1. Introduction
Fourier-transform mid-infrared spectrometry (FT-IR) is a recognized and widely used method to rapidly determine the compositional quality of raw milk and other liquid milk products. With the use of sophisticated multivariate statistical models, it is possible to calculate from the FT-IR spectrum the concentration of fat, protein, lactose, urea, fatty acid groups, individual fatty acids [
1,
2,
3], and other milk characteristics such as pH and freezing point. FT-IR spectrometry is also an attractive technology to screen for possible adulteration of liquid milk products [
4,
5,
6]. In fact, various papers have been published on how infrared spectroscopy can be used to screen spectra for atypical milk composition.
Approaches for screening atypical FT-IR milk spectra can be classified into targeted and untargeted methods [
4]. Targeted methods rely on mathematical models trained to detect the presence—or estimate the quantity—of specific adulterants in the milk. Development of these models requires the adulteration of a sufficiently large and representative collection of milk samples with an adulterant, possibly at different concentrations. Due to the characteristic effect of the adulterant on the milk’s FT-IR spectrum, mathematical models can be trained to distinguish spectra belonging to adulterated milk from those belonging to normal milk. This way, mathematical models have been developed to identify milk adulterated with melamine [
7], urea [
3], water, starch, sodium citrate, formaldehyde, sucrose, and other adulterants [
8,
9]. The advantage of targeted methods is that they can indicate the presence of specific adulterants in the milk. Moreover, mathematical models tuned to specific adulterants typically exhibit lower detection limits (i.e., are more sensitive) compared to untargeted methods. The main disadvantage of targeted methods is that they are only capable of detecting known adulterants. In reality, however, it is often not known which adulterants are currently used or will be used in the future. Adulteration with substances (or complex blends thereof) that the mathematical models were not explicitly trained to detect can therefore go undetected.
This is where untargeted screening methods excel. These methods do not rely on mathematical models trained to detect a specific deviation in the FT-IR spectrum. Instead, they are sensitive to any deviation present in the spectrum by creating a mathematical model based on FT-IR spectra from authentic milk samples that still contain all normal variation (e.g., seasonal variation, different cow breeds and farm management practices). This mathematical model acts as a normal FT-IR milk fingerprint that can be compared with spectra from milk samples that are to be examined. If a spectrum deviates above a stated threshold from the normal milk fingerprint, it is denoted atypical. Since untargeted methods only rely on regular milk spectra, their development often is less expensive, and only requires a little statistical fine-tuning while offering a broad protection for possible adulteration of milk samples. This has made untargeted screening methods a popular subject of scientific research [
6,
10] and commercial applications [
11]. Although untargeted screening methods are capable of revealing any type of adulteration with a strong effect on the spectrum, their disadvantage is that they do not comprehensibly characterize in what way a spectrum is atypical and what the root cause is.
However, information about how and why a milk sample is atypical is crucial for effective and appropriate follow-up action (e.g., contacting the farm or manufacturer, identifying the root cause, and evaluating the possible risks for safety and quality of dairy products). In this paper, we therefore evaluate the potential of combining untargeted methods for atypical spectra screening with cluster algorithms to reveal in what way an individual milk spectrum is atypical and what the underlying cause might be. To do so, untargeted spectra screening was applied to a large dataset of bovine herd bulk milk spectra. This way, a dataset of atypical milk spectra was created. Cluster algorithms were then used to identify possible categories of atypical milk spectra. We show how information in the spectra (e.g., the compositional milk profile) and meta-data associated with their acquisition (e.g., time of measurement) can be used to understand in what way the spectrum is atypical and how it can be used to form hypotheses about the underlying causes.
3. Results and Discussion
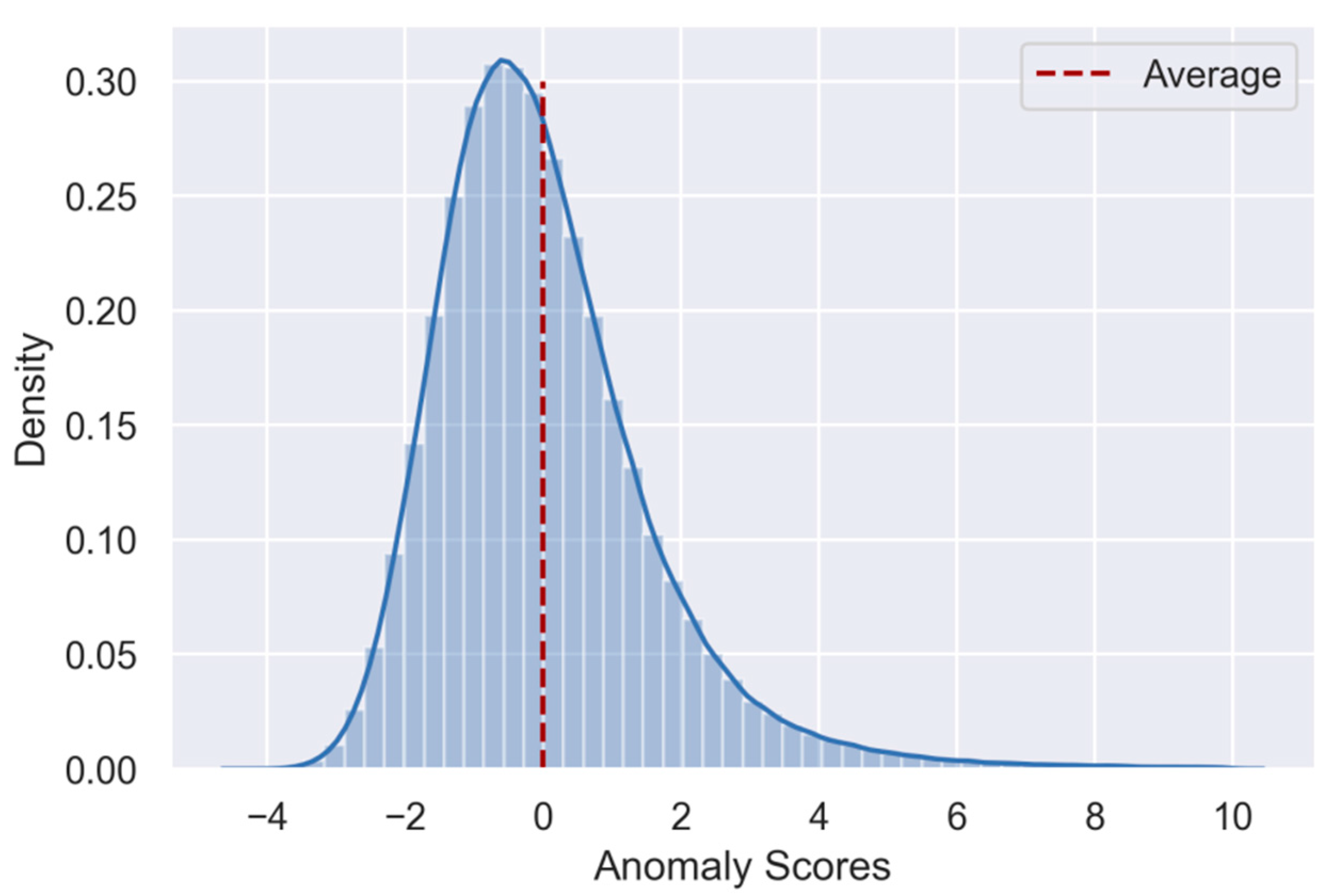
The goal of the study was an evaluation of the potential of combining untargeted methods for atypical milk spectra screening with cluster algorithms to reveal in what way a milk sample is atypical and what the underlying causes could be. How useful a combined approach is depends on whether atypical milk spectra can be clustered into robust and meaningful categories that can, in turn, be linked to possible root causes. However, identifying meaningful and generalizable clusters in complex data is inherently difficult. Because the true number of distinct clusters and their actual meaning cannot be known a priori, the challenge lies in selecting one out of many possible cluster solutions. In the current study, we tried to balance generalizability and interpretability and found that categorizing atypical milk spectra into ten distinct clusters provided a robust and meaningful description of our dataset.
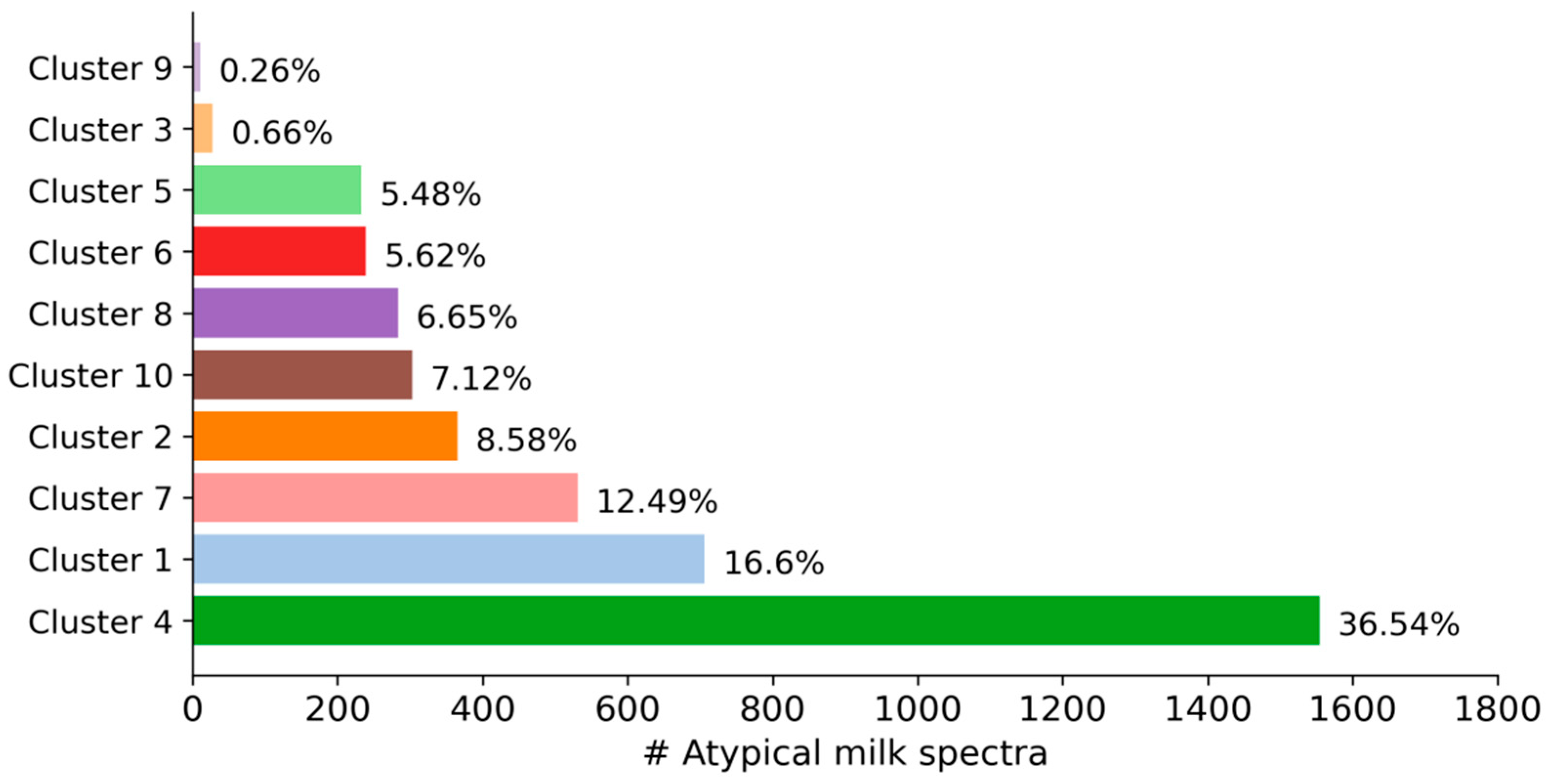
As can be seen in
Figure 2, the size of the clusters varied from 11 milk spectra (0.26% of all spectra) in cluster 9 to 1554 milk spectra (36.54%) in cluster 4. Because some factors that cause milk spectra to be atypical are more likely than others, variability in the size of the clusters is expected. Interestingly, out of 4253 spectra, only 11 milk samples with similar spectral characteristics were necessary to form a distinct cluster. This indicates how sensitive cluster algorithms can be in detecting very small groups of atypical spectra—even within large datasets.
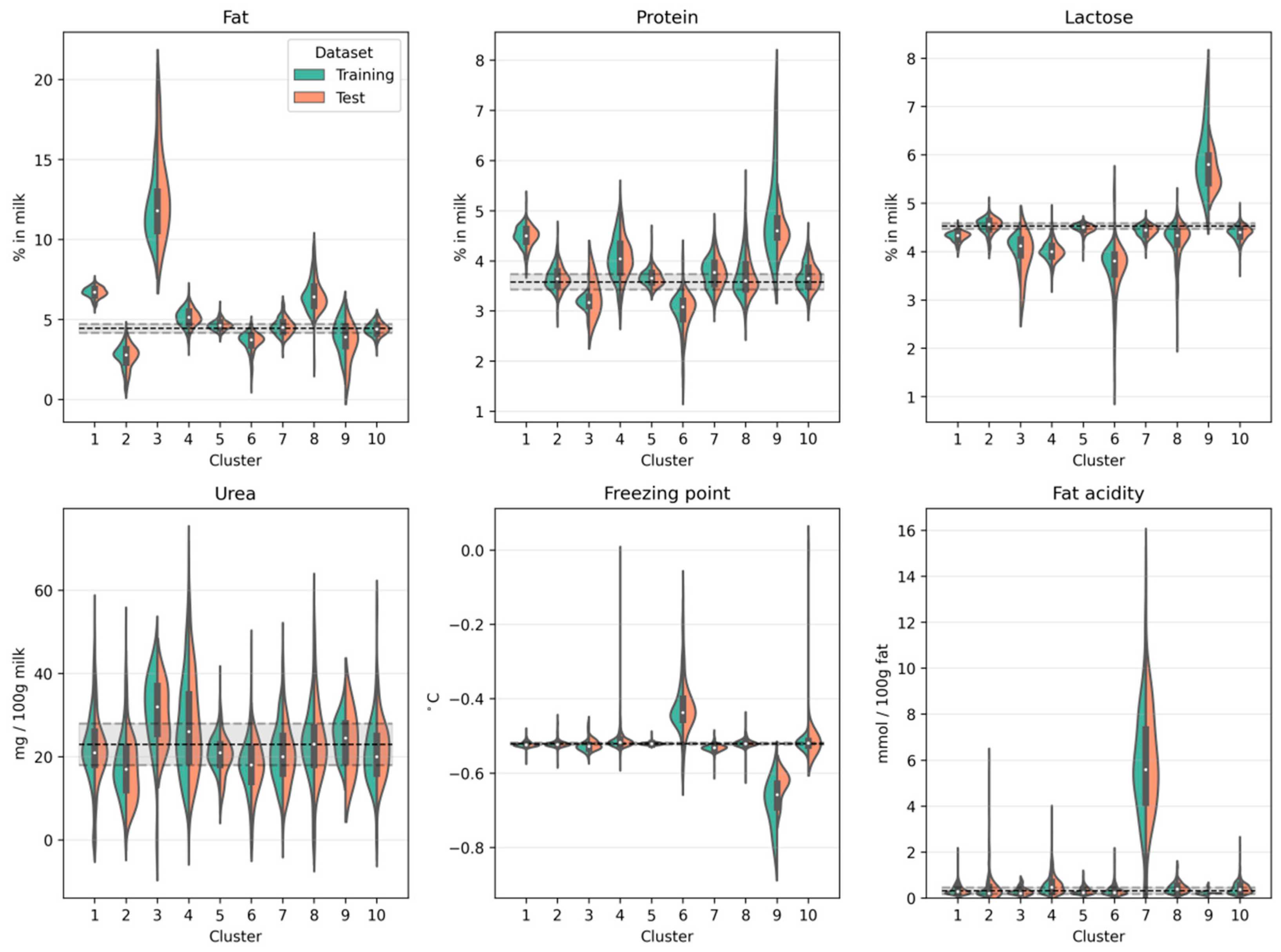
Figure 3 shows for both the training dataset and the test dataset the compositional milk profile on a cluster-by-cluster basis. Notice the similarity of the distributions when comparing the training and test dataset. This shows that our cluster solution generalizes to new data and does not merely describe peculiarities in our specific training set. The compositional milk profile also allows for an initial characterization of the clusters so that hypotheses can be made about the underlying root cause. For example, cluster 3 is characterized by an increased fat content of up to 22%. This can be the consequence of insufficient mixing of milk before sampling. Therefore, if untargeted screening methods identify an atypical milk spectrum which is subsequently classified as belonging to cluster 3, it is most likely because the milk has a drastically increased fat content as it was not homogeneously sampled. Cluster 6, on the other hand, is marked by a decreased protein and lactose concentration but an increased freezing point. This is typical for extraneous water in the milk [
18]. This can be the result of an influx of water due to improper functioning of valves or an insufficient draining of water residues after cleansing the milking system or the tank. Cluster 7 is marked by a drastic increase in free fatty acids in the milk. This can be the consequence of the lipase activity [
19] in sensitive milk (e.g., due to an increased somatic cell count in late lactation cows [
20]) or milk that is subject to mechanical strain (e.g., air inclusion in milking systems) [
21]. Because increased free fatty acid concentration in the milk can result in rancid flavors of milk products [
21,
22] and loss of fat in whey during cheese production [
23], identifying such atypical milk samples therefore is of direct economic relevance. Finally, cluster 9 is characterized by an increased protein and lactose concentration combined with a decreased freezing point. This could be indicative of adulteration with protein-rich (e.g., milk powder, whey protein isolate) and carbohydrate-based adulterants (e.g., glucose, starch) to increase the apparent concentration of protein and lactose [
24]. Individual milk spectra categorized as belonging to cluster 9 could therefore indicate economically motivated adulteration. The ability to identify such cases makes it possible to initiate further target-oriented chemical analyses and contact with the farm for clarification. Although informative, milk spectra can also be atypical for reasons other than those found in the compositional milk profile. For clusters 5 and 10, for instance, the compositional milk profile does not seem to provide relevant diagnostic information. It is therefore also important to focus on the characteristic spectra of the clusters.
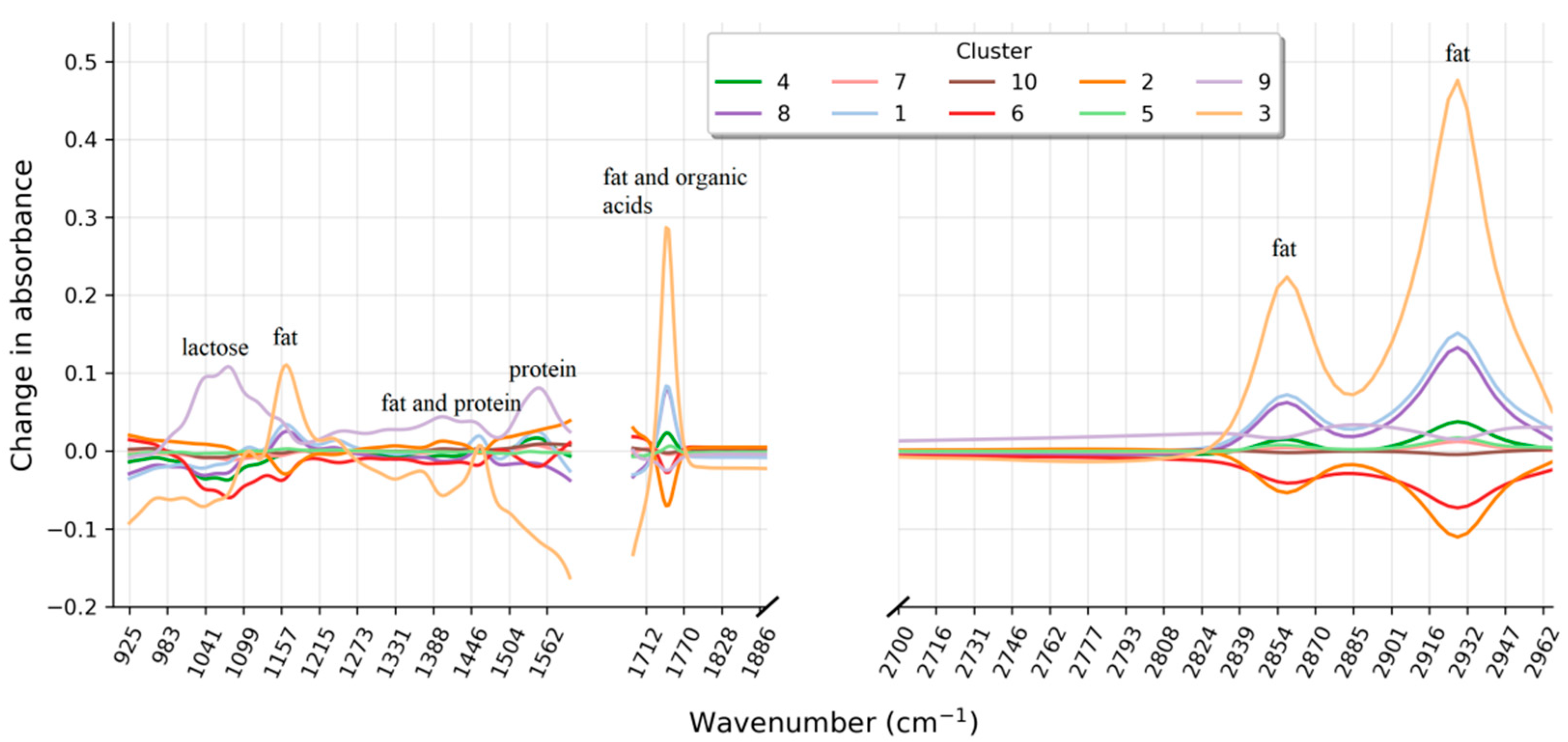
Figure 4 gives an illustration of how the average milk spectrum per cluster deviates from a typical milk spectrum. Inspecting the average spectra reveals that the change in absorbance of some clusters mainly occurs in spectral regions that are directly related to the concentration of fat (around 1750 and 2860 cm
−1), protein (around 1540 cm
−1), and lactose (around 1040 cm
−1). Cluster 3, for example, is marked by increased absorption in the regions around 1750 and 2860 cm
−1 which corresponds to the C = O bonds (carboxyl group) and C-H bonds (ethylene group) characteristic for fat. For cluster 6, on the other hand, decreased absorption around 1540 (H-N-C = O amides) and 1040 cm
−1 (C-O carbohydrates) correspond to a decreased protein and lactose concentration, respectively. However, some clusters also show a structurally abnormal absorbance profile. A closer look at the spectral region between 2740 and 2970 cm
−1 reveals an interesting pattern for cluster 9. The spectral absorbance pattern in this region differs from the remaining clusters and the natural variation encountered in typical FT-IR spectra. Similarly, for cluster 2 in the region between 926 and 1100 cm
−1. Such structural abnormalities can go unnoticed when only the compositional milk profile is analyzed but may provide important information about the nature of the atypical appearance. This information can then be used to generate hypotheses about a possible explanation that can be tested by target-oriented chemical analyses or further exploration on the spot.
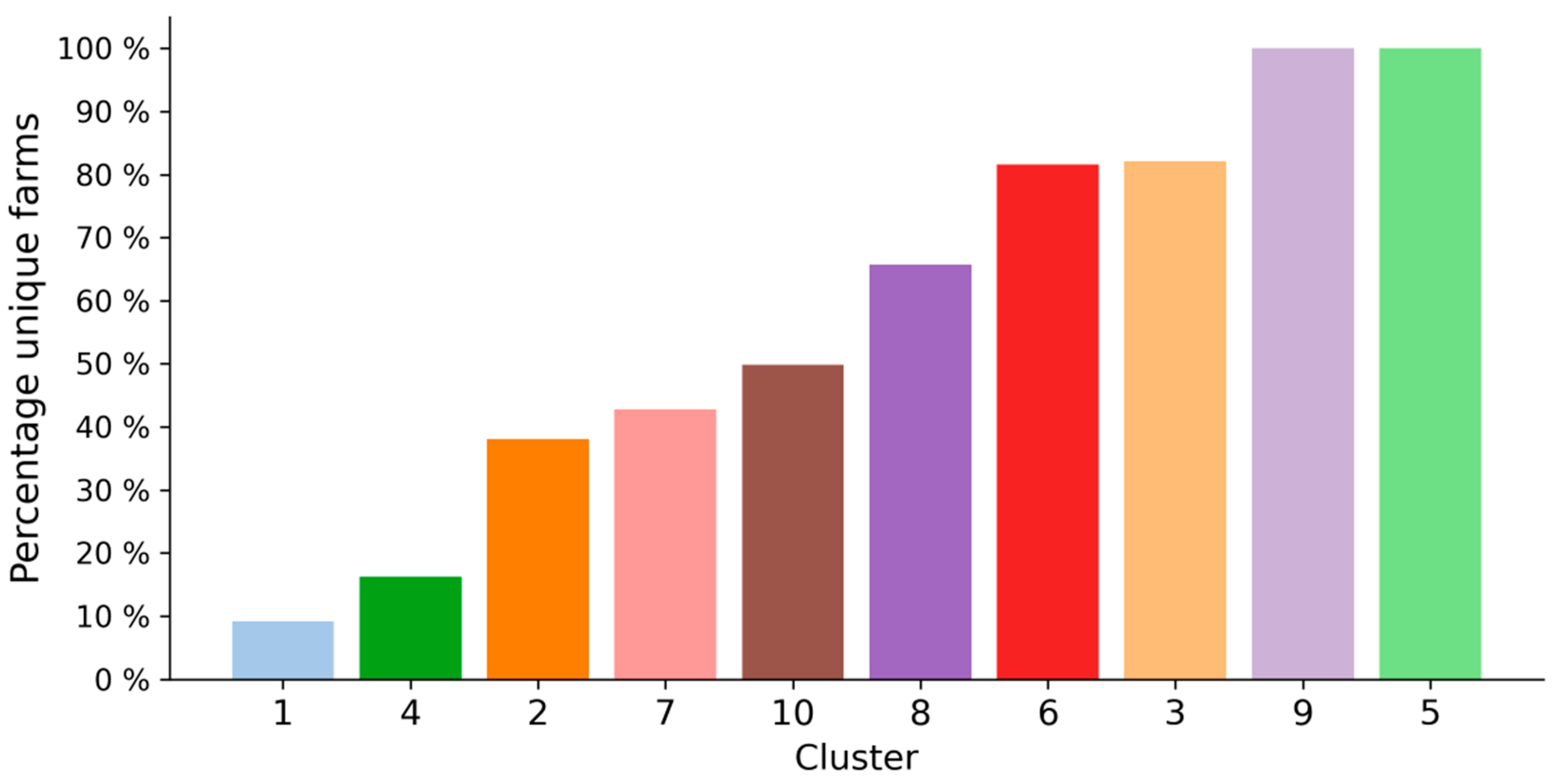
Describing clusters on the basis of their compositional milk profile and the underlying milk spectra provides important information and allows for an assessment of whether classifying atypical milk spectra is feasible in the first place. However, we believe that an evaluation of the applicability of a cluster solution also depends on important meta-information regarding the atypical spectra. One such source of information relates to the proportion of unique farms in relation to the size of each cluster. This gives an indication of the extent to which the root cause underlying atypical milk spectra within a cluster can be found at many different farms, or only a few farms. As can be seen in
Figure 5, most of the distinct clusters have a proportion of unique farms in between 40 and 80%. For example, 43% of all the spectra in cluster 7 belong to different farms. Considering the actual size of the cluster, it can be calculated that the 529 spectra in cluster 7 belong to 227 different farms. This also means that, considering a period of almost 3 years, a farm in cluster 7 will have on average 2.3 atypical spectra in this cluster. This could be indicative of certain factors more prominent at these farms. Knowledge about these factors (e.g., factors leading to increased mechanical strain of milk) could then be used to take preventive measures. We also found two clusters, cluster 1 and cluster 4, where the proportion of unique farms was particularly low (9.33 and 16.34%, respectively). This indicates that the majority of atypical spectra correspond to milk supplies from relatively few farms. In cluster 5 and cluster 9, on the contrary, all spectra belong to different farms. This could either mean that the underlying cause affects farms only incidentally or that the reason is not located at the farms but instead at the laboratory where the FT-IR measurements take place.
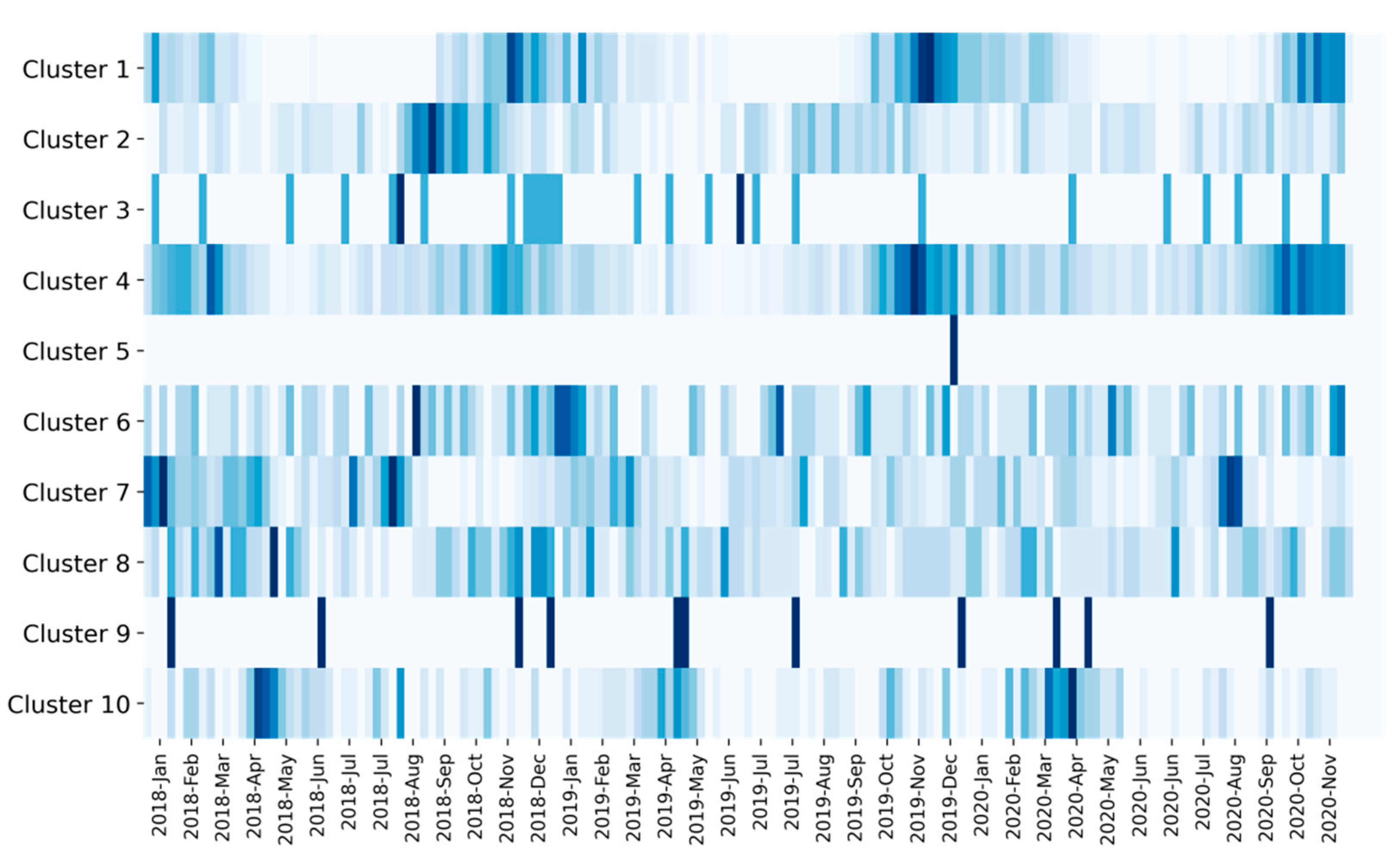
We also analyzed the temporal profile of the clusters. In
Figure 6, we visualized per cluster the distribution of milk samples over time. This makes it possible to identify the extent to which a cluster primarily describes milk that is atypical because the underlying root cause has a seasonal profile (such as feeding and variation in temperature). Moreover, it can be useful to know whether the underlying root cause is confined to a temporally isolated event or whether it is more permanent. In our dataset, most of the clusters do not appear to have a seasonal profile but are evenly distributed across the three-year period. This suggests that the clusters are stable in the sense that the underlying root causes exist throughout the year and are likely to be permanent. For example, the few spectra belonging to cluster 9 were discovered evenly throughout a period of three years and each spectrum corresponds to milk from a different farm. This is not indicative of a systematic intentional adulteration, where multiple milk samples from the same farms are expected to be affected over a certain period of time. Nevertheless, evidence suggests that atypical spectra belonging to cluster 9 will also be found in the future, and this may justify further target-oriented analyses concerning these milk samples or the farms they are coming from. We also identified clusters with a seasonal profile. As can be seen in
Figure 6, clusters 1, 4, and 10 have a yearly seasonal profile. In cluster 10 the majority of spectra originate from milk samples collected between April and May. This period of the year typically marks the start of the grazing season, which goes along with a transition from silage-based feeding to autonomous grazing. It is well known that this leads to characteristic changes in the milk’s fatty acid profile [
25,
26]. Because over 80% of the farms in the Netherlands have their cows out in the meadow during the grazing season, natural variation due to grazing is already incorporated in our mathematical model. Cluster 10 could therefore contain milk from farms where the transition to the grazing season leads to particularly drastic changes in the fatty acid profile of the milk—perhaps due to abrupt changes in the food intake. Note that a change in the fatty acid profile does not have to be reflected in the milk’s overall fat percentage. Another two clusters that follow a yearly seasonal profile are cluster 1 and, albeit being less pronounced, cluster 4. Both clusters mainly contain milk samples collected around November and December. This period is likely associated with the transition from autonomous grazing to silage-based feeding at the end of the grazing season.
Another discovery that can be made from the temporal profiles relates to cluster 5. More than 99% of the spectra in cluster 5 were measured on two consecutive days. We found that all but two spectra were measured on a single FT-IR instrument. This highly suggests that the spectra in cluster 5 are atypical due to temporary instabilities related to the particular FT-IR instrument. For example, multiple reflections inside the layers within the measurement cell of the infrared instrument can produce characteristic artifacts in the spectrum known as fringes [
27]. This is an interesting and important discovery. On the one hand, it indicates that such instrument instabilities produce characteristic spectral patterns that can be detected by a cluster algorithm. On the other hand, it shows that such instabilities can lead to drastic changes in the spectrum that go unnoticed when only the milk’s compositional profile or even the raw spectra are analyzed. Moreover, mathematical models used for the calculation of chemical compounds that are present only in small concentrations (e.g., individual fatty acids) can produce invalid results if the instabilities affect spectral features used by these models. In addition, such instabilities can, and in our case did, lead to anomaly scores higher than 99.9% for all other scores in this period. In other words, these spectra were considered as atypical for reasons that are unrelated to the chemical composition of the milk. By analyzing the time-course of anomaly scores on an instrument-by-instrument basis, it is possible to identify periods of instrument instabilities that are reflected as increased anomaly scores. This way, atypical milk spectrum screening can also be used to simultaneously screen FT-IR instruments for instabilities that can lead to systematic changes in milk spectra. Moreover, such instrument instabilities can indicate that the instrument requires maintenance.
Implications, Limitations, and Future Outlook
Despite the promising results obtained from our cluster-level analyses, we also acknowledge some limitations. First, it may not always be obvious or even possible to describe what it means for the actual milk if it has a specific atypical absorbance pattern. This is particularly the case for categories characterized by non-trivial absorbance patterns rather than isolated peaks. In our case, we found that clusters 5 and 10 were interesting in this regard. Both clusters could hardly be characterized by inspection of the spectra or their compositional milk profile. Nevertheless, information about why they were atypical could be inferred from the corresponding meta-data. It is therefore possible to identify categories for which an explanation can be given as to why milk spectra are atypical without being able to explain in what way they are atypical.
Another limitation concerns the classification of new atypical milk spectra. We demonstrated that is possible to identify meaningful categories of atypical milk spectra that also generalize to new datasets. Although generalizability of a cluster solution is crucial, it should be clear that a spectrum is always more similar to one cluster than to another. This means that a milk spectrum will always be assigned to a category, no matter how well it actually fits the category. This can lead to erroneous conclusions about how and why the milk is atypical. Ideally, only those milk spectra that are sufficiently similar to the spectra that were used by the cluster algorithm to identify the categories in the first place should be assigned to a category. Probabilistic generative models such as Gaussian Mixture Models can, theoretically, do exactly that. Since they can be used to generate synthetic data, they can also be used to calculate the probability that a particular milk spectrum can be randomly generated from this model. When the probability for a milk spectrum is particularly low, the model may not be appropriate for categorizing the spectrum. This way, it is also important to keep in mind that a cluster solution will necessarily become outdated over time. This is because the chemical composition of normal milk changes over the years and the reasons that cause milk spectra to be atypical do so too. This requires that the cluster algorithm also be updated. Monitoring, over time, the proportion of atypical spectra that cannot be categorized by the model can give important information about the up-to-datedness of the models. However, more research needs to be done in this area.
A final limitation concerns untargeted milk spectra screening methods in general. Untargeted spectra screening relies on a comparison of an individual milk spectrum with a reference FT-IR milk fingerprint. The reference milk fingerprint represents the gold standard of what is assumed to reflect normal milk. However, milk with a spectrum that deviates from this fingerprint does not automatically reflect poor quality. In fact, even the opposite can be the case. With the use of cluster algorithms and a thorough analysis of the resulting categories, it may be possible to distinguish milk that is atypical for undesired reasons (e.g., adulteration with water) from milk that is atypical for desirable reasons (e.g., particularly good farm management practices or feeding regimes). On the basis of our findings, we believe that a combined approach for atypical spectra screening can serve as a valuable complementary quality assurance tool in routine FT-IR milk analysis. After meaningful and generalizable categories of atypical milk spectra have been identified, new atypical milk spectra can be classified into these categories. Knowledge about the different categories, in terms of what they reflect and what the possible root causes are, can then be used to describe why and in what way a new milk spectrum is atypical. Moreover, our approach is computationally efficient, scalable, and can be implemented in large-scale routine screening for atypical milk. Being able to give an indication about how and why a milk spectrum is atypical is necessary for taking appropriate actions (e.g., rejecting the milk) and is an indispensable prerequisite when contacting the farm for clarification. Our discovery that instrument instabilities can also be responsible for atypical spectra is of particular relevance here. It is important to differentiate between spectra that are atypical because the milk is chemically abnormal or because the FT-IR instrument produces measurement artifacts in the spectrum. This way, atypical spectra screening can be used as a tool for monitoring the quality and authenticity of milk, and for monitoring the FT-IR instruments from which the spectra are obtained and the compositional milk profile is computed.