Structural and Rheological Properties of Yanang Gum (Tiliacora triandra)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structural Characterization
2.2.1. Chemical Composition
2.2.2. Linkage Analysis (Methylation and GC-MS of Partially Methylated Alditol Acetates (PMAA))
2.2.3. Spectroscopic Analysis FT-IR and NMR
2.3. Molecular Characterization
2.4. Rheological Measurements
2.4.1. Preparation of Solutions
2.4.2. Steady Shear Measurements
2.4.3. Dynamic Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Monosaccharide Compositions
3.2. Glycosidic Linkage Position
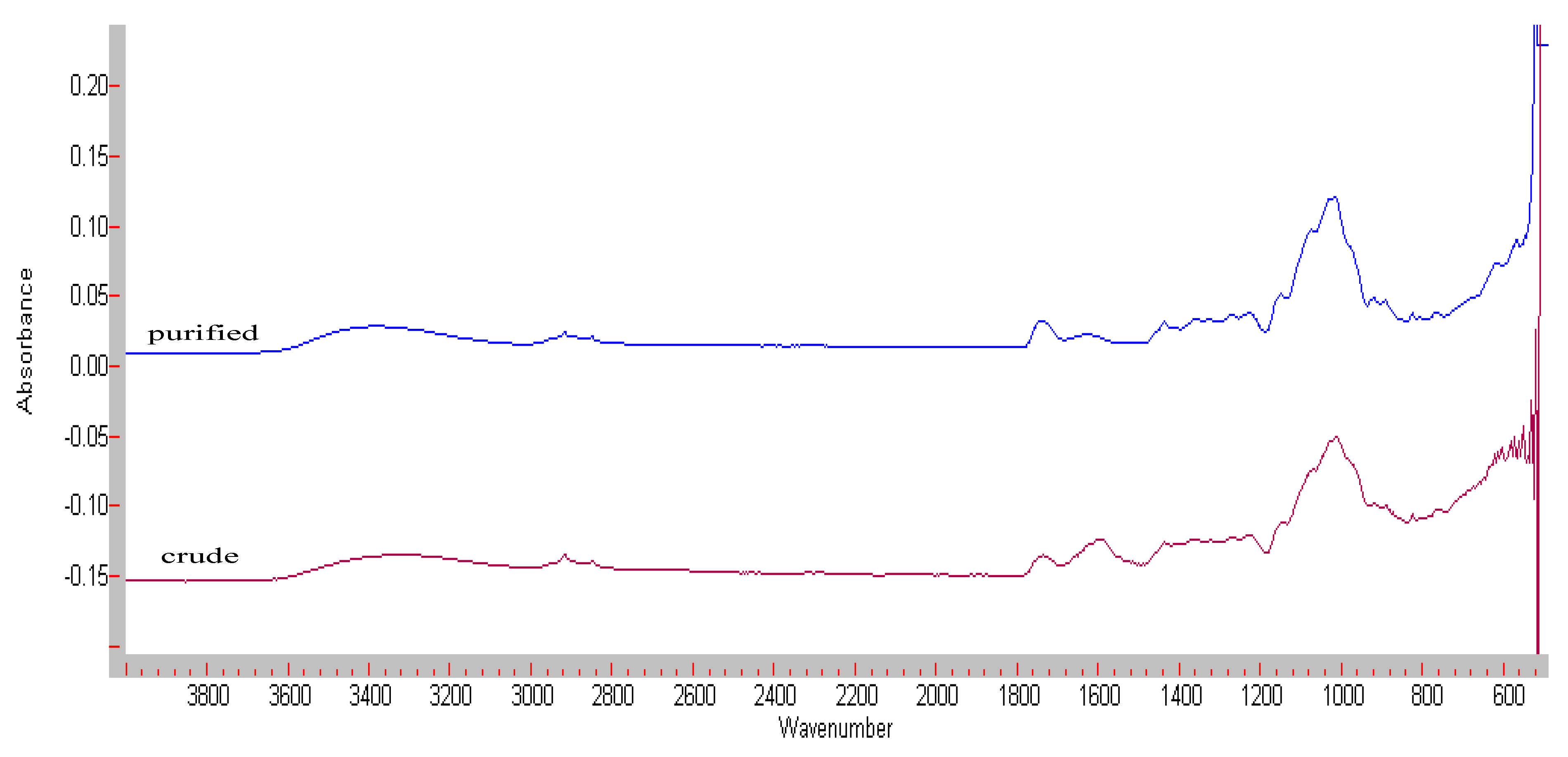
3.3. FT-IR Spectroscopy
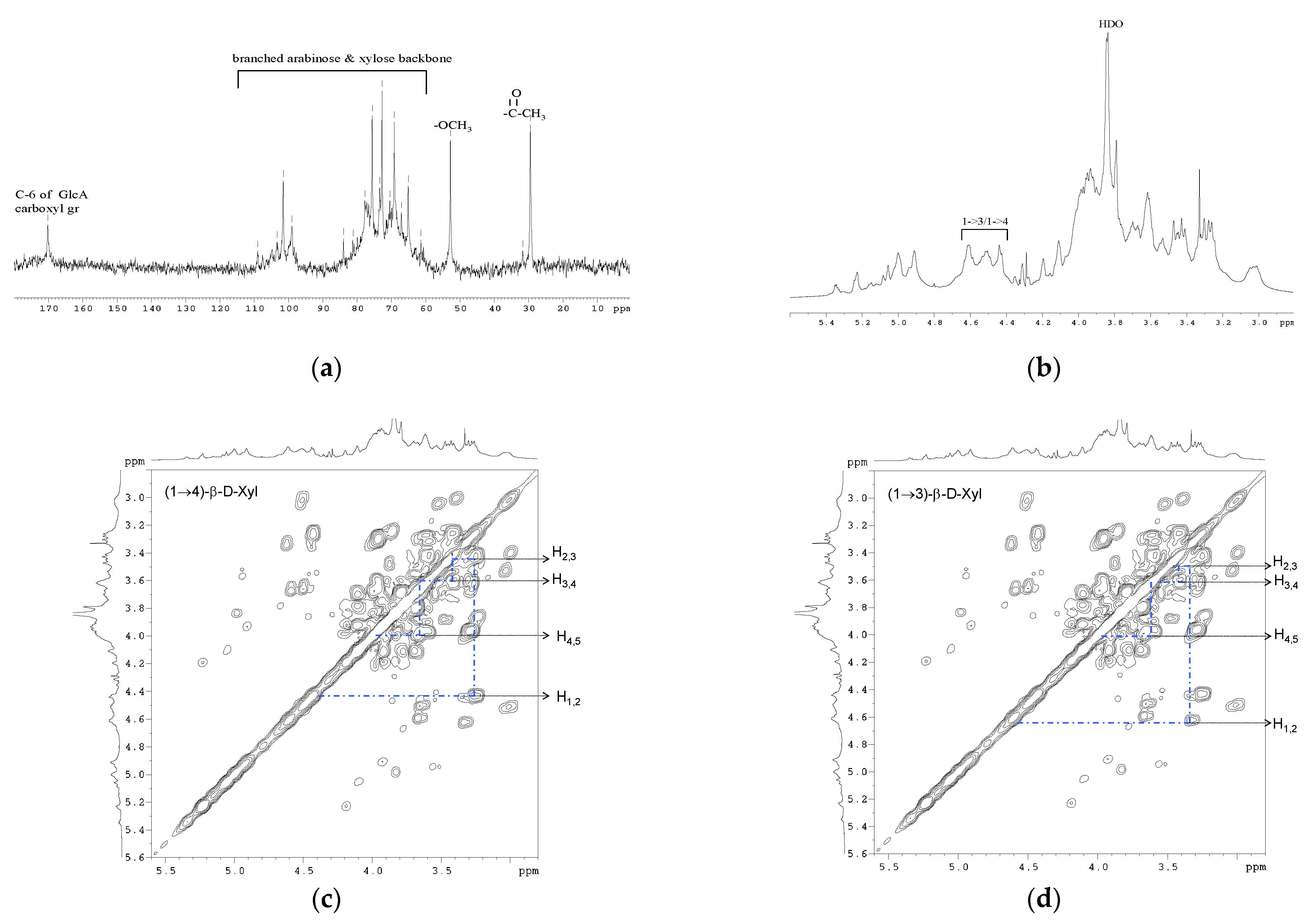
3.4. NMR Spectroscopy
3.5. Molecular Characterization
3.6. Rheological Properties
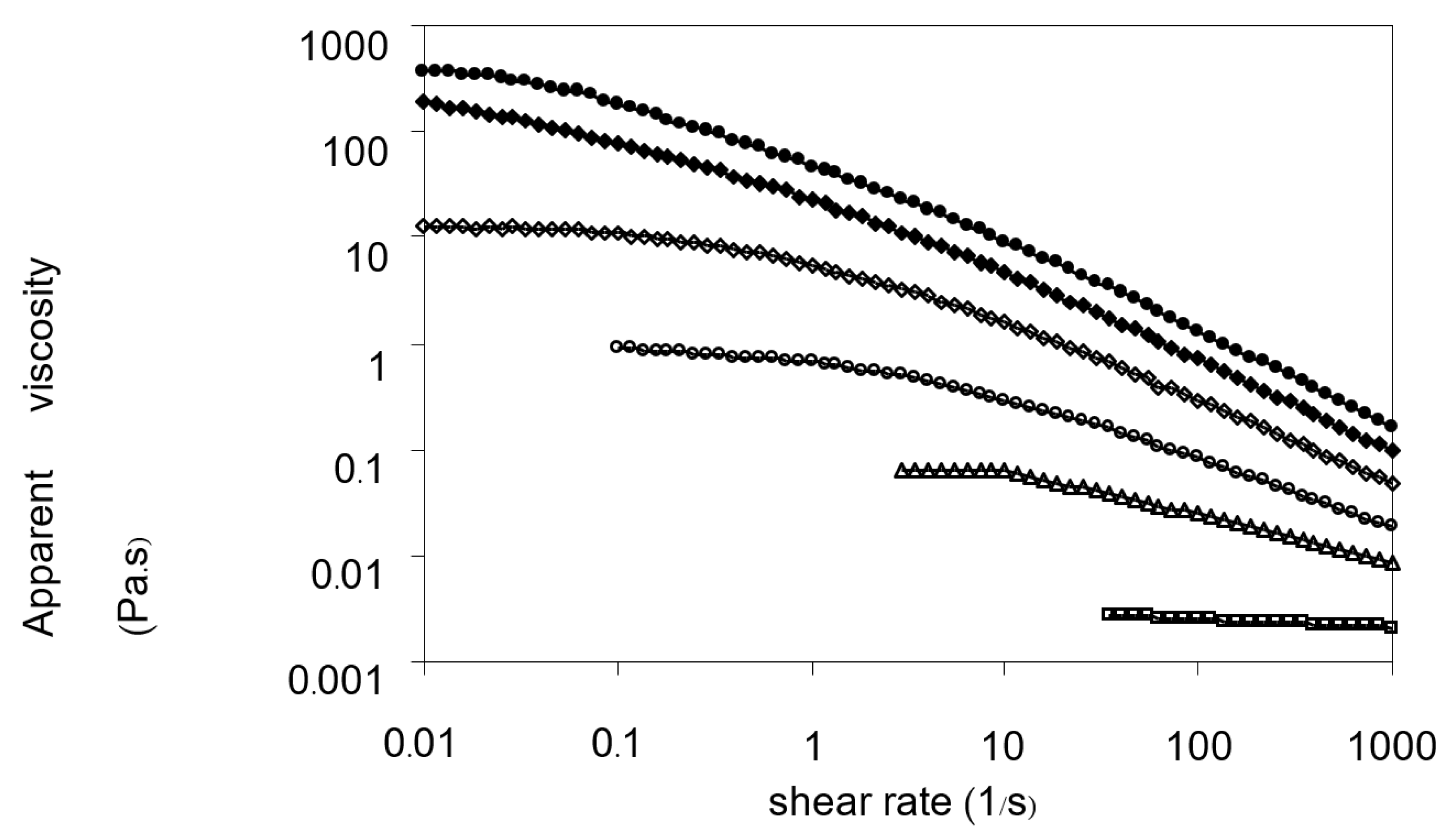
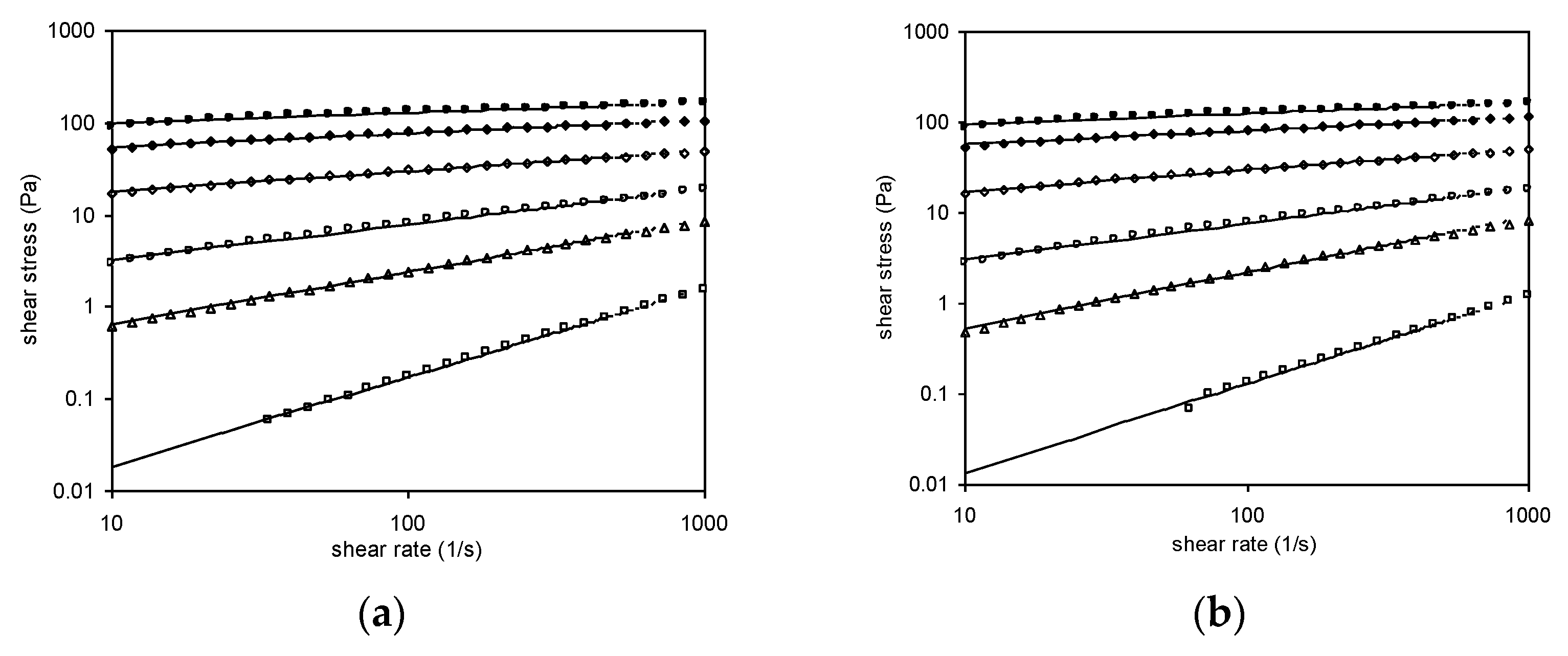
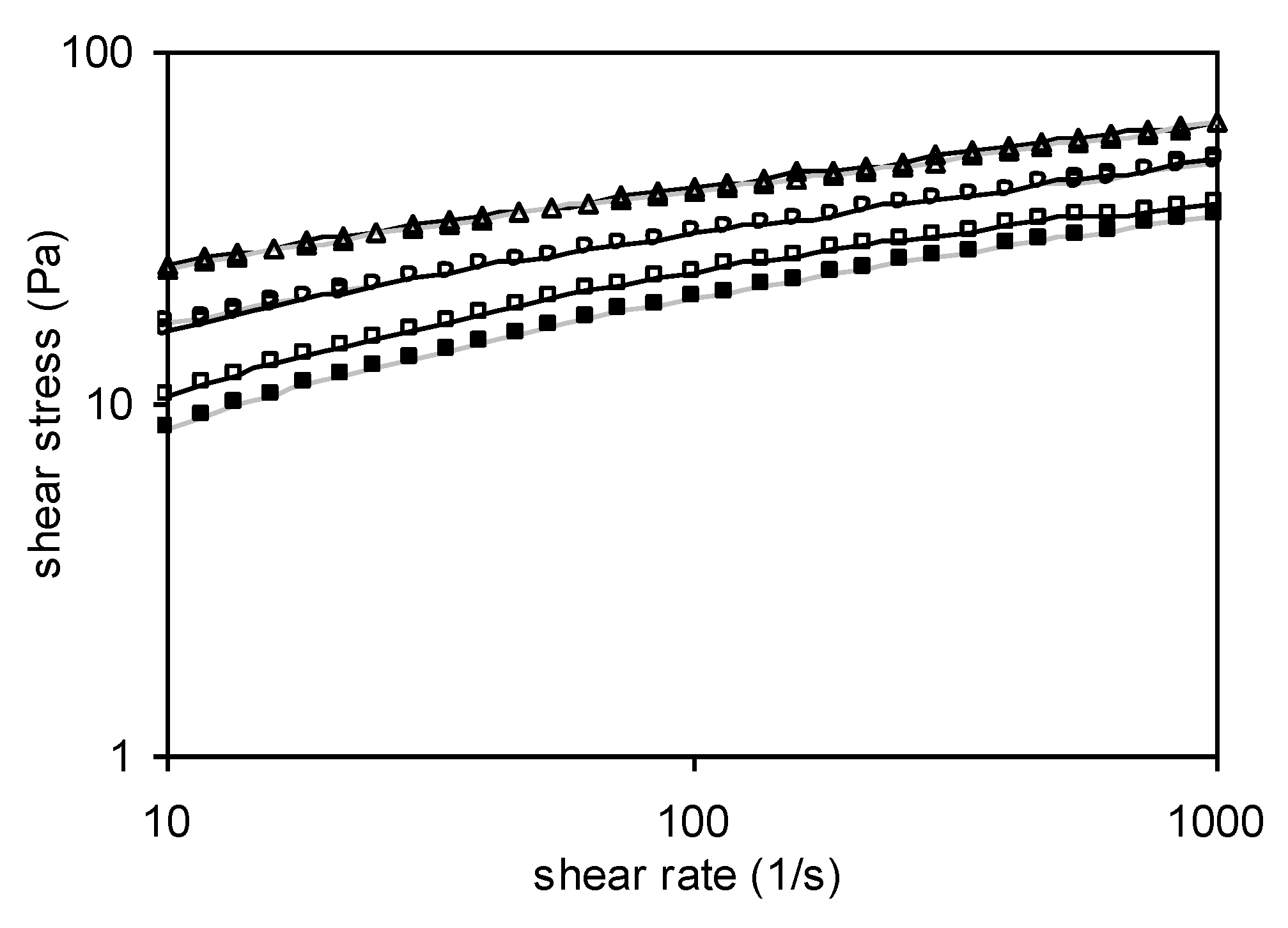
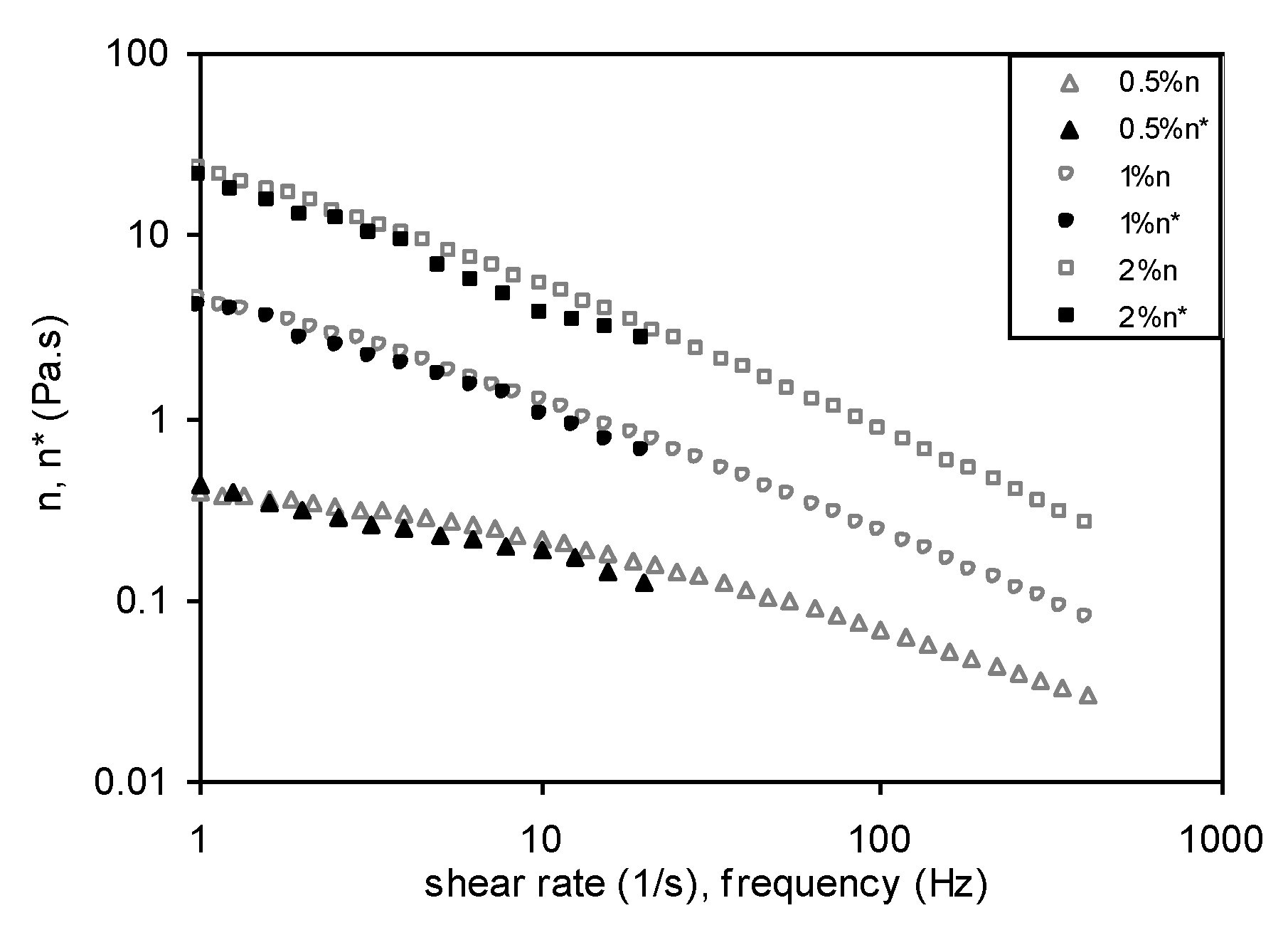
3.6.1. Steady Shear Measurements
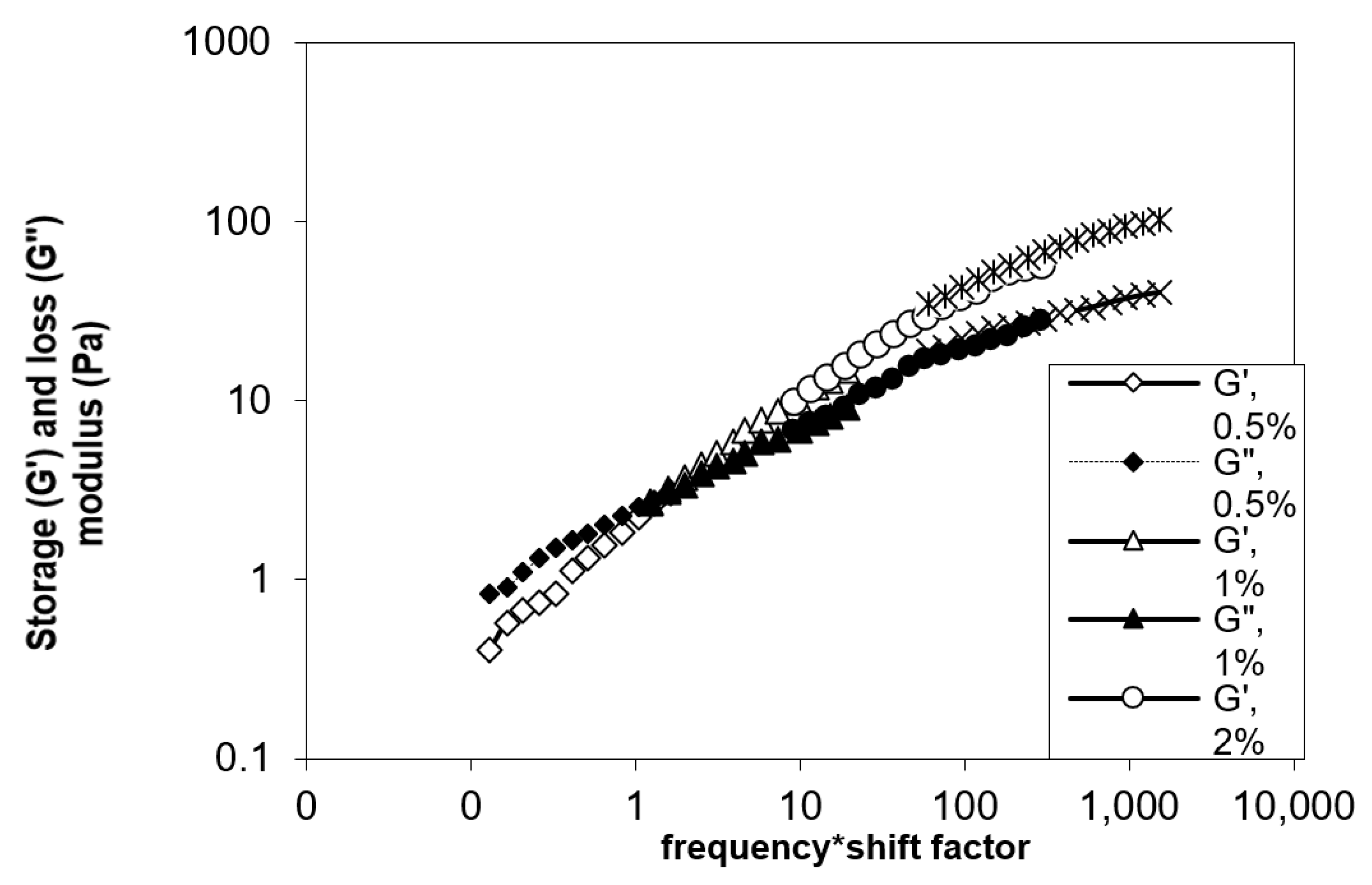
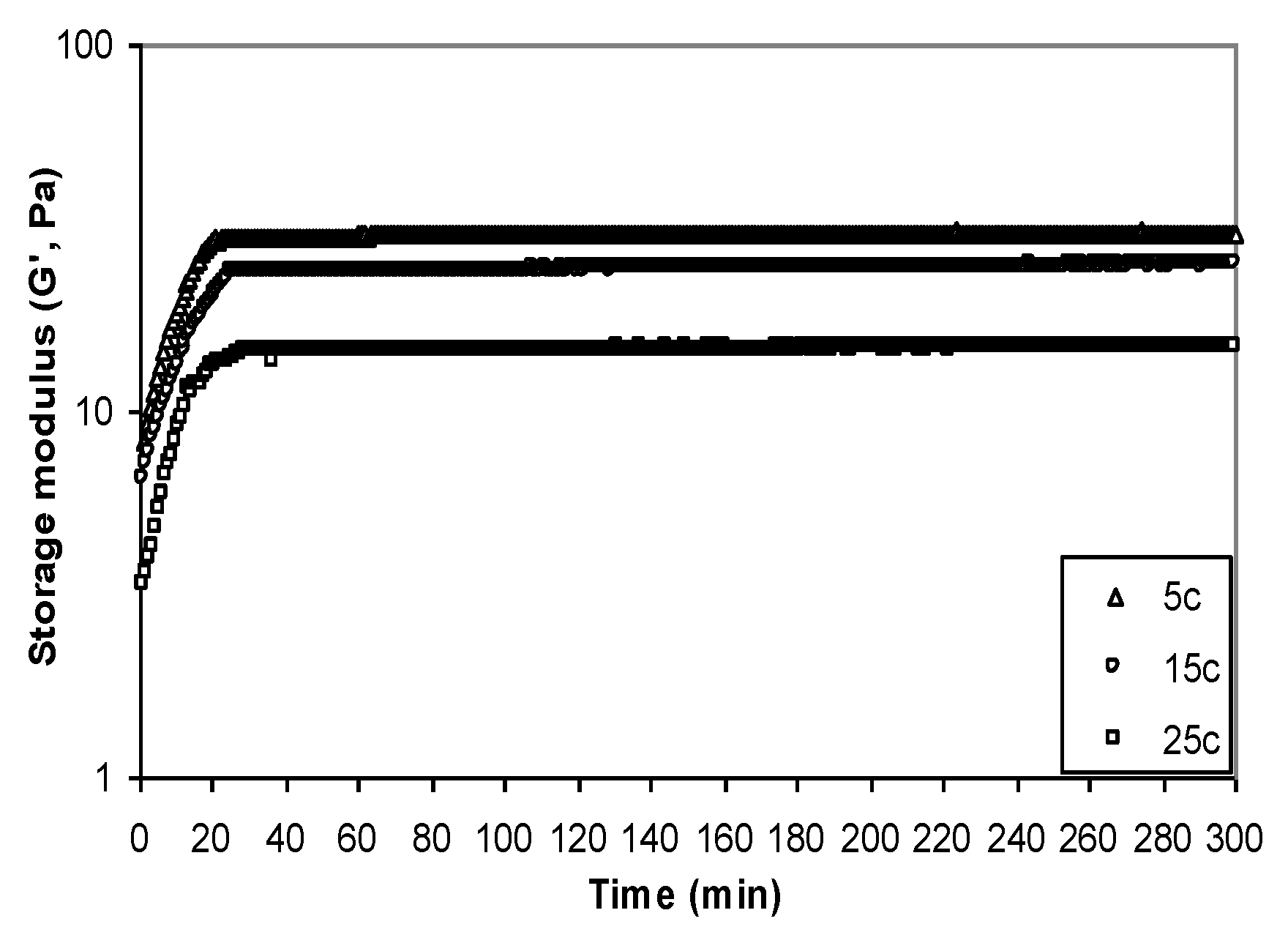
3.6.2. Dynamic Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singthong, J.; Ningsanond, S.; Cui, S.W. Extraction and physicochemical characterization of polysaccharide gum from Yanang (Tiliacora triandra) leaves. Food Chem. 2009, 114, 1301–1307. [Google Scholar] [CrossRef]
- Chaikham, P.; Kemsawasd, V.; Seesuriyachan, P. Spray drying probiotics along with maoluang juice plus Tiliacora triandra gum for exposure to the in vitro gastrointestinal environments. LWT-Food Sci. Technol. 2017, 78, 31–40. [Google Scholar] [CrossRef]
- Singthong, J.; Oonsivilai, R.; Oonmetta-aree; Ningsanond, S. Bioactive compounds and encapsulation of Yanang (Tiliacora Triandra) leaves. Afr. J. Tradit. Complement. Altern. Med. 2014, 931–932, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wood, P.J.; Huang, X.; Cui, W. Preparation and characterization of molecular weight standards of low polydispersity from oat and barley (1→3) (1→4)-β-D-glucan. Food Hydrocoll. 2003, 17, 845–853. [Google Scholar] [CrossRef]
- Blanshard, J.M.V.; Mitchell, J.R. Polysaccharides in Food; Butterworth: Massachusetts, MA, USA, 1978. [Google Scholar]
- BeMiller, J.N.; Whistler, R.L. Carbohydrates. In Food Chemistry, 3rd ed.; Fennema, O.R., Ed.; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Işikli, N.D.; Karababa, E. Rheological characterization of fenugreek paste (cemen). Food Eng. 2005, 69, 185–190. [Google Scholar] [CrossRef]
- Eliason, A.-C. Carbohydrates in Food; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Singthong, J.; Ningsanond, S.; Cui, S.W.; Goff, H.D. Extraction and physicochemical characterization of Krueo Ma Noy pectin. Food Hydrocoll. 2005, 19, 793–801. [Google Scholar] [CrossRef]
- Singthong, J.; Ningsanond, S.; Cui, S.W.; Goff, H.D. Structural characterization, degree of esterification and some gelling of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid and Semisolid Foods: Principle and Application; Aspen: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Biermann, C.J.; McGinnis, G.D. Analysis of Carbohydrates by GLC and MS; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Shi, L.; Dong, Q.; Ding, K. Structure elucidation and immunomodulatory activity in vitro of xylan from roots of Cudrania tricuspidata. Food Chem. 2014, 152, 291–296. [Google Scholar] [CrossRef]
- Kabel, M.A.; Borne, H.; Vincken, J.-P.; Voragen, A.G.J.; Schols, H.A. Structural differences of xylans affect their interaction with cellulose. Carbohydr. Polym. 2007, 69, 94–105. [Google Scholar] [CrossRef]
- Åkerholm, M.; Salmén, L. Interactions between wood polymers studied by dynamic FT-IR spectroscopy. Polymer 2001, 42, 963–969. [Google Scholar] [CrossRef]
- Cerná, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Copìková, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Kačuráková, M.; Wilson, R.H. Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 2001, 44, 291–303. [Google Scholar] [CrossRef]
- Deniaud, E.; Quemener, B.; Fleurence, J.; Lahaye, M. Structural studies of the mix-linked β-(1→3)/β-(1→4)-D-xylans from the cell wall of Palmaria palmate (Rhodophyta). Int. J. Biol. Macromol. 2003, 33, 9–18. [Google Scholar] [CrossRef]
- Lahaye, M.; Rondeau-Mouro, C.; Deniaud, E.; Buléon, A. Solid-state 13C NMR spectroscopy studies of xylans in the cell wall of Palmaria palmate (L. Kuntze, Rhodophyta). Carbohydr. Res. 2003, 38, 1559–1569. [Google Scholar] [CrossRef]
- Simas, F.F.; Gorin, P.A.J.; Guerrini, M.; Naggi, A.; Sassaki, G.L.; Delgobo, C.L.; Iacomini, M. Structure of a heteroxylan of gum exudates of the palm Scheelea phalerata (uricuri). Phytochemistry 2004, 65, 2347–2355. [Google Scholar] [CrossRef]
- Marvelys, L.; Maritza, M.; Lilian, S.; Gladys, L.; Julio, H. Structural elucidation of the polysaccharide from Sterculia apetala gum by a combination of chemical methods and NMR spectroscopy. Food Hydrocoll. 2006, 20, 908–913. [Google Scholar] [CrossRef]
- Sarbu, A.; Gonçalves, F.; Norberta de Pinho, M. Oat spelts xylan molecular mass estimation by size exclusion chromatography. Carbohydr. Polym. 2003, 53, 297–304. [Google Scholar] [CrossRef]
- Girhammar, U.; Nair, B.M. Isolation, separation and characterization of water-soluble non-starch polysaccharides from wheat and rye. Food Hydrocoll. 1992, 6, 285–299. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Rigby, N.M.; Selvendran, R.R.; Waldron, K.W. Investigation of occurrence of xylan-xyloglucan complexes in the cell walls of olive pulp (Olea europaea). Carbohydr. Polym. 1995, 27, 277–284. [Google Scholar] [CrossRef]
- Shiga, T.M.; Lajolo, F.M. Cell wall polysaccharides of common beans (Phaseolus vulgaris L.)-composition and structure. Carbohydr. Polym. 2006, 63, 1–12. [Google Scholar] [CrossRef]
- Sims, I.M.; Newman, R.H. Structural studies of acidic xylans exuded from leaves of the monocotyledonous plants Phormium tenax and Phormium cookianum. Carbohydr. Polym. 2006, 63, 379–384. [Google Scholar] [CrossRef]
- Abu-Jdayil, B.; Al-Malah, K.; Asoud, H. Rheological characterization of milled sesame (tehineh). Food Hydrocoll. 2002, 16, 55–61. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sanchez, B.; Katthain, R. Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Lapasin, R.; Pricl, S. Rheology of Industrial Polysaccharides: Theory and Applications; Blackie Academic & Professional: London, UK, 1995. [Google Scholar]
- Ross-Murphy, S.B. Structure-property relationships in food biopolymer gels and solutions. J. Rheol. 1995, 39, 1451–1463. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Zhang, L. Rheological behavior of Aeromonas gum in aqueous solutions. Food Hydrocoll. 2006, 20, 723–729. [Google Scholar] [CrossRef]
- Ren, Y.; Ellis, P.R.; Sutherland, I.W.; Ross-Murphy, S.B. Dilute and semi-dilute solution properties of an exopolysaccharide from Escherichia coli strain S61. Carbohydr. Polym. 2003, 52, 189–195. [Google Scholar] [CrossRef]



| Chemical Name | Deduced Linkage | Relative Abundance (%) * |
|---|---|---|
| 1,4-di-O-acetyl-(1-deuterio)-2,3,5-tri-O-methyl pentitol | Terminal-Araf | 5.755 ± 0.18 |
| 1,3,4,5-tetra-O-acetyl-(1-deuterio)-2-O-methyl pentitol | 1,3,5-Araf | 0.504 ± 0.08 |
| 1,5-di-O-acetyl-(1-deuterio)-2,3,4-tri-O-methyl pentitol | Terminal Xylp | 14.553 ± 0.54 |
| 1,3,5-tri-O-acetyl-(1-deuterio)-2,4-di-O-methyl pentitol | 1,3-Xylp | 21.884 ± 0.64 |
| 1,4,5-tri-O-acetyl-(1-deuterio)-2,3-di-O-methyl pentitol | 1,4-Xylp | 49.637 ± 0.71 |
| 1,2,3,5-tetra-O-acetyl-(1-deuterio)-4-O-methyl pentitol | 1,2,3-Xylp | 0.549 ± 0.08 |
| 1,2,4,5-tetra-O-acetyl-(1-deuterio)-3,6-di-O-methyl hexitol | 1,2,4-Galp | 1.687 ± 0.09 |
| 1,2,3,5-tetra-O-acetyl-(1-deuterio)-4,6-di-O-methyl hexitol | 1,2,3-Galp | 3.767 ± 0.12 |
| 1,3,5,6-tetra-O-acetyl-(1-deuterio)-2,4-di-O-methyl hexitol | 1,3,6-Glup | 1.663 ± 0.07 |
| Type of Linkage | Chemical Shifts (ppm) | ||||
|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | |
| 1,3-Xylp | 4.64 | 3.36 | 3.49 | 3.65 | 3.98/3.77 |
| 1,4-Xylp | 4.46 | 3.29 | 3.46 | 3.57 | 3.98/3.65 |
| Concentration (%) | Forward Measurements | Backward Measurements | ||||
|---|---|---|---|---|---|---|
| k (Pa·sn) | n | R2 | k (Pa·sn) | n | R2 | |
| 0.10 | 0.0020 | 0.9711 | 0.9995 | 0.0014 | 0.9817 | 0.9968 |
| 0.25 | 0.1716 | 0.5716 | 0.9991 | 0.1297 | 0.6140 | 0.9962 |
| 0.50 | 1.3133 | 0.3889 | 0.9957 | 1.2028 | 0.4004 | 0.9959 |
| 1.00 | 10.5880 | 0.2248 | 0.9960 | 10.1860 | 0.2330 | 0.9952 |
| 1.50 | 39.71 | 0.1452 | 0.9903 | 39.308 | 0.1520 | 0.9889 |
| 2.00 | 75.581 | 0.1143 | 0.9743 | 72.474 | 0.1193 | 0.9721 |
| Temperature (°C) | Forward Measurements | Backward Measurements | ||||
|---|---|---|---|---|---|---|
| k (Pa·sn) | n | R2 | k (Pa·sn) | n | R2 | |
| 5 | 16.231 | 0.2002 | 0.9981 | 15.548 | 0.2021 | 0.9983 |
| 15 | 13.712 | 0.2064 | 0.9977 | 12.949 | 0.2156 | 0.9971 |
| 25 | 10.588 | 0.2248 | 0.9960 | 10.186 | 0.2330 | 0.9952 |
| 35 | 10.545 | 0.2294 | 0.9889 | 10.543 | 0.2419 | 0.9930 |
| 45 | 9.6783 | 0.2397 | 0.9777 | 8.9007 | 0.2632 | 0.9877 |
| 55 | 7.8844 | 0.2529 | 0.9886 | 7.7036 | 0.2751 | 0.9895 |
| 65 | 6.4982 | 0.2675 | 0.9785 | 4.9293 | 0.2928 | 0.9854 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singthong, J.; Oonsivilai, R. Structural and Rheological Properties of Yanang Gum (Tiliacora triandra). Foods 2022, 11, 2003. https://doi.org/10.3390/foods11142003
Singthong J, Oonsivilai R. Structural and Rheological Properties of Yanang Gum (Tiliacora triandra). Foods. 2022; 11(14):2003. https://doi.org/10.3390/foods11142003
Chicago/Turabian StyleSingthong, Jittra, and Ratchadaporn Oonsivilai. 2022. "Structural and Rheological Properties of Yanang Gum (Tiliacora triandra)" Foods 11, no. 14: 2003. https://doi.org/10.3390/foods11142003
APA StyleSingthong, J., & Oonsivilai, R. (2022). Structural and Rheological Properties of Yanang Gum (Tiliacora triandra). Foods, 11(14), 2003. https://doi.org/10.3390/foods11142003






