the WTPO group, the diet named as HD + PO was made by replacing 25% of lard with edible PO, resulting in a concentration of 8% (w/w) of PO in the diet. The PO used was cold-pressed, refined oil obtained from a local market. The fatty acid composition of PO used in the study is given in Table 1.

Table 1.
Fatty acid composition of PO.
Body weight and feed intake were measured weekly and thrice a week, respectively, throughout the 16 weeks of the experiment. The diet composition is given in Table 2.

Table 2.
Ingredients of the experimental diet.
Low-fat diet (LD), 10% kcal from fat; high-fat diet (HD), 60% kcal from fat; HD + PO diet, 60% kcal from fat diet containing 8% perilla oil in diet. The sign “-“ indicates that the component is not added to the diet.
2.2. Sample Collection
Mice were euthanized following 12 h of fasting. Tissues were collected, frozen immediately, and stored at −72 °C, and some parts of the tissues were fixed in formalin for histological evaluation. After dissection of the colon, edema or ulceration level was recorded as previously reported [20].
2.3. Histological Analysis
Longitudinally dissected colon tissue was fixed in 10% formalin, embedded in paraffin, sectioned and stained using hematoxylin and eosin (H&E). Stained slides were visualized using an Axiophot Zeiss Z1 microscope (Carl Zeiss, Gottingen, Germany).
2.4. Fecal Bacterial Count
Samples diluted using autoclaved PBS were cultured for 24 h on Desoxycholate agar for Enterobacteriaceae and for 48 h on BL agar for Bifidobacteria at 37 °C. Agar was obtained from MB cell, Seoul, Korea.
2.5. Serum Parameters
Biochemical assays to measure serum triglyceride (TG), total cholesterol (TC), and HDL-C were performed using appropriate kits (ASAN Bio, Seoul, Korea). Pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, in serum and colon, were measured using kits (R&D Systems, Minneapolis, MN, USA). Endotoxin levels in serum were quantified using a Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fischer Scientific, Rockford, IL, USA).
2.6. RT-qPCR and Western Blot
RT-qPCR was used to measure colon-inflammation-related mRNA expression. The purity of RNA was confirmed after extraction using the QIAGEN RNA easy kit (QIAGEN GmbH, Hilden, Germany). Further, using the cDNA synthesized by a cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA), qPCR was carried out using the primers presented in Table 3.

Table 3.
Primer list for qPCR.
For Western blot, colon tissue lysate was obtained using RIPA buffer consisting of protease and phosphatase inhibitors and was quantified by BCA assay. In total, 20 μg of protein were loaded and separated using 10% SDS-PAGE and transferred to a PVDF membrane. The target proteins were detected after incubation with primary and secondary antibodies, followed by detection using an ATTO LuminoGraph II (ATTO, Tokyo, Japan) imaging system.
2.7. Statistical Analysis
One-way analysis of variance using SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses. Values with p < 0.05 obtained after Duncan’s test were considered statistically significant and are indicated with different superscripts (a, b, and c). Data are expressed as mean ± standard deviation.
3. Results
3.1. PO-Supplemented WT and fat-1 Transgenic Mice Exhibited Improved Anthropometric Parameters and Serum Lipids
Consumption of HFD significantly increased body weight in all three HFD-fed groups compared to LD-fed groups (Figure 1A). However, WTPO and TRHD groups showed significantly lower body weights than the WTHD group. The liver and epididymal fat weights showed similar patterns (Figure 1B,C). Compared to the WTHD group, WTPO and TRHD groups had significantly lower organ weights. In the case of LD-fed mice, both wild-type and transgenic mice did not show a difference in body weight and organ weights, although TRLD had slightly lower values. The feed intake was not significant between groups (WTLD—2.83 ± 0.07 ns; WTHD—2.59 ± 0.32 ns; WTPO—2.83 ± 0.37 ns; TRLD—3.18 ± 0.64 ns; TRHD—2.51 ± 0.05 ns).
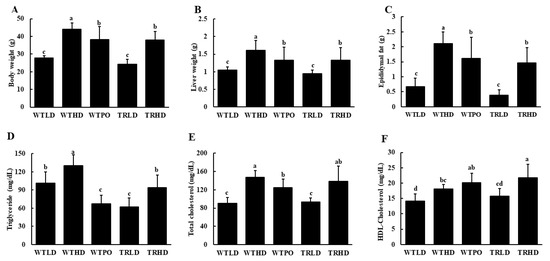
Figure 1.
(A) Final body weight, (B) liver weight, (C) epididymal fat weight, (D) serum TG, (E) serum TC, and (F) HDL-C. Mice were divided into five groups (n = 8); WTLD and TRLD fed a low-fat diet (LD, 10% kcal from fat); WTHD and TRHD fed a high-fat diet (HD, 60% kcal from fat); WTPO fed an HD + PO diet (60% kcal from fat diet, supplemented with 8% perilla oil in the diet). Values are expressed as mean ± SD, and those with different superscript (a, b, c) letters indicate significance between groups by ANOVA with Duncan’s test at p < 0.05.
Analysis of serum lipids showed that PO supplementation in wild-type mice and endogenously produced omega-3 fatty acids in transgenic mice significantly reversed HFD-induced rise in serum TG levels (Figure 1D). The WTHD group exhibited significantly higher TG levels than the other experimental groups. Interestingly, the WTLD and TRHD groups had similar TG levels. Notably, serum TG of the WTPO group was similar to that of the TRLD group. Compared to the WTHD group, WTPO had significantly reduced levels of serum TC, and the TRHD group showed a tendency to reduce TC levels (Figure 1E). The TRHD group had significantly higher levels of HDL-C while the WTPO showed the same increasing tendency, compared to that of WTHD group (Figure 1F).
3.2. PO-Supplemented WT and fat-1 Transgenic Mice Had a Longer Colon, Lower Macroscopic Score, and Improved Histological Conditions
As shown in Figure 2A, HFD intake resulted in a decreased colon length in the WTHD group compared to that in the LD-fed groups. Interestingly, WTPO and TRHD groups showed significantly improved colon lengths than the WTHD group, and the colon length of the WTPO group was significantly longer than that of WTLD, TRLD, and TRHD groups, suggesting the protective effect of PO on colon health. The macroscopic score was significantly increased in the WTHD group, whereas WTPO and TRHD showed significantly lower macroscopic scores (Figure 2B). The H&E staining showed that the endothelia of HFD-fed groups were disrupted (Figure 2C) while WTPO and TRHD showed an improved colon with tightly arranged goblet cells and undisrupted epithelia.
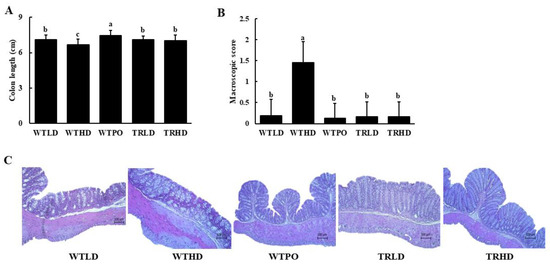
Figure 2.
(A) Colon length, (B) macroscopic score, and (C) histological changes. Mice were divided into five groups (n = 8); WTLD and TRLD fed a low-fat diet (LD, 10% kcal from fat); WTHD and TRHD fed a high-fat diet (HD, 60% kcal from fat); WTPO fed an HD + PO diet (60% kcal from fat diet, supplemented with 8% perilla oil in the diet). Values are expressed as mean ± SD, and those with different superscript (a, b, c) letters indicate significance between groups by ANOVA with Duncan’s test at p < 0.05.
3.3. PO-Supplemented WT and fat-1 Transgenic Mice Showed Improved Microbial Population and Decreased Endotoxin Levels in Serum
The composition of intestinal microbiota was altered in the WTHD group, with an increase in the number of Enterobacteriaceae and a decrease in the number of Bifidobacteria (Table 4). Compared to the WTHD group, all the other groups, including WTLD, TRLD, WTPO, and TRHD, showed a significantly reduced number of Enterobacteriaceae and an increased number of Bifidobacteria. Interestingly, Bifidobacteria colonies were the highest in the TRHD group, followed by the WTPO group. The analysis of endotoxin levels in serum revealed that WTLD, TRLD, WTPO, and TRHD groups had significantly lower levels of serum endotoxin than the WTHD group.

Table 4.
Fecal count of Enterobacteriaceae, Bifidobacteria, and endotoxin levels in serum.
3.4. PO-Supplemented WT and fat-1 Transgenic Mice Showed Lower Levels of Pro-Inflammatory Cytokines in Serum and Colon
As shown in Figure 3, the WTHD group had increased levels of pro-inflammatory cytokines in serum and colon. In serum, WTPO and TRHD groups showed similar levels of TNF-α, IL-6, and IL-1β, which were significantly lower compared to those in the WTHD group (Figure 3A). Similarly, compared with the WTHD group, WTPO and TRHD groups showed significantly reduced levels of TNF-α, IL-6, and IL-1β in the colon (Figure 3B). Furthermore, the TRHD group had significantly lower levels of these cytokines than the WTPO group. The WTLD and TRLD groups had similar levels of pro-inflammatory cytokines, which were lower than the HFD-fed groups.
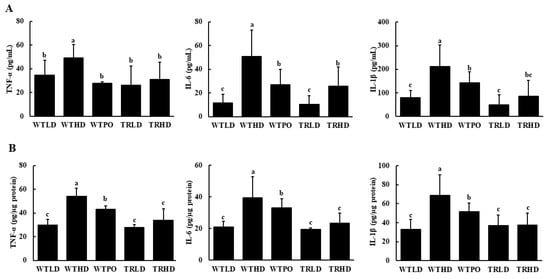
Figure 3.
Pro-inflammatory cytokine levels in (A) serum and (B) colon. Mice were divided into five groups (n = 8); WTLD and TRLD fed a low-fat diet (LD, 10% kcal from fat); WTHD and TRHD fed a high-fat diet (HD, 60% kcal from fat); WTPO fed an HD + PO diet (60% kcal from fat diet, supplemented with 8% perilla oil in the diet). Values are expressed as mean ± SD, and those with different superscript (a, b, c) letters indicate significance between groups by ANOVA with Duncan’s test at p < 0.05.
3.5. mRNA and Protein Expression of Inflammatory Markers in the Colon Was Lower in PO-Supplemented WT and fat-1 Transgenic Mice
The mRNA expression levels of TNF-α, IL-6, and IL-1β were significantly upregulated in the WTHD group (Figure 4A). In contrast, WTPO and TRHD groups showed significantly lower levels, similar to that of the LD-fed groups. In addition, WTPO showed enhanced expression of tight junction proteins, such as claudin-1 and Zo-1, and mucins, such as Muc1 and Muc3, compared to the WTHD group (Figure 4B). The TRHD group showed an increasing tendency in claudin-1 expression and significantly higher expression levels of Zo-1, Muc1, and Muc3. Additionally, the mRNA expression of G-protein-coupled receptor 120 (GPR120), a cell membrane receptor stimulated by long-chain fatty acids, was significantly higher in the WTPO and TRHD groups than in the WTHD group (Figure 4C).
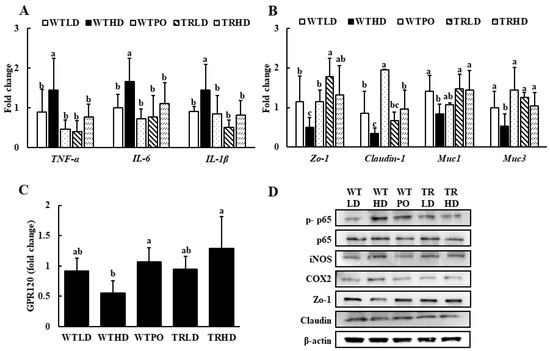
Figure 4.
The mRNA expression of (A) pro-inflammatory markers, (B) epithelial tight junction markers, (C) GPR120, and (D) protein expression in colon. Mice were divided into five groups (n = 8); WTLD and TRLD fed a low-fat diet (LD, 10% kcal from fat); WTHD and TRHD fed a high-fat diet (HD, 60% kcal from fat); WTPO fed an HD + PO diet (60% kcal from fat diet, supplemented with 8% perilla oil in the diet). Values are expressed as mean ± SD, and those with different superscript (a, b, c) letters indicate significance between groups by ANOVA with Duncan’s test at p < 0.05.
As shown in Figure 4D, the protein expression of p-p65, iNOS, and COX2 was lowered in WTPO and TRHD groups compared to the WTHD group. In addition, treatment with PO increased the expression of tight junction protein, Zo-1, and its expression was similar to that of TRLD and TRHD.
4. Discussion
There has been an increase in the incidence of IBD worldwide due to various factors such as smoking, infections, antibiotics, high-fat and low-fiber diets. Studies have shown that about 15–40% of IBD patients are obese, and an additional 20–40% are overweight [21]. Inflammatory reactions occurring as a result of a chronic HFD consumption is one of the main mechanisms of initiation of obesity-associated dysfunction of several organs.
Consumption of a HFD for approximately 16 weeks induces gut microbial changes and inflammation in mice [22]. In the present study, we used an HFD-induced colon inflammation mouse model consisting of wild-type C57BL/6J mice and fat-1 transgenic mice. Our study showed that supplementation with PO, which has an omega-6 to omega-3 fatty acid ratio of 1:4.8, in WT mice and endogenous production of omega-3 fatty acids by fat-1 mice exerted similar effects in preventing HFD-induced body weight gain, as evidenced by the reduced final body weight and organ weights. Furthermore, the serum lipid-lowering effects were also similar in WTPO and TRHD mice. This suggested that omega-3 fatty acids present in PO could attenuate obesity which was in agreement with some previous reports on PO and fat-1 transgenic mice [23,24].
After the experimental period, significant differences were observed in the colon lengths between WTHD and WTLD groups. The WT mice fed PO, and transgenic mice that can produce endogenous omega-3 fatty acids prevented colon length shortening, improved macroscopic score, and alleviated serum and colon pro-inflammatory cytokines. The culture of bacteria in the stool showed that both WTPO and TRHD reduced the levels of Enterobacteriaceae, which in turn reflected in the reduced levels of endotoxins. A previous study on fat-1 transgenic mice and fat-2 transgenic mice (consists of a gene that converts monounsaturated fatty acids to omega-6 fatty acids) showed that the amount of Enterobacteriaceae was lower and Bifidobacteria was more abundant in fat-1 transgenic mice than that in fat-2 transgenic mice [25]. The results obtained in the present study corroborate these results, with fat-1 transgenic mice showing the highest number of Bifidobacteria among all groups, especially when compared with the WTHD group. PO-fed WT mice also showed a similar effect indicating that the ameliorating effect is majorly mediated through omega-3 fatty acids.
It was evident from the colon histology and reduction of pro-inflammatory cytokines in the colon that omega-3 fatty acids suppressed the LPS-induced activation of nuclear factor-kappa B (NF-κB). TNF-α have a significant role in the pathogenesis of IBD, initiating the cytotoxic, apoptotic, and acute-phase reactions and increasing the levels of other pro-inflammatory cytokines, such as IL-1β and IL-6. Supplementation of PO showed lower levels of inflammatory cytokines compared to the WTHD group. Additionally, the suppression of NF-κB activation and the production of iNOS and COX2 in the WTPO and TRHD groups indicated that omega-3 fatty acids downregulated the inflammatory process. A previous study on transgenic mice with TNBS-induced colitis showed that it suppresses NF-κB and increased nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathways, which was in agreement with our results [17].
Claudin-1 and Zo-1 are major constituents of tight junctions, where epithelial cells are connected, forming a paracellular seal. Mucins are a group of proteins with different functions in the mucus layer barrier of the colon defense system. Three layers of the colon mucosa include (a) glycocalyx formed by the membrane-anchored mucins and epithelial cells, (b) interlinked MUC2 protein layer, and (c) less dense and less viscous outermost layer. Muc1 and Muc3 are membrane-anchored mucins. IBD is characterized by a defective mucosal layer and tight junction proteins [26]. The expression levels of Muc1, Muc3, claudin-1, and Zo-1 were increased in WTPO and fat-1 transgenic mice, indicating that omega-3 fatty acids improve the intestinal epithelial barrier; thus, preventing the infiltration of bacterial endotoxins into the lamina propria of the intestinal tract and attenuating colon inflammation.
GPR120 is a membrane G-protein-coupled receptor that is known to be activated by omega-3 fatty acids. GPR120 is known to initiate anti-inflammatory responses in cells including macrophages and adipocytes [27]. Activation of GPR120 suppresses the TNF-α or LPS-induced activation of transforming growth factor beta-activated kinase 1 (TAK1), which further inhibits the initiation of the NF-κB signaling pathway. A previous colitis study using an IL-10 knockout mouse reported that GPR120 acts as an anti-inflammatory agent [28]. In our study, the expression of GPR120 was upregulated in WTPO and TRHD compared to the WTHD group, indicating that the improvement effect of omega-3 fatty acids in the colon could be mediated through the stimulation of the GPR120 receptor.
5. Conclusions
PO attenuated HFD-induced colon inflammation in WT mice by attenuating the intestinal barrier protection, suppressing the NF-κB pathway, and decreasing the expression of pro-inflammatory genes, which might be attributed to the activation of GPR120. Comparing the effect of PO provided through the diet to that of the endogenously produced omega-3 fatty acids in the mice, we observed that their anti-inflammatory activities were identical. Through this study, we could establish that PO could be considered as a rich omega-3 fatty acid supplement and its consumption can decrease the omega-6 to omega-3 ratio, therefore, should be encouraged to maintain healthy dietary habits. In addition, clinical trials are needed to validate the daily intake amount of perilla oil in humans.
Author Contributions
Conceptualization—K.-A.K., Y.-S.C.; data curation—S.S.T.; formal analysis—S.S.T., K.-A.K.; funding acquisition—K.-A.K.; investigation—S.S.T., K.-A.K.; methodology—K.-A.K., S.S.T.; supervision—K.-A.K.; visualization—S.S.T., K.-A.K.; writing—original draft—S.S.T.; writing—review and editing—K.-A.K., Y.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Education) (2017R1D1A1B0302941814).
Institutional Review Board Statement
All experimental procedures carried out were permitted by the Animal Use and Care Committee of Chungnam National University (CNU-01038).
Data Availability Statement
Data can be made available upon request to the corresponding author.
Acknowledgments
The authors would like to thank Byung Hyun Park, Jeonbuk National University Medical School, Jeonju, Korea for his kind offering of fat-1 transgenic mice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Segula, D. Complications of obesity in adults: A short review of the literature. Malawi Med. J. 2014, 26, 20–24. [Google Scholar] [PubMed]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory links between high fat diets and diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 fatty acids and neurodegenerative diseases: New evidence in clinical trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef] [Green Version]
- Cleland, L.G.; Caughey, G.E.; James, M.J.; Proudman, S.M. Reduction of cardiovascular risk factors with longterm fish oil treatment in early rheumatoid arthritis. J. Rheumatol. 2006, 33, 1973–1979. [Google Scholar]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: Present, past and future. Expert Rev. Clin. Pharmacol. 2017, 10, 865–873. [Google Scholar] [CrossRef]
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coeffier, M.; Charlène, G.; Ghosh, S.; Guillaume, S.; Marion-Letellier, R. Dietary n-3 pufa may attenuate experimental colitis. Mediat. Inflamm. 2018, 2018, 8430614. [Google Scholar] [CrossRef]
- Sharma, M.; Kaur, R.; Kaushik, K.; Kaushal, N. Redox modulatory protective effects of omega-3 fatty acids rich fish oil against experimental colitis. Toxicol. Mech. Methods 2019, 29, 244–254. [Google Scholar] [CrossRef]
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606. [Google Scholar] [CrossRef]
- Cha, Y.; Jang, J.Y.; Ban, Y.H.; Guo, H.; Shin, K.; Kim, T.S.; Kim, Y.B. Anti-atherosclerotic effects of perilla oil in rabbits fed a high-cholesterol diet. Lab. Anim. Res. 2016, 32, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chen, C.S.; Lin, J.Y. Protective effect of dietary perilla oil on allergic inflammation in asthmatic mice. Eur. J. Lipid Sci. Technol. 2012, 114, 1007–1015. [Google Scholar] [CrossRef]
- Kim, S.R.; Je, J.; Jeong, K.; Kim, S.J.; Lee, K.Y.; Choi, S.G.; Kim, H.; Park, S.W. Perilla oil decreases aortic and hepatic lipid accumulation by modulating lipogenesis and lipolysis in high-fat diet-fed mice. J. Med. Food 2019, 22, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Igarashi, J.; Ohtuka, Y.; Oguchi, S.; Kaneko, K.; Yamashiro, Y. Effects of n-3 polyunsaturated fatty acids and vitamin E on colonic mucosal leukotriene generation, lipid peroxidation, and microcirculation in rats with experimental colitis. Digestion 2001, 63, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Shoda, R.; Matsueda, K.; Yamato, S.; Umeda, N. Therapeutic efficacy of n-3 polyunsaturated fatty acid in experimental Crohn's disease. J. Gastroenterol. 1995, 30, 98–101. [Google Scholar]
- Hudert, C.A.; Weylandt, K.H.; Lu, Y.; Wang, J.; Hong, S.; Dignass, A.; Serhan, C.N.; Kang, J.X. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. USA 2006, 103, 11276–11281. [Google Scholar] [CrossRef] [Green Version]
- Yum, H.W.; Kang, J.X.; Hahm, K.B.; Surh, Y.J. Constitutive omega-3 fatty acid production in fat-1 transgenic mice and docosahexaenoic acid administration to wild type mice protect against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Biochem. Biophys. Res. Commun. 2017, 487, 847–855. [Google Scholar] [CrossRef]
- Thomas, S.S.; Cha, Y.S.; Kim, K.A. Perilla oil alleviates high-fat diet-induced inflammation in the colon of mice by suppressing nuclear factor-kappa b activation. J. Med. Food 2020, 23, 818–826. [Google Scholar] [CrossRef]
- Jang, H.Y.; Koo, J.H.; Lee, S.M.; Park, B.H. Atopic dermatitis-like skin lesions are suppressed in fat-1 transgenic mice through the inhibition of inflammasomes. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.S.; Cha, Y.S.; Kim, K.A. Effect of vegetable oils with different fatty acid composition on high-fat diet-induced obesity and colon inflammation. Nutr. Res. Pract. 2020, 14, 425–437. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Li, J.; Tang, R.; Zhang, G.; Zeng, H.; Wood, R.J.; Liu, Z. High fat diet alters gut microbiota and the expression of paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediat. Inflamm. 2017, 2017, 9474896. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, H.; Yuan, F.; Li, N.; Huang, Q.; He, L.; Wang, L.; Liu, Z. Perilla oil has similar protective effects of fish oil on high-fat diet-induced nonalcoholic fatty liver disease and gut dysbiosis. Biomed Res. Int. 2016, 2016, 9462571. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Bae, J.S.; Hahm, K.B.; Cha, J.Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem. Pharmacol. 2012, 84, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Kaliannan, K.; Li, X.Y.; Wang, B.; Pan, Q.; Chen, C.Y.; Hao, L.; Xie, S.; Kang, J.X. Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun. Biol. 2019, 2, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Chen, L.; Wang, Y.; Wei, X.; Zeng, S.; Zheng, Y.; Gao, C.; Liu, H. Activation of the omega-3 fatty acid receptor GPR120 protects against focal cerebral ischemic injury by preventing inflammation and apoptosis in mice. J. Immunol. 2019, 202, 747–759. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wang, H.; Shi, P.; Wang, W.; Sun, Y. GPR120, a potential therapeutic target for experimental colitis in IL-10 deficient mice. Oncotarget 2017, 8, 8397–8405. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).