Aqueous Extract of Phyllanthus emblica L. Alleviates Functional Dyspepsia through Regulating Gastrointestinal Hormones and Gut Microbiome In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Identification of Aqueous Extract from Phyllanthus Emblica
2.2. Animal Treatment
2.3. Measurement and Analysis of Gastric Emptying (GE) Rate and Small Intestinal Transit Rate (SIT)
2.4. Detection of Serum Gastrointestinal Hormones
2.5. Microbiome Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition Characterization of APE
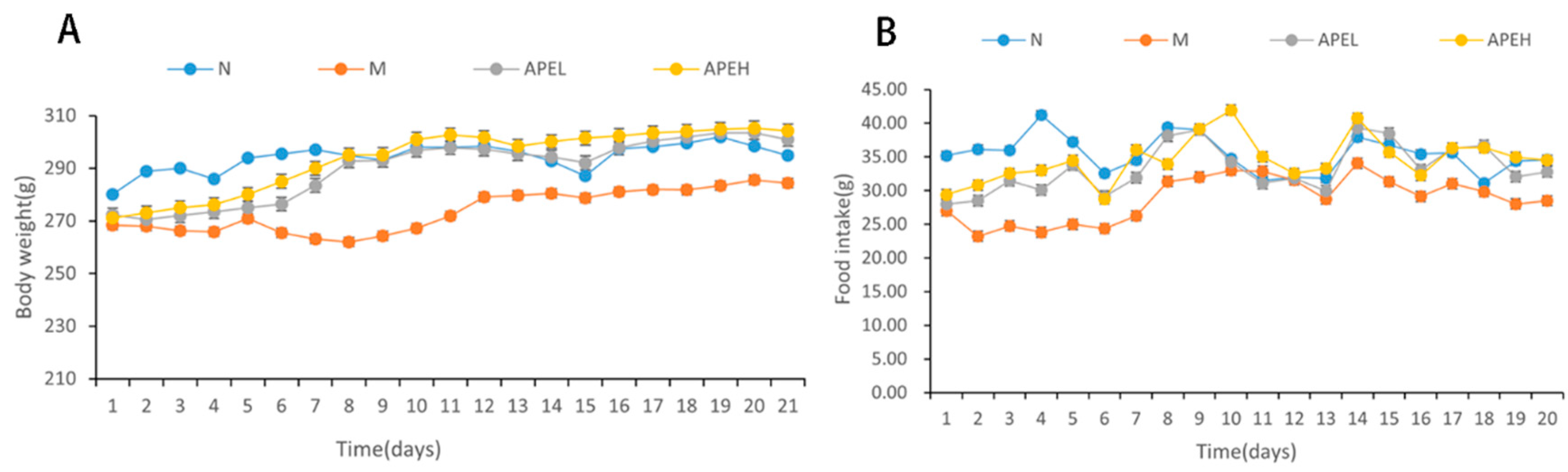
3.2. Food Intake and Body Weight
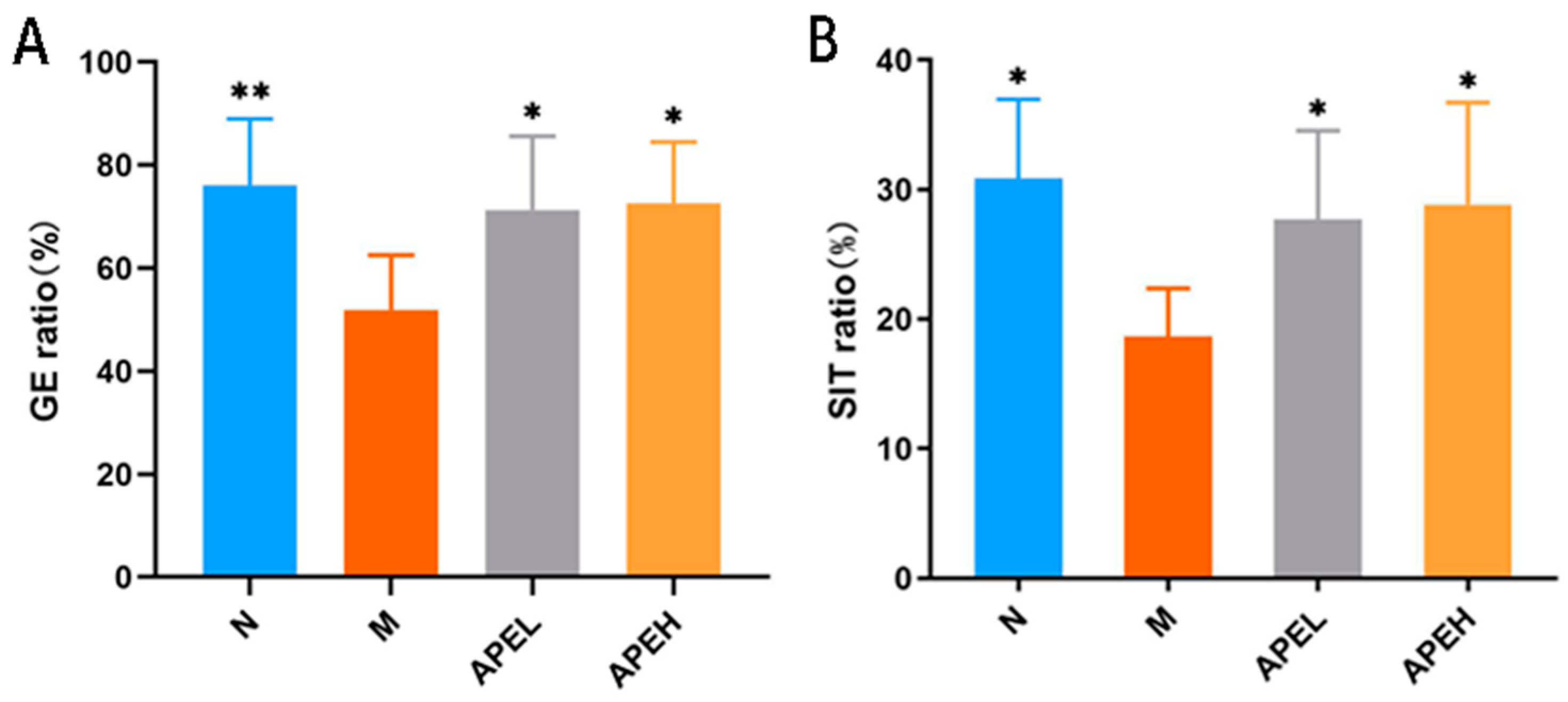
3.3. Gastrointestinal Motility
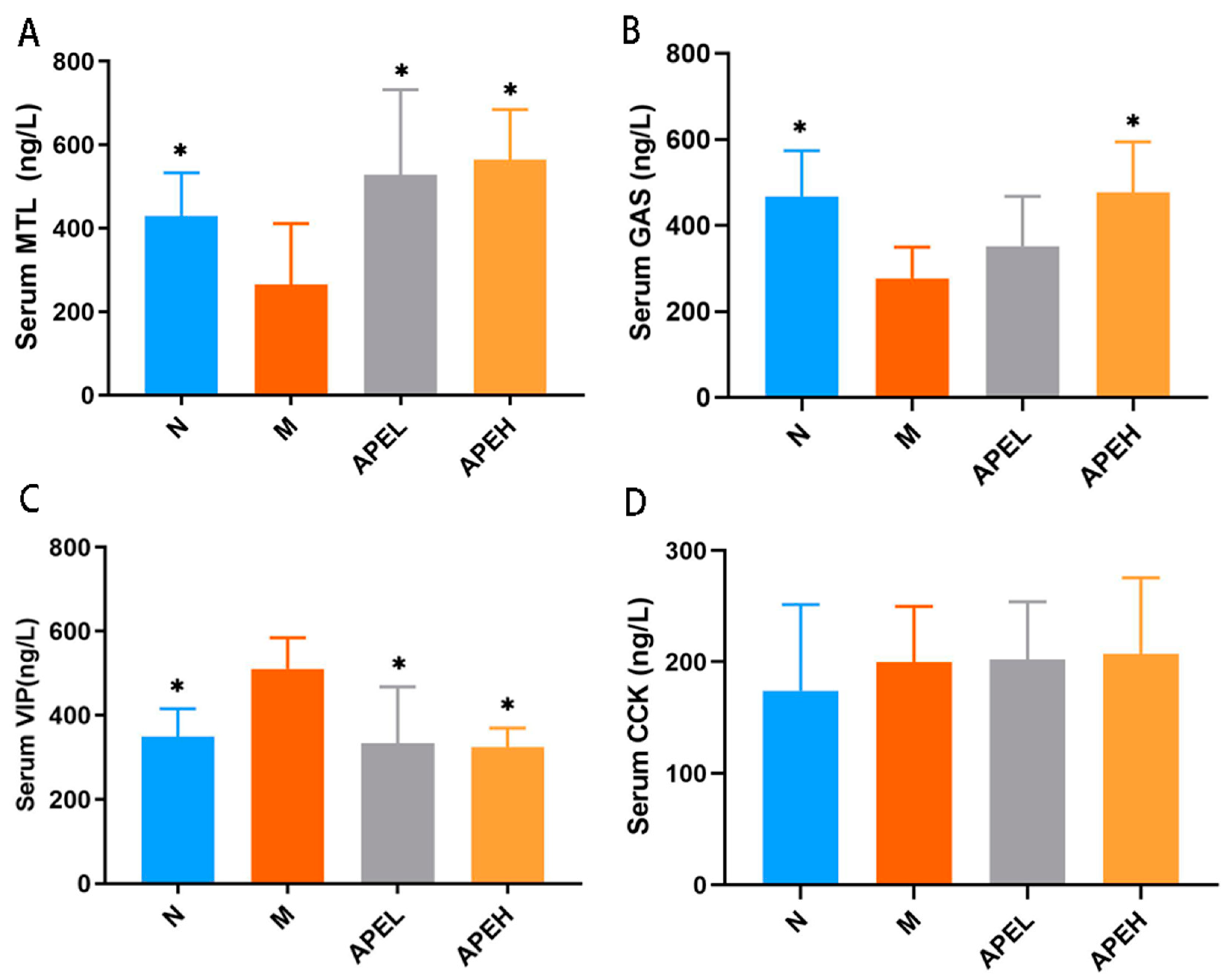
3.4. Serum Gastrointestinal Hormones
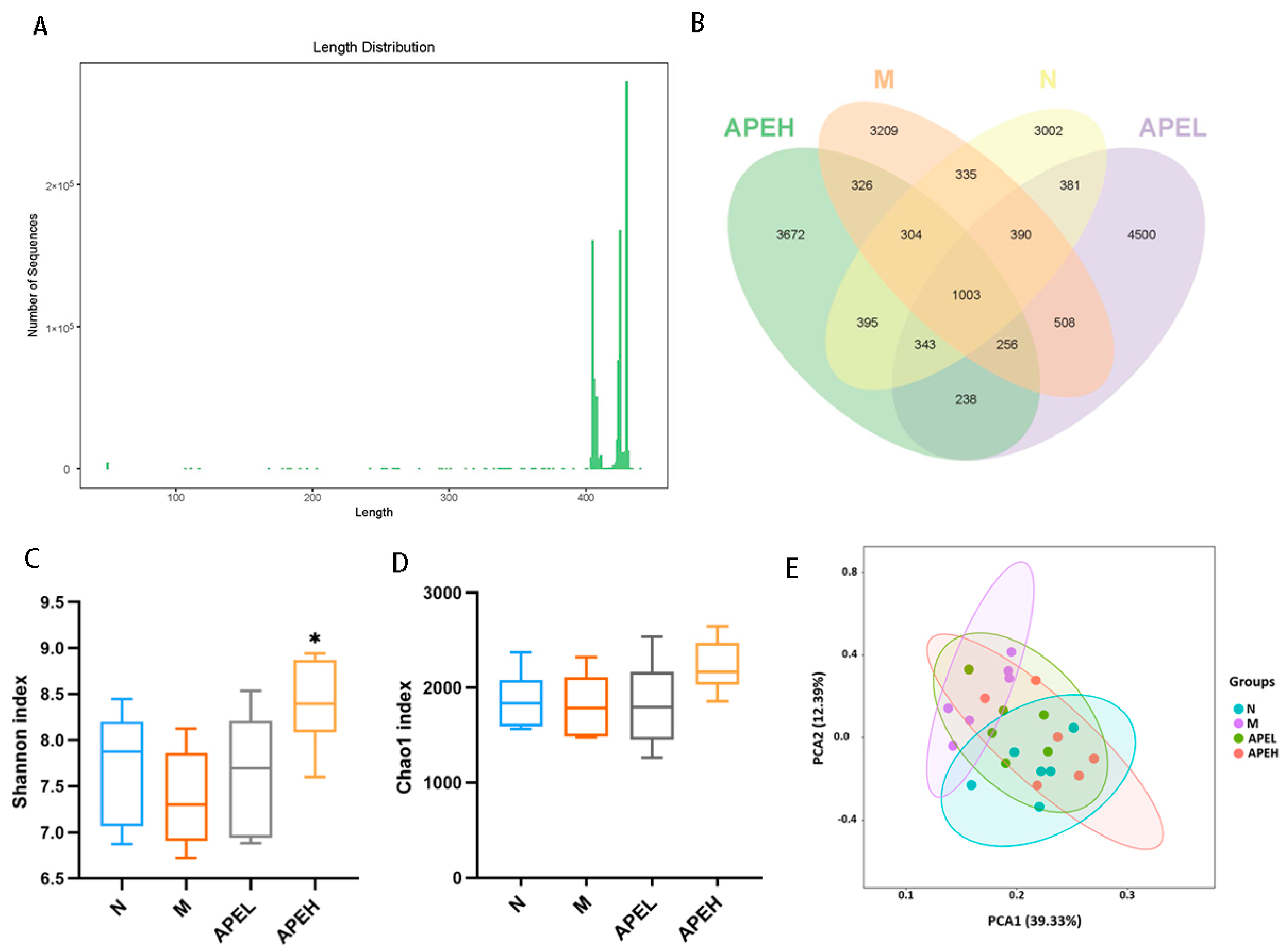
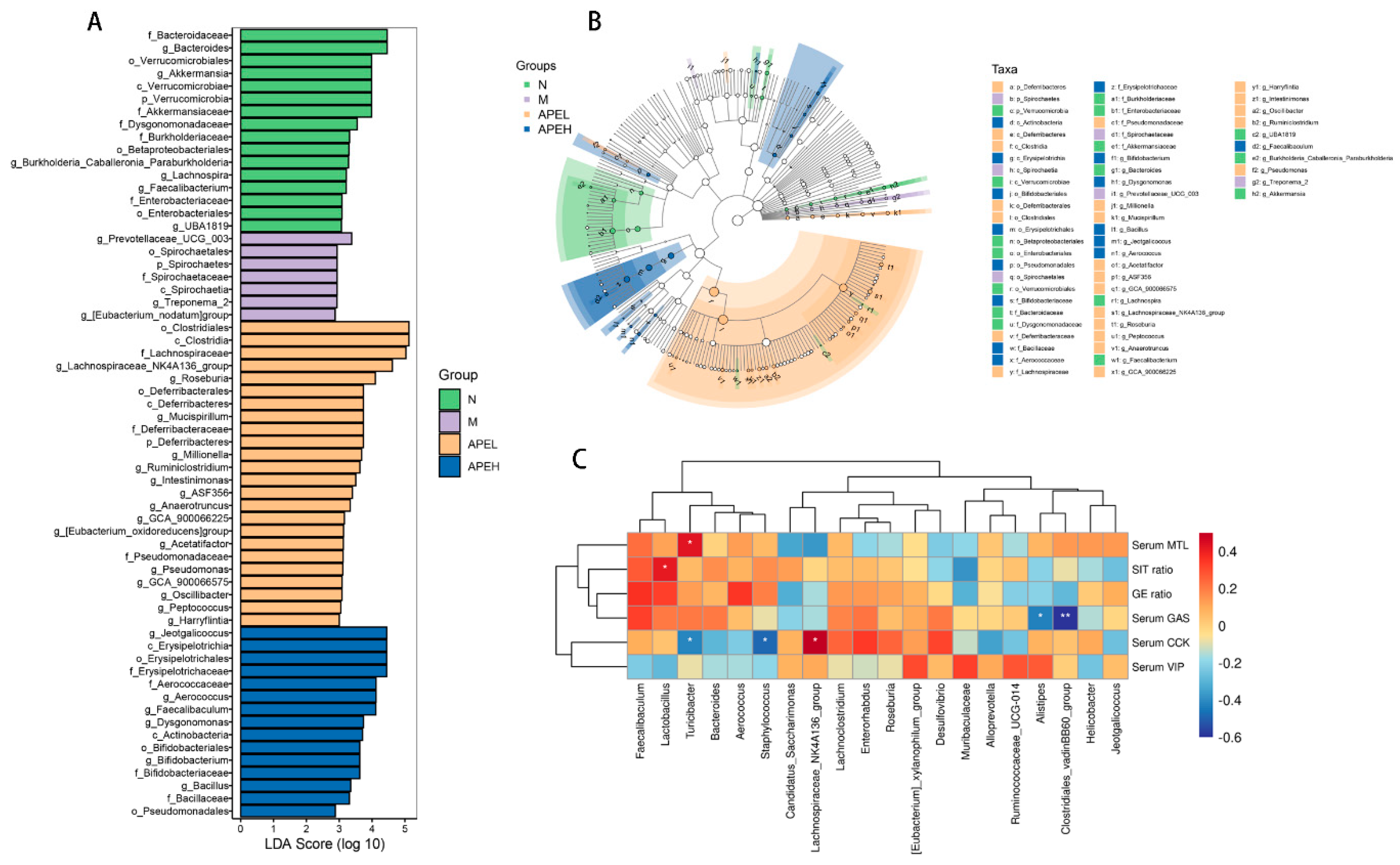
3.5. Microbiome Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wauters, L.; Talley, N.J.; Walker, M.M.; Tack, J.; Vanuytsel, T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 2019, 69, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Talley, N.J. Functional dyspepsia and duodenal eosinophilia: A new model. J. Dig. Dis. 2017, 18, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Walker, M.M.; Holtmann, G. Functional dyspepsia. Curr. Opin. Gastroenterol. 2016, 32, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahadeva, S.; Ford, A. Clinical and epidemiological differences in functional dyspepsia between the East and the West. Neurogastroenterol. Motil. 2015, 28, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Azpiroz, F.; Boeckxstaens, G.; Elsenbruch, S.; Feinle-Bisset, C.; Holtmann, G.; Lackner, J.M.; Ronkainen, J.; Schemann, M.; Stengel, A.; et al. Functional dyspepsia. Nat. Rev. Dis. Primers. 2017, 3, 17081. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Shanahan, E.R.; Raj, A.; Koloski, N.A.; Fletcher, L.; Morrison, M.; Walker, M.M.; Talley, N.J.; Holtmann, G. Dyspepsia and the microbiome: Time to focus on the small intestine. Gut 2016, 66, 1168–1169. [Google Scholar] [CrossRef]
- Ford, A.C.; Mahadeva, S.; Carbone, M.F.; Lacy, B.E.; Talley, N.J. Functional dyspepsia. Lancet 2020, 396, 1689–1702. [Google Scholar] [CrossRef]
- Ding, J.-H.; Jin, Z.; Yang, X.-X.; Lou, J.; Shan, W.-X.; Hu, Y.-X.; Du, Q.; Liao, Q.-S.; Xie, R.; Xu, J.-Y. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J. Gastroenterol. 2020, 26, 6141–6162. [Google Scholar] [CrossRef]
- Zhu, J.; Tong, H.; Ye, X.; Zhang, J.; Huang, Y.; Yang, M.; Zhong, L.; Gong, Q. The Effects of Low-Dose and High-Dose Decoctions of Fructus aurantii in a Rat Model of Functional Dyspepsia. Med. Sci. Monit. 2020, 26, e919815. [Google Scholar] [CrossRef]
- Ford, A.; Marwaha, A.; Sood, R.; Moayyedi, P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: A meta-analysis. Gut 2014, 64, 1049–1057. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, W.; Liu, X.; Hu, Y.; Zhu, X.; Ju, L.; Feng, F.; Qu, W.; Liu, W.; Xu, J. Digestive promoting effect and mechanism of Jiao Sanxian in rats. J. Ethnopharmacol. 2021, 278, 114334. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, C.; Wang, P.; Yang, L.; Wu, H.; Liu, H.; Qi, M.; Guo, Z.; Li, J.; Shi, H.; et al. Child compound Endothelium corneum attenuates gastrointestinal dysmotility through regulating the homeostasis of brain-gut-microbiota axis in functional dyspepsia rats. J. Ethnopharmacol. 2019, 240, 111953. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, B.; Aili, M.; Huo, S.; Han, M.-T.; Aibai, S.; Li, Z.-J. Effect of Artemisia rupestris L. Extract on Gastrointestinal Hormones and Brain-Gut Peptides in Functional Dyspepsia Rats. Evid. Based Complement. Altern. Med. 2020, 2020, 2528617. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Dsouza, J.J. Amla (Emblica officinalis Gaertn), a wonder berry in the treatment and prevention of cancer. Eur. J. Cancer Prev. 2011, 20, 225–239. [Google Scholar] [CrossRef]
- Wang, C.C.; Yuan, J.R.; Wang, C.F.; Yang, N.; Chen, J.; Liu, D.; Song, J.; Feng, L.; Tan, X.B.; Jia, X.B. Anti-inflammatory effects of Phyllanthus emblica L. on benzopyrene-induced precancerous lung lesion by regulating the IL-1β/miR-101/Lin28B signaling pathway. Integr. Cancer Ther. 2017, 16, 505–515. [Google Scholar] [CrossRef]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, T.; Rizwan, M.; Khan, N. Comparative therapeutic efficacy of Phyllanthus emblica (Amla) fruit extract and procaine penicillin in the treatment of subclinical mastitis in dairy buffaloes. Microb. Pathog. 2018, 115, 8–11. [Google Scholar] [CrossRef]
- Fatima, N.; Pingali, U.; Muralidhar, N. Study of pharmacodynamic interaction of Phyllanthus emblica extract with clopidogrel and ecosprin in patients with type II diabetes mellitus. Phytomedicine 2014, 21, 579–585. [Google Scholar] [CrossRef]
- Al-Rehaily, A.J.; Al-Howiriny, T.A.; Al-Sohaibani, M.O.; Rafatullah, S. Gastroprotective effects of ‘Amla’ Emblica officinalis on in vivo test models in rats. Phytomedicine 2002, 9, 515–522. [Google Scholar] [CrossRef]
- Yin, K.; Li, X.; Luo, X.; Sha, Y.; Gong, P.; Gu, J.; Tan, R. Hepatoprotective effect and potential mechanism of aqueous extract from Phyllanthus emblica on carbon-tetrachloride-induced liver fibrosis in rats. Evid. Based Complement. Altern. Med. 2021, 2021, 5345821. [Google Scholar] [CrossRef]
- Huang, C.Z.; Tung, Y.T.; Hsia, S.M.; Wu, C.H.; Yen, G.C. The hepatoprotective effect of Phyllanthus emblica L. fruit on high fat diet-induced non-alcoholic fatty liver disease (NAFLD) in SD rats. Food Funct. 2017, 8, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Hashem-Dabaghian, F.; Ziaee, M.; Ghaffari, S.; Nabati, F.; Kianbakht, S. A systematic review on the cardiovascular pharmacology of Emblica officinalis Gaertn. J. Cardiovasc. Thorac. Res. 2018, 10, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikhale, R.V.; Sinha, S.K.; Khanal, P.; Gurav, N.S.; Ayyanar, M.; Prasad, S.K.; Gurav, S.S. Computational and network pharmacology studies of Phyllanthus emblica to tackle SARS-CoV-2. Phytomedicine Plus. 2021, 1, 100095. [Google Scholar] [CrossRef] [PubMed]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, Q.; Marques, M.; Witcher, M. Anticancer Properties of Phyllanthus emblica (Indian Gooseberry). Oxid Med. Cell. Longev. 2015, 2015, 950890. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Liu, P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014, 62, 529–541. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chaudhuri, S.R.; Chattopadhyay, S.; Bandyopadhyay, S.K. Healing properties of some indian medicinal plants against indomethacin-induced gastric ulceration of rats. J. Clin. Biochem. Nutr. 2007, 41, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Peng, Y.; Meng, H.; Li, X. Protective effect of polysaccharides fractions from sijunzi decoction in reserpine-induced spleen deficiency rats. RSC Adv. 2016, 6, 60657–60665. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, M.; Wang, T.; Sun, J.; Wang, Y.; Xia, M.; Jiang, Y.; Zhou, X.; Wan, J. Research on mechanism of charred hawthorn on digestive through modulating “brain-gut” axis and gut flora. J. Ethnopharmacol. 2019, 245, 112166. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H. Why dyspepsia can occur without organic disease: Pathogenesis and management of functional dyspepsia. J. Gastroenterol. 2012, 47, 862–871. [Google Scholar] [CrossRef]
- Carbone, F.; Tack, J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig. Dis. 2014, 32, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; Caenepeel, P.; Geypens, B.; Janssens, J.; Tack, J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am. J. Gastroenterol. 2003, 98, 783–878. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. Gastrointestinal hormones and their targets. Advances in experimental medicine and biology. Adv. Exp. Med. Biol. 2014, 817, 157–175. [Google Scholar] [PubMed]
- Camilleri, M. Gastrointestinal hormones and regulation of gastric emptying. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 3–10. [Google Scholar] [CrossRef]
- Friedenberg, F.K.; Desipio, J.; Korimilli, A.; Bohning, M.; Sum, E.; Parkman, H.P.; Richter, J.E.; Fisher, R.S. Tonic and phasic pyloric activity in response to CCK-octapeptide. Dig. Dis. Sci. 2008, 53, 905–911. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef] [Green Version]
- Karakan, T.; Ozkul, C.; KüpeliAkkol, E.; Bilici, S.; Sobarzo-Sánchez, E.; Capasso, R. Gut-Brain-Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef]
- Xie, X.; Geng, C.; Li, X.; Liao, J.; Li, Y.; Guo, Y.; Wang, C. Roles of gastrointestinal polypeptides in intestinal barrier regulation. Peptides 2022, 151, 170753. [Google Scholar] [CrossRef]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology 2016, 150, 1305–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mars, R.; Frith, M.; Kashyap, P.C. Functional gastrointestinal disorders and the microbiome-what is the best strategy for moving microbiome-based therapies for functional gastrointestinal disorders into the clinic? Gastroenterology 2021, 160, 538–555. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, Y.; Shi, M.; Tian, M.; Ji, J.; Liao, X.; Hu, X.; Chen, F. Dietary Luffa cylindrica (L.) Roem promotes branched-chain amino acid catabolism in the circulation system via gut microbiota in diet-induced obese mice. Food Chem. 2020, 320, 126648. [Google Scholar] [CrossRef]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.; Chan, A.; Sung, J.; et al. High-Fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lin, Y.; Jiang, Y.; Wu, B.; Yu, Y. Aqueous Extract of Phyllanthus emblica L. Alleviates Functional Dyspepsia through Regulating Gastrointestinal Hormones and Gut Microbiome In Vivo. Foods 2022, 11, 1491. https://doi.org/10.3390/foods11101491
Li X, Lin Y, Jiang Y, Wu B, Yu Y. Aqueous Extract of Phyllanthus emblica L. Alleviates Functional Dyspepsia through Regulating Gastrointestinal Hormones and Gut Microbiome In Vivo. Foods. 2022; 11(10):1491. https://doi.org/10.3390/foods11101491
Chicago/Turabian StyleLi, Xiaoqing, Yilin Lin, Yiqi Jiang, Binbin Wu, and Yigang Yu. 2022. "Aqueous Extract of Phyllanthus emblica L. Alleviates Functional Dyspepsia through Regulating Gastrointestinal Hormones and Gut Microbiome In Vivo" Foods 11, no. 10: 1491. https://doi.org/10.3390/foods11101491
APA StyleLi, X., Lin, Y., Jiang, Y., Wu, B., & Yu, Y. (2022). Aqueous Extract of Phyllanthus emblica L. Alleviates Functional Dyspepsia through Regulating Gastrointestinal Hormones and Gut Microbiome In Vivo. Foods, 11(10), 1491. https://doi.org/10.3390/foods11101491





