Characterization of Key Aroma Compounds in Fermented Bamboo Shoots Using Gas Chromatography-Olfactometry-Mass Spectrometry, Odor Activity Values, and Aroma Recombination Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Extraction of Volatile Compounds Using HS-SPME
2.3. Identification of Aroma Compounds Using GC-O-MS
2.4. Quantitative Assessment of Aroma Compounds
2.5. OAVs
2.6. Quantitative Descriptive Sensory Analysis
2.7. Recombination and Omission Experiments
2.8. Statistical Analysis
3. Results
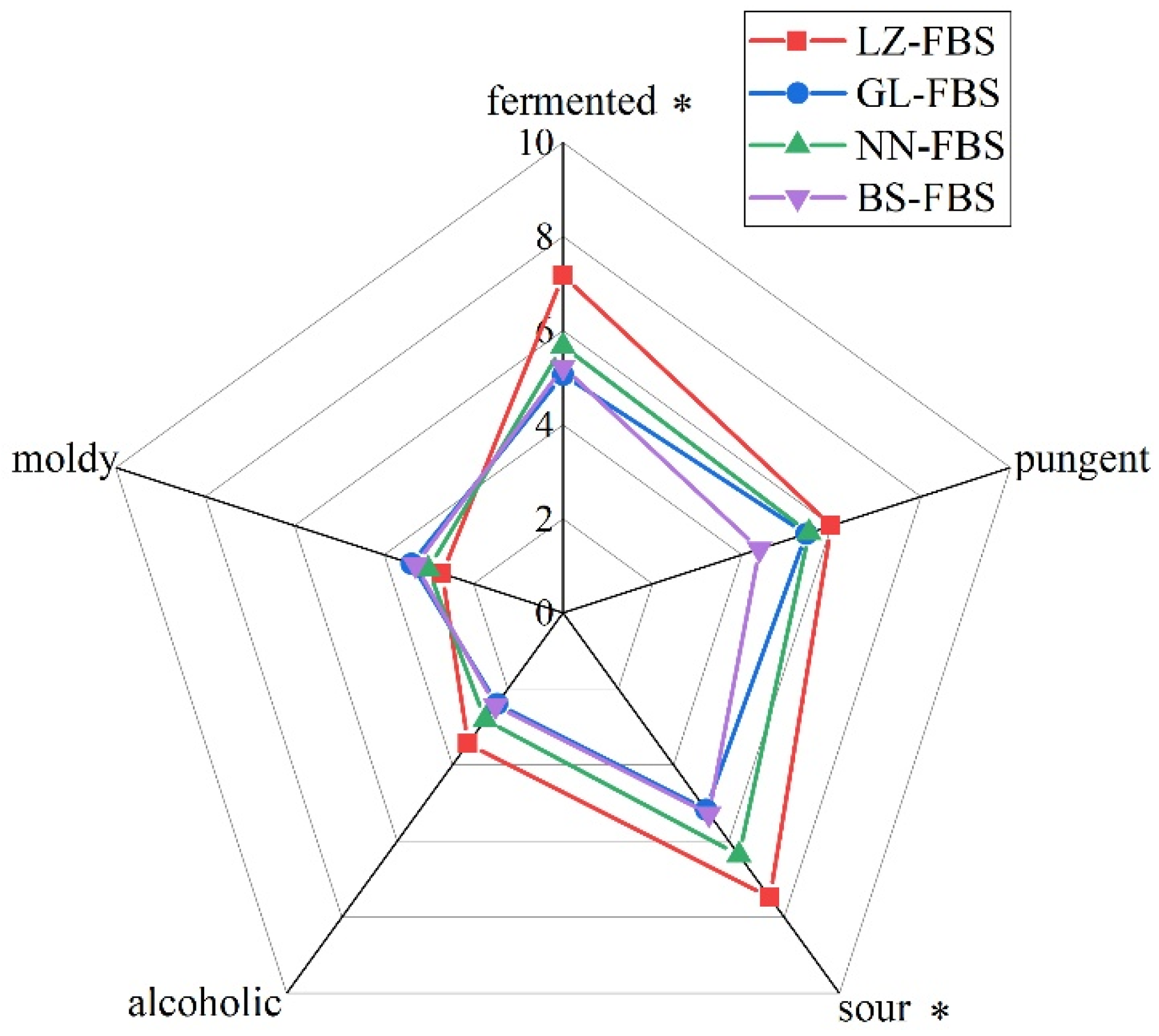
3.1. Sensory Analysis
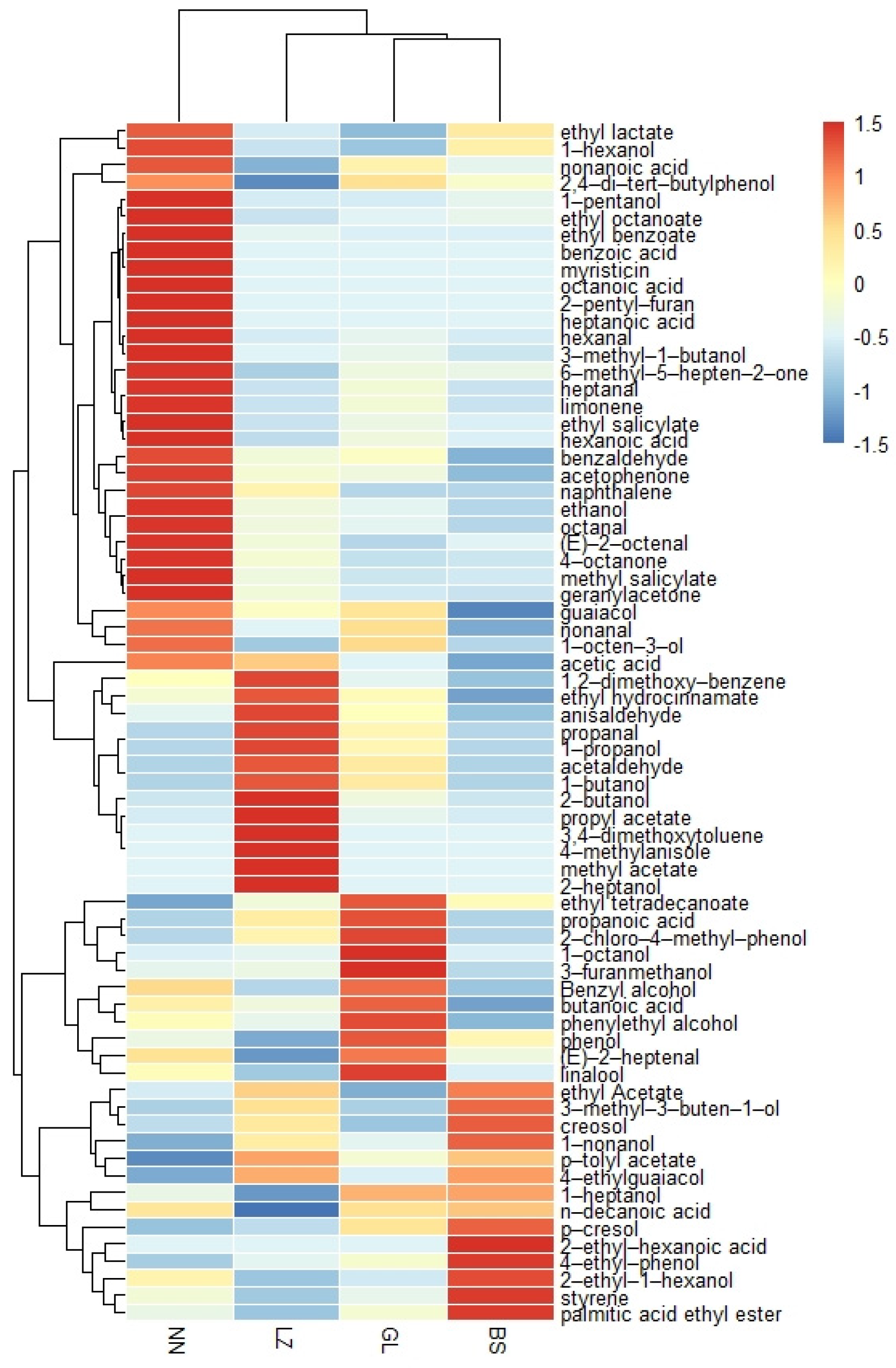
3.2. Identification and Quantification of GFBS with GC-MS
3.3. Identification of Aroma-Active Compounds by GC-MS-OSME and Their OAV Analysis
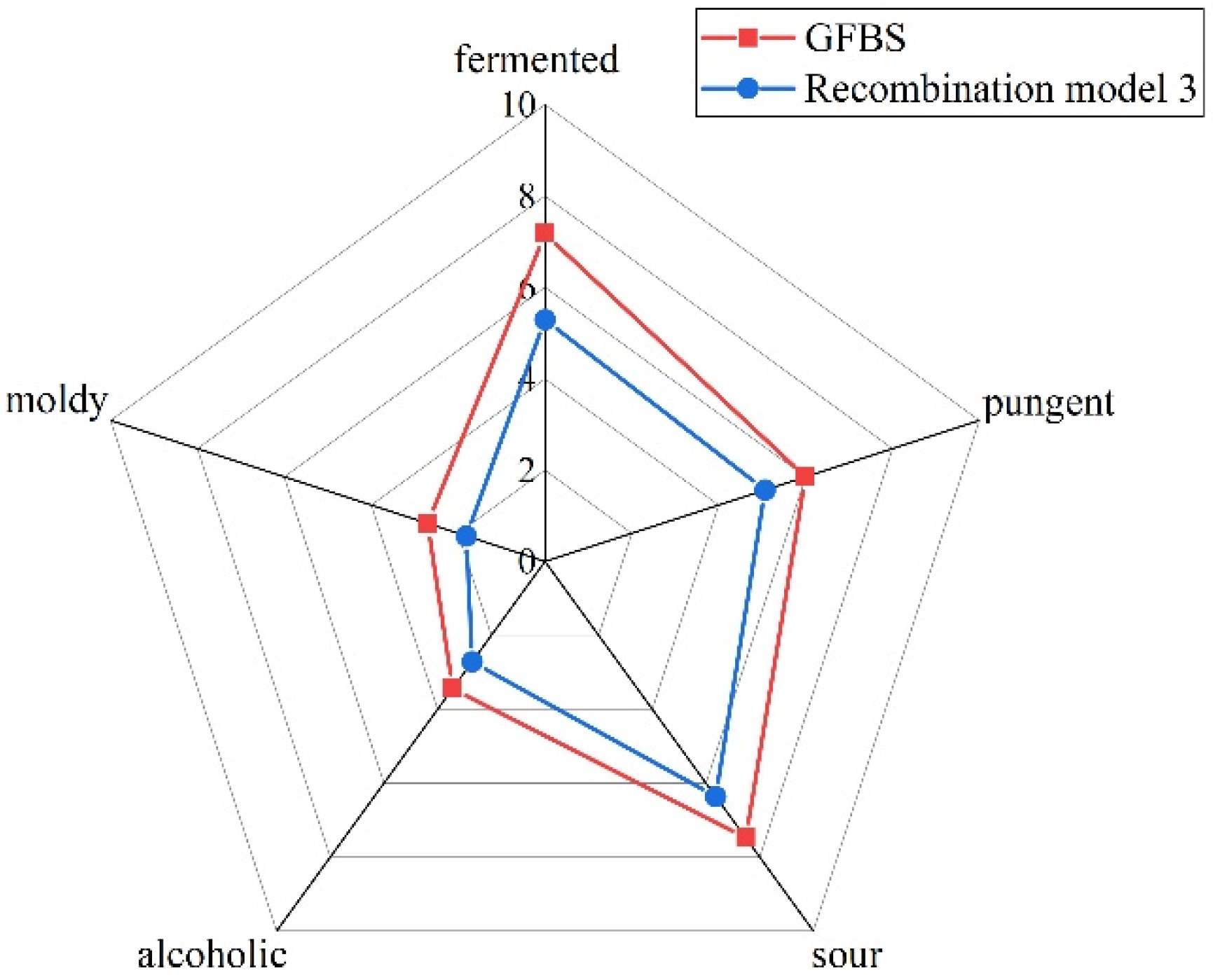
3.4. Determination of Key Aroma Compounds Using Recombination Experiments
4. Discussion
4.1. Dominant Aroma Compounds in GFBS
4.2. Subdominant Aroma Compounds in GFBS
4.3. Other Substances with Important Contributions to GFBS Odor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample | Species of RAW BAMBOO SHOOTS | Guangxi Fermented Bamboo Shoots Location | Fermentation Time (Month) | pH |
|---|---|---|---|---|
| LZ-FBS | Dendrocalamus latiflorus Munro | Liuzhou | 1 | 3.42 ± 0.02 |
| NN-FBS | Dendrocalamus latiflorus Munro | Nanning | 1 | 3.85 ± 0.03 |
| GL-FBS | Dendrocalamus latiflorus Munro | Guiling | 1 | 3.93 ± 0.03 |
| BS-FBS | Dendrocalamus latiflorus Munro | Baise | 1 | 3.82 ± 0.04 |
References
- Cheigh, H.S.; Park, K.Y. Biochemical, microbiological, and nutritional aspects of kimchi (Korean fermented vegetable products). Crit. Rev. Food Sci. Nutr. 1994, 34, 175–203. [Google Scholar] [CrossRef] [PubMed]
- Campbellplatt, G. Fermented Foods—A World Perspective. Food Res. Int. 1994, 27, 253–257. [Google Scholar] [CrossRef]
- Behera, P.; Balaji, S. Health Benefits of Fermented Bamboo Shoots: The Twenty-First Century Green Gold of Northeast India. Appl. Biochem. Biotechnol. 2021, 193, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Satya, S.; Bal, L.M.; Singhal, P.; Naik, S.N. Bamboo shoot processing: Food quality and safety aspect (a review). Trends Food Sci. Technol. 2010, 21, 181–189. [Google Scholar] [CrossRef]
- Fu, S.G.; Yoon, Y.; Bazemore, R. Aroma-active components in fermented bamboo shoots. J. Agric. Food Chem. 2002, 50, 549–554. [Google Scholar] [CrossRef]
- Guo Rongcan, W.C.; Hongrui, J.; Lian, Y.; Xiaoling, L.; Mouming, Z. Optimization and Comparison Analysis of Extraction Methods of Odorant from Guangxi Fermentated Bamboo Shoots. Sci. Technol. Food Ind. 2019, 40, 202–210+220. [Google Scholar]
- Zheng, W.; Guan, Q.; Liu, Z.; Wei, B.; Xiong, S.; Xiong, T. Comparison of flavors of Suancai and Suansun in Guangxi based on GC-MS. Food Ferment. Ind. 2020, 46, 253–257. [Google Scholar]
- Guo, R.; Yu, F.; Wang, C.; Jiang, H.; Yu, L.; Zhao, M.; Liu, X. Determination of the Volatiles in Fermented Bamboo Shoots by Head Space—Solid-Phase Micro Extraction (HS-SPME) with Gas Chromatography—Olfactory—Mass Spectrometry (GC-O-MS) and Aroma Extract Dilution Analysis (AEDA). Anal. Lett. 2020, 54, 1162–1179. [Google Scholar] [CrossRef]
- Zhao, C.; Fan, W.; Xu, Y. Characterization of key aroma compounds in pixian broad bean paste through the molecular sensory science technique. Lwt Food Sci. Technol. 2021, 148, 111743. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, H.; Cadwallader, K.R. Aroma-active compounds in Kimchi during fermentation. J. Agric. Food Chem. 1998, 46, 1944–1953. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Luo, S.; Zhang, J.; Huang, M.; Chen, F.; Zheng, F.; Sun, X.; Li, H. Characterization of key aroma compounds in Meilanchun sesame flavor style baijiu by application of aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission/addition experiments. Rsc Adv. 2018, 8, 23757–23767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma Characterization of Chinese Rice Wine by Gas Chromatography-Olfactometry, Chemical Quantitative Analysis, and Aroma Reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of Key Aroma Compounds in Beijing Roasted Duck by Gas Chromatography-Olfactometry-Mass Spectrometry, Odor-Activity Values, and Aroma-Recombination Experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wu, Q.; Niu, Y.; Wu, M.; Zhu, J.; Zhou, X.; Chen, X.; Wang, H.; Li, J.; Kong, J. Characterization of the Key Aroma Compounds in Five Varieties of Mandarins by Gas Chromatography-Olfactometry, Odor Activity Values, Aroma Recombination, and Omission Analysis. J. Agric. Food Chem. 2017, 65, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Au, C.; Acevedo, N.C.; Horner, H.T.; Wang, T. Determination of the Gelation Mechanism of Freeze-Thawed Hen Egg Yolk. J. Agric. Food Chem. 2015, 63, 10170–10180. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Ma, J.; Lv, Y.; Tong, Q.; Guo, H. Characterization of key off-odor compounds in thermal duck egg gels by GC-olfactometry-MS, odor activity values, and aroma recombination. Lwt Food Sci. Technol. 2021, 143, 111182. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis-General Guidance for the Design of Test Rooms. ISO (International Organisation for Standardisation): Geneva, Switzerland, 2007.
- Roessler, E.B.; Pangborn, R.M.; Sidel, J.L.; Stone, H. Expanded statistical tables for estimating significance in paired-preference, paired-difference, duo-trio and triangle tests. J. Food Sci. 1978, 43, 940–943. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Z.; Yu, H.; Xu, Z.; Tian, H. Flavoromic determination of lactones in cheddar cheese by GC-MS-olfactometry, aroma extract dilution analysis, aroma recombination and omission analysis. Food Chem. 2022, 368, 130736. [Google Scholar] [CrossRef]
- Bressanello, D.; Liberto, E.; Cordero, C.; Sgorbini, B.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.R.; Bicchi, C. Chemometric Modeling of Coffee Sensory Notes through Their Chemical Signatures: Potential and Limits in Defining an Analytical Tool for Quality Control. J. Agric. Food Chem. 2018, 66, 7096–7109. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Cui, C.; Su, G.; Lin, L.; Zhao, M. Approaches of aroma extraction dilution analysis (AEDA) for headspace solid phase microextraction and gas chromatography-olfactometry (HS-SPME-GC-O): Altering sample amount, diluting the sample or adjusting split ratio? Food Chem. 2015, 187, 44–52. [Google Scholar] [CrossRef]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and other Media, 2nd ed.; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Lu, Y.; Wang, Y.; Zhao, G.; Yao, Y. Identification of aroma compounds in Zhuhoujiang, a fermented soybean paste in Guangdong China. Lwt Food Sci. Technol. 2021, 142, 111057. [Google Scholar] [CrossRef]
- Rothe, M.; Wolm, G.; Tunger, L.; Siebert, H.J. Threshold values of aromatic compounds and their use for evaluation of aroma analysis. Die Nahr. 1972, 16, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.A. Inheritance and flavor contribution of 2-isobutyl-thiazole, methyl salicylate and eugenol in tomatoes. Proc. Amer. Soc. Hort. Sci. 1970, 95, 9–13. [Google Scholar]
- Abe, K.; Hori, Y.; Myoda, T. Characterization of key aroma compounds in aged garlic extract. Food Chem. 2020, 312, 126081. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, M.; Song, H.; Meng, Q. Characterization of key aroma compounds in traditional Chinese soy sauce through the molecular sensory science technique. Lwt Food Sci. Technol. 2020, 128, 109413. [Google Scholar] [CrossRef]
- Sadecka, J.; Sakova, N.; Pangallo, D.; Korenova, J.; Kolek, E.; Puskarova, A.; Buckova, M.; Valik, L.; Kuchta, T. Microbial diversity and volatile odour-active compounds of barrelled ewes' cheese as an intermediate product that determines the quality of winter bryndza cheese. Lwt Food Sci. Technol. 2016, 70, 237–244. [Google Scholar] [CrossRef]
- Kumazawa, K.; Kaneko, S.; Nishimura, O. Identification and Characterization of Volatile Components Causing the Characteristic Flavor in Miso (Japanese Fermented Soybean Paste) and Heat-Processed Miso Products. J. Agric. Food Chem. 2013, 61, 11968–11973. [Google Scholar] [CrossRef]
- Fan, S.; Tang, K.; Xu, Y.; Chen, S. Characterization of the potent odorants in Tibetan Qingke Jiu by sensory analysis, aroma extract dilution analysis, quantitative analysis and odor activity values. Food Res. Int. 2020, 137, 109349. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, L.; Zhan, P.; Tian, H.; Liu, J. Characterization of the aroma compounds of Millet Huangjiu at different fermentation stages. Food Chem. 2022, 366, 130691. [Google Scholar] [CrossRef]
- Qian, Y.L.; An, Y.; Chen, S.; Qian, M.C. Characterization of Qingke Liquor Aroma from Tibet. J. Agric. Food Chem. 2019, 67, 13870–13881. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Han, Y. Quantification of Volatile Compounds in Chinese Ciders by Stir Bar Sorptive Extraction (SBSE) and Gas Chromatography-Mass Spectrometry (GC-MS). J. Inst. Brew. 2011, 117, 61–66. [Google Scholar] [CrossRef]
| Compound a | RT b (min) | RI c | Method f | Concentration g,a | ||||
|---|---|---|---|---|---|---|---|---|
| Calculated d | Literature e | LZ-FBS | GL-FBS | NN-FBS | BS-FBS | |||
| Acetaldehyde | 4.097 | - | - | MS | 66.45 ± 15.01 | 36.06 ± 5.08 | 0 | 0 |
| Propanal | 4.765 | - | - | MS, S, O | 22.06 ± 7.37 | 9.39 ± 0.9 | 0 | 0 |
| Methyl acetate | 5.175 | 818 | 834 | MS | 10.58 ± 4.14 | 0 | 0 | 0 |
| Ethyl Acetate | 5.97 | 881 | 894 | MS, RI, S, O | 206.49 ± 88.05 | 57.35 ± 7.27 | 104.11 ± 28.9 | 251.16 ± 88 |
| Ethanol | 6.822 | 931 | 939 | MS, RI, S | 2107.09 ± 120.17 | 2052.18 ± 204.02 | 2719.03 ± 50.77 | 1930.17 ± 521.31 |
| Propyl acetate | 7.611 | 971 | 982 | MS, RI | 213.79 ± 103.65 | 15.28 ± 8.48 | 0 | 0 |
| 2-butanol | 8.6 | 1022 | 1031 | MS, RI | 159.98 ± 43.24 | 28.49 ± 2.86 | 0 | 0 |
| 1-propanol | 8.972 | 1033 | 1045 | MS, RI | 1222.33 ± 133 | 523.13 ± 47.68 | 0 | 0 |
| Hexanal | 10.211 | 1085 | 1081 | MS, RI | 0 | 8.4 ± 1.34 | 133.41 ± 15.37 | 0 |
| 1-butanol | 11.607 | 1142 | 1147 | MS, RI | 25.95 ± 5.78 | 14.15 ± 3.11 | 0 | 0 |
| Heptanal | 12.824 | 1191 | 1194 | MS, RI | 0 | 2.96 ± 0.34 | 14.68 ± 2.29 | 0 |
| Limonene | 13.146 | 1204 | 1203 | MS, RI | 0 | 0.64 ± 0.29 | 3.08 ± 0.56 | 0 |
| 3-methyl-1-butanol | 13.12 | 1203 | 1212 | MS, RI | 19.68 ± 11.36 | 23.07 ± 9.02 | 70.7 ± 3.87 | 16.55 ± 2.96 |
| 4-octanone | 13.662 | 1225 | - | MS | 5.31 ± 2.36 | 2.84 ± 1.67 | 13.19 ± 3.04 | 3.14 ± 1.71 |
| 2-pentyl-furan | 13.958 | 1237 | 1234 | MS, RI | 0 | 0 | 15.08 ± 3.75 | 0 |
| 1-pentanol | 14.165 | 1245 | 1254 | MS, RI | 3.25 ± 0.93 | 3.31 ± 0.31 | 14.84 ± 0.82 | 4.17 ± 0.48 |
| 3-methyl-3-buten-1-ol | 14.32 | 1251 | 1251 | MS, RI | 1.43 ± 0.27 | 0 | 0 | 2.2 ± 0.4 |
| Styrene | 14.596 | 1263 | 1264 | MS, RI | 1.58 ± 0.1 | 2.6 ± 0.86 | 2.96 ± 0.82 | 6.28 ± 1.69 |
| Octanal | 15.332 | 1292 | 1296 | MS, RI, S, O | 4.29 ± 1.35 | 3.54 ± 0.29 | 12.13 ± 0.43 | 1.96 ± 1.71 |
| 2-heptanol | 15.814 | 1313 | 1318 | MS, RI | 22.06 ± 8.91 | 0 | 0 | 0 |
| (E)-2-heptenal | 16.224 | 1330 | 1332 | MS, RI, | 3.73 ± 1.85 | 22.33 ± 10.49 | 16.9 ± 4.35 | 11.21 ± 5.05 |
| 6-methyl-5-hepten-2-one | 16.601 | 1346 | 1338 | MS, RI | 0 | 2.75 ± 0.07 | 11.48 ± 2.56 | 2.35 ± 0.6 |
| Ethyl lactate | 16.542 | 1343 | 1356 | MS, RI | 132.13 ± 17.55 | 60.05 ± 4.31 | 423.33 ± 10.12 | 275.68 ± 37.12 |
| 1-hexanol | 16.639 | 1348 | 1361 | MS, RI | 26.34 ± 8.06 | 18.64 ± 0.5 | 82.25 ± 8.59 | 50.48 ± 6.32 |
| Nonanal | 17.937 | 1403 | 1400 | MS, RI, S, O | 8.79 ± 2.2 | 13.5 ± 2.08 | 16.44 ± 0.39 | 5.93 ± 0.76 |
| Ethyl octanoate | 18.787 | 1439 | 1436 | MS, RI | 0 | 1.49 ± 0.38 | 19.18 ± 15.41 | 2.24 ± 0.56 |
| 1-octen-3-ol | 19.015 | 1448 | 1452 | MS, RI, S, O | 8.04 ± 2.42 | 67.05 ± 8.43 | 93.01 ± 19.52 | 13.21 ± 5.36 |
| 4-methylanisole | 18.956 | 1446 | 1441 | MS, RI | 25.37 ± 15.07 | 0 | 0 | 0 |
| (E)-2-octenal | 19.066 | 1451 | 1425 | MS, RI, S, O | 5.81 ± 2.91 | 2 ± 0.85 | 17.5 ± 6.56 | 4.26 ± 2 |
| 1-heptanol | 19.132 | 1453 | 1457 | MS, RI | 0 | 19.48 ± 1.91 | 8.77 ± 1.34 | 20.34 ± 5.55 |
| Acetic acid | 19.572 | 1472 | 1473 | MS, RI, S, O | 3378.09 ± 188.06 | 2521.16 ± 238.03 | 3710.7 ± 163.78 | 2005.91 ± 258.52 |
| 2-ethyl-1-hexanol | 19.962 | 1489 | 1490 | MS, RI | 0 | 23.48 ± 1.93 | 72.38 ± 66.92 | 150.08 ± 38.07 |
| Benzaldehyde | 21.227 | 1535 | 1541 | MS, RI, S, O | 83.51 ± 8.58 | 94.06 ± 3.13 | 184.72 ± 123.63 | 28.87 ± 14.2 |
| Linalool | 21.493 | 1544 | 1551 | MS, RI, S | 5.23 ± 3.75 | 62.14 ± 15.96 | 28.27 ± 8.29 | 14.2 ± 12.53 |
| Propanoic acid | 21.616 | 1548 | 1564 | MS, RI, S | 402.64 ± 35.87 | 793.05 ± 32.39 | 0 | 0 |
| 1-octanol | 21.873 | 1557 | 1562 | MS, RI | 14.99 ± 10.99 | 285.36 ± 387.74 | 5.65 ± 1.39 | 2 ± 0.43 |
| Butanoic acid | 24.635 | 1643 | 1652 | MS, RI | 18.79 ± 2.34 | 48.63 ± 2.15 | 28.39 ± 0.34 | 0 |
| 3-furanmethanol | 25.155 | 1658 | - | MS | 4.97 ± 0.31 | 12.57 ± 1.7 | 4.61 ± 0.07 | 3.22 ± 0.89 |
| 1-nonanol | 25.252 | 1661 | 1664 | MS, RI, S, O | 11.1 ± 3.6 | 10.52 ± 3.2 | 9.97 ± 4.22 | 11.85 ± 0.99 |
| Acetophenone | 25.447 | 1666 | 1671 | MS, RI | 7.63 ± 1.54 | 6.79 ± 0.09 | 21.73 ± 9.79 | 0 |
| Ethyl benzoate | 26.005 | 1682 | 1681 | MS, RI | 9.7 ± 4.27 | 0 | 246.73 ± 63.83 | 0 |
| 1,2-dimethoxy-benzene | 27.718 | 1729 | 1737 | MS, RI | 138.35 ± 29.34 | 56.73 ± 1.49 | 75.86 ± 5.9 | 32.42 ± 2.68 |
| P-tolyl acetate | 28.043 | 1737 | - | MS | 20.03 ± 4.81 | 10.57 ± 4.12 | 0 | 18.02 ± 2.42 |
| Naphthalene | 28.72 | 1755 | 1765 | MS, RI, S | 5.61 ± 2.68 | 0 | 12.95 ± 2.38 | 0 |
| Methyl salicylate | 29.934 | 1788 | 1798 | MS, RI, S, O | 39.04 ± 13.06 | 15.77 ± 1.94 | 174.04 ± 40.59 | 16.84 ± 8.34 |
| 3,4-dimethoxytoluene | 30.809 | 1813 | 1806 | MS, RI | 25.58 ± 6.45 | 0 | 0 | 0 |
| Ethyl salicylate | 31.28 | 1827 | - | MS | 0 | 2.83 ± 0.51 | 17.75 ± 4.62 | 0.94 ± 0.85 |
| Hexanoic acid | 32.407 | 1861 | 1849 | MS, RI, S | 0 | 105.1 ± 6.13 | 504.44 ± 32.38 | 37.76 ± 2.11 |
| Guaiacol | 32.522 | 1865 | 1859 | MS, RI, S, O | 103.44 ± 10.6 | 120.39 ± 5.45 | 143.74 ± 1.91 | 51.22 ± 4.22 |
| Geranylacetone | 32.839 | 1874 | 1868 | MS, RI, S, O | 10.21 ± 2.37 | 5.11 ± 1.9 | 35.38 ± 30.77 | 3.94 ± 2.05 |
| Benzyl alcohol | 33.012 | 1880 | 1880 | MS, RI | 63.22 ± 9.38 | 247.65 ± 5.31 | 187.64 ± 4.95 | 47.17 ± 4.07 |
| Ethyl hydrocinnamate | 33.596 | 1898 | 1903 | MS, RI | 48.79 ± 13.55 | 25.09 ± 3.83 | 20.23 ± 3.85 | 0 |
| Phenylethyl alcohol | 34.283 | 1921 | 1929 | MS, RI, S, O | 113.03 ± 22.64 | 277.87 ± 5.17 | 153.16 ± 10.71 | 49.87 ± 5.3 |
| 2-chloro-4-methyl-phenol | 34.953 | 1945 | - | MS | 16.61 ± 11.92 | 38.17 ± 19.42 | 0 | 0 |
| 2-ethyl-hexanoic acid | 35.48 | 1964 | - | MS | 0 | 21.26 ± 7.59 | 15.1 ± 3.01 | 6734.16 ± 446.45 |
| Creosol | 35.65 | 1970 | 1981 | MS, RI, S, O | 658.4 ± 104.45 | 307.7 ± 6.6 | 363.39 ± 339.52 | 913.7 ± 58.26 |
| Heptanoic acid | 35.641 | 1969 | - | MS | 0 | 0 | 58.73 ± 5.28 | 0 |
| Phenol | 36.816 | 2011 | 2037 | MS, RI, S | 305.51 ± 31.79 | 5757.22 ± 54.07 | 2122.09 ± 20.76 | 3151.9 ± 115.36 |
| 4-ethylguaiacol | 37.511 | 2038 | 2032 | MS, RI, S, O | 226.08 ± 49.7 | 118.08 ± 2.87 | 69.04 ± 10.95 | 235.61 ± 20.37 |
| Anisaldehyde | 37.905 | 2053 | 2058 | MS, RI, S, O | 6.8 ± 1.43 | 4.27 ± 1.16 | 3.49 ± 0.59 | 2.51 ± 0.59 |
| Octanoic acid | 38.543 | 2077 | 2084 | MS, RI | 0 | 0 | 300.93 ± 115.1 | 0 |
| Ethyl tetradecanoate | 38.65 | 2081 | - | MS | 4.29 ± 3.15 | 11.33 ± 1.19 | 0 | 5.72 ± 4.8 |
| P-cresol | 38.814 | 2087 | 2085 | MS, RI, S, O | 16083.21 ± 470.61 | 19120.68 ± 88.86 | 15435.89 ± 123.97 | 21313.09 ± 490.83 |
| 4-ethyl-phenol | 41.372 | 2176 | 2186 | MS, RI, S, O | 83.35 ± 16.6 | 96.39 ± 27.33 | 64.89 ± 6.28 | 167.54 ± 13.07 |
| Nonanoic acid | 41.583 | 2183 | 2192 | MS, RI | 182.91 ± 65.85 | 369.44 ± 63.03 | 530.72 ± 335.93 | 283.5 ± 17.01 |
| Palmitic acid ethyl ester | 43.804 | 2251 | 2248 | MS, RI | 122.12 ± 27.93 | 145.01 ± 94.92 | 139.31 ± 17.11 | 192.8 ± 16.95 |
| Myristicin | 44.548 | 2274 | 2258 | MS, RI | 0 | 0 | 926.59 ± 96.33 | 0 |
| N-decanoic acid | 44.956 | 2286 | 2300 | MS, RI | 0 | 41.13 ± 8.35 | 39.16 ± 19.4 | 44.66 ± 7.63 |
| 2,4-di-tert-butylphenol | 45.74 | 2309 | 2315 | MS, RI | 284.77 ± 153.05 | 461.93 ± 48.1 | 510.82 ± 211.08 | 406.54 ± 175.48 |
| Benzoic acid | 50.683 | - | - | MS | 0 | 0 | 1287.17 ± 292.22 | 0 |
| Compound | Ions (m/z) a | Calibration Equation b | R2 | Odor Description c |
|---|---|---|---|---|
| Propanal | 58.1, 57.1, 44.0, 42.0 | 0.9905 | Pungent | |
| Ethyl acetate | 43.1, 61.1, 70.1, 45.1 | 0.9945 | Fruit, wine | |
| Octanal | 41.1, 43.1, 57.1, 84.1 | 0.9983 | Citrus, fresh | |
| Nonanal | 57.1, 41.1, 55.1, 98.1 | 0.9994 | Citrus, fatty | |
| 1-octen-3-ol | 57.1, 43.1, 72.1, 85.1 | 0.9989 | Green, mushroom | |
| (E)-2-octenal | 70.1, 55.1, 41.1, 83.1 | 0.9908 | Unpleasant, nut, fatty | |
| Acetic acid | 43.1, 45.0, 60.1, 42.1 | 0.9980 | Vinegar, sour | |
| Benzaldehyde | 105.1, 106.1, 77.1, 55.1 | 0.9948 | Almond, cherry-like | |
| 1-nonanol | 56.1, 70.1, 41.1, 83.1 | 0.9996 | Rose, floral | |
| Methyl salicylate | 120.1, 92.1, 152.1, 65.1 | 0.9906 | ||
| Guaiacol | 109.1, 124.1, 81.1, 53.1 | 0.9997 | Smoky, sweet | |
| Geranylacetone | 69.1, 43.1, 151.1, 136.1 | 0.9993 | Sweet, rose | |
| Phenylethyl alcohol | 91.1, 92.1, 122.1, 65.1 | 0.9948 | Floral, rose, honey | |
| Creosol | 138.1, 123.1, 95.1, 67.1 | 0.9984 | Dusty, woody | |
| 4-ethylguaiacol | 137.1, 152.1, 122.1, 91.1 | 0.9989 | Smoky | |
| Anisaldehyde | 135.1, 136.1, 77.1, 92.1 | 0.9960 | Phenolic | |
| P-cresol | 107.1, 108.1, 77.1, 79.1 | 0.9997 | Phenolic, animal | |
| 4-ethyl-phenol | 107.1, 122.1, 77.1, 91.1 | 0.9986 | Herbal, phenolic |
| Compound | Threshold a (μg/kg in Water) | LZ | GL | NN | BS |
|---|---|---|---|---|---|
| Propanal | 60 b | 32.83 | 15.83 | 0 | 0 |
| Ethyl acetate | 97.8 c | 30.83 | 0.12 | 9.75 | 40.03 |
| Octanal | 0.7 d | 34.56 | 31.56 | 66.14 | 25.17 |
| Nonanal | 1.1 e | 4.71 | 8.15 | 10.31 | 2.61 |
| 1-octen-3-ol | 1 d | 9.09 | 28.1 | 36.46 | 10.75 |
| (E)-2-octenal | 3 f | 5.4 | 3.87 | 10.12 | 3.87 |
| Acetic acid | 1200 b | 3537.23 | 2674.52 | 3872.09 | 2155.79 |
| Benzaldehyde | 50 b | 0.06 | 0.12 | 0.71 | 0 |
| 1-nonanol | 2 g | 3.04 | 2.97 | 2.91 | 3.13 |
| Methyl salicylate | 60 h | 0.06 | 0.05 | 0.15 | 0.05 |
| Guaiacol | 0.84 i | 79.68 | 95.93 | 118.32 | 29.62 |
| Geranylacetone | 10 b | 0.12 | 0 | 0.43 | 0 |
| Phenylethyl alcohol | 53.95 e | 1.64 | 5.33 | 2.53 | 0.22 |
| Creosol | 8.92 e | 30.27 | 14.44 | 16.96 | 41.8 |
| 4-ethylguaiacol | 1.3 j | 21.63 | 4.91 | 1.15 | 23.11 |
| Anisaldehyde | 10 b | 4.05 | 3.70 | 3.78 | 3.84 |
| P-cresol | 3.9 e | 38684.24 | 57826.67 | 34997.95 | 71409.51 |
| 4-ethyl-phenol | 13 i | 2.42 | 2.71 | 2.02 | 4.25 |
| Odorants Omitted from the Recombination Model | N a | Significance b |
|---|---|---|
| Propanal | 5 | - |
| Ethyl acetate | 4 | - |
| Octanal | 6 | * |
| Nonanal | 3 | - |
| 1-octen-3-ol | 4 | - |
| (E)-2-octenal | 6 | * |
| Acetic acid | 8 | *** |
| 1-nonanol | 3 | - |
| Guaiacol | 8 | *** |
| Phenylethyl alcohol | 7 | ** |
| Creosol | 7 | ** |
| 4-ethylguaiacol | 6 | * |
| Anisaldehyde | 3 | - |
| P-cresol | 9 | *** |
| 4-ethyl-phenol | 2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Tian, Y.; Sun, M.; Liu, J.; Bai, Y.; Liu, X.; Guo, Y. Characterization of Key Aroma Compounds in Fermented Bamboo Shoots Using Gas Chromatography-Olfactometry-Mass Spectrometry, Odor Activity Values, and Aroma Recombination Experiments. Foods 2022, 11, 2106. https://doi.org/10.3390/foods11142106
Li S, Tian Y, Sun M, Liu J, Bai Y, Liu X, Guo Y. Characterization of Key Aroma Compounds in Fermented Bamboo Shoots Using Gas Chromatography-Olfactometry-Mass Spectrometry, Odor Activity Values, and Aroma Recombination Experiments. Foods. 2022; 11(14):2106. https://doi.org/10.3390/foods11142106
Chicago/Turabian StyleLi, Shubo, Yufeng Tian, Minghao Sun, Jiaojiao Liu, Yunxia Bai, Xiaoling Liu, and Yuan Guo. 2022. "Characterization of Key Aroma Compounds in Fermented Bamboo Shoots Using Gas Chromatography-Olfactometry-Mass Spectrometry, Odor Activity Values, and Aroma Recombination Experiments" Foods 11, no. 14: 2106. https://doi.org/10.3390/foods11142106
APA StyleLi, S., Tian, Y., Sun, M., Liu, J., Bai, Y., Liu, X., & Guo, Y. (2022). Characterization of Key Aroma Compounds in Fermented Bamboo Shoots Using Gas Chromatography-Olfactometry-Mass Spectrometry, Odor Activity Values, and Aroma Recombination Experiments. Foods, 11(14), 2106. https://doi.org/10.3390/foods11142106