Hapten Synthesis and Monoclonal Antibody Preparation for Simultaneous Detection of Albendazole and Its Metabolites in Animal-Origin Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of hapten and conjugates
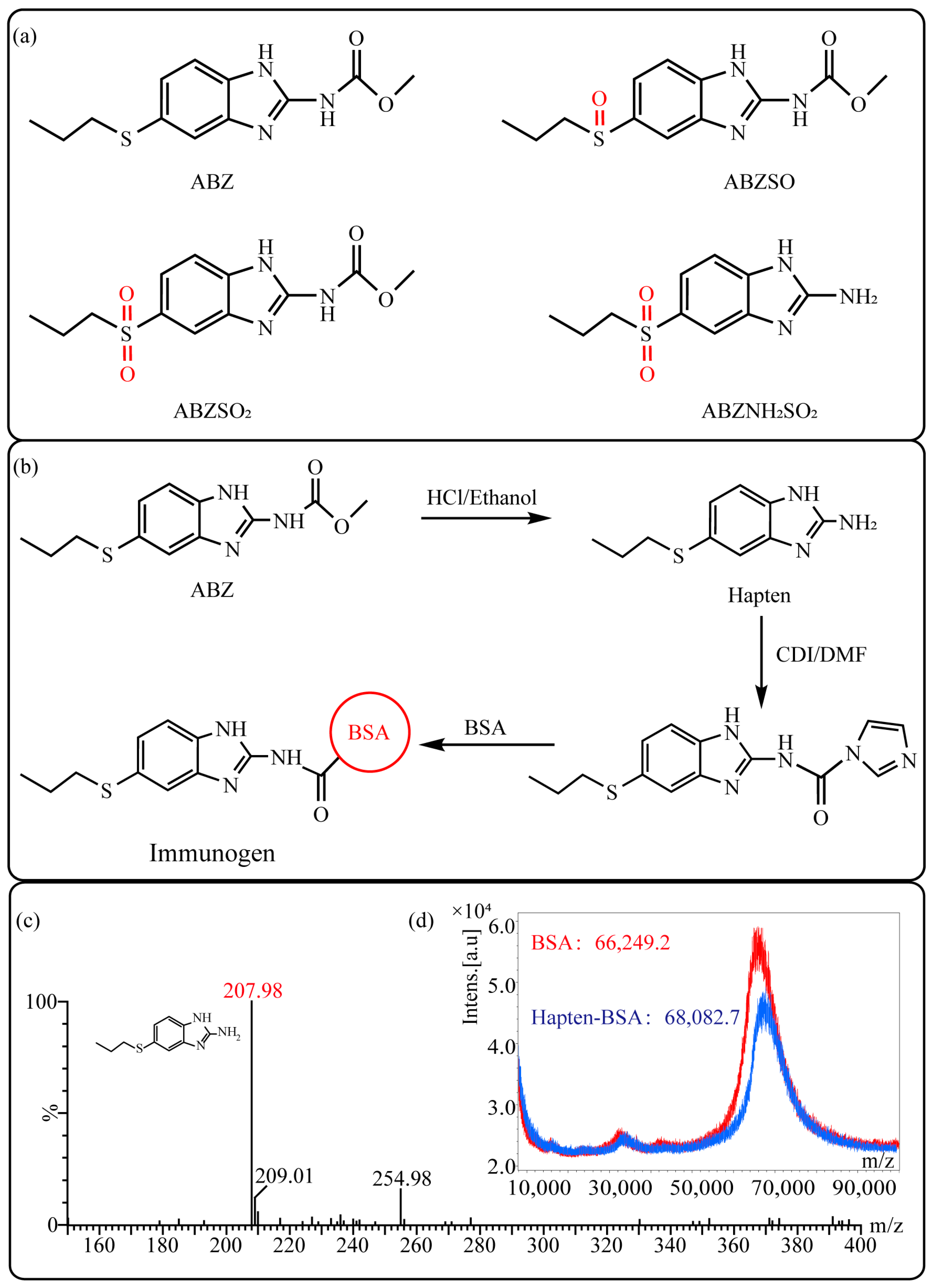
2.2.1. Synthesis and Identification of Hapten
2.2.2. Synthesis and Identification of Immunogen and Coating Antigen
2.3. Production of Monoclonal Antibody
2.4. Development and Optimization of ic-ELISA
2.4.1. Effect of pH
2.4.2. Effect of Ionic Strength
2.5. Cross-Reactivities and Computational Chemistry Analysis
2.6. Sample Preparation
3. Results and Discussion
3.1. Hapten Design and Characterization
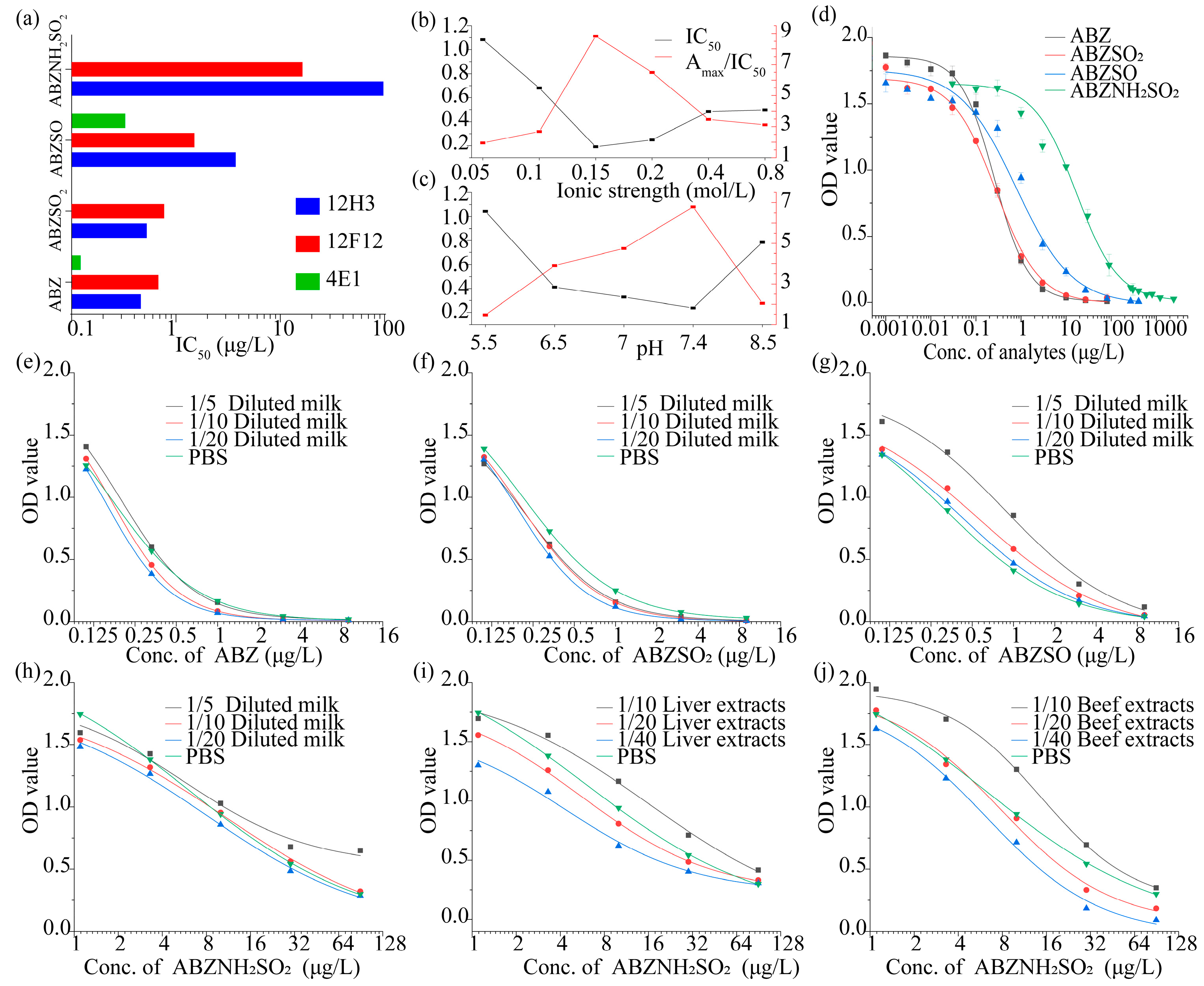
3.2. Development and Optimization of the ic-ELISA
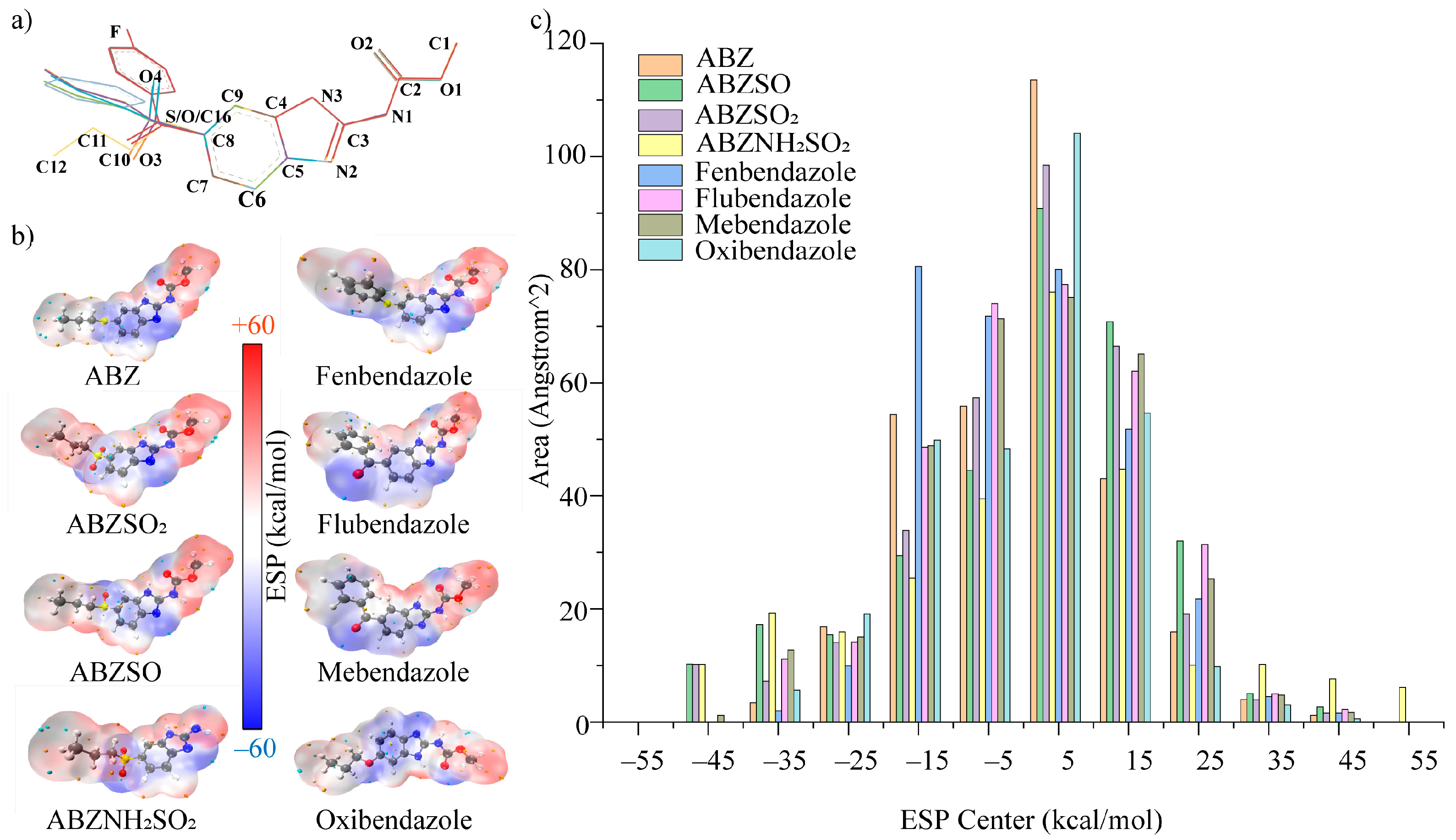
3.3. Cross-Reactivities and Structure-Activity Relationship Study by Computational Chemistry
3.4. Matrix Effect and Recovery in Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Tamarozzi, F.; Degani, M.; Angheben, A.; Bisoffi, Z. Efficacy of high-dose albendazole with ivermectin for treating imported loiasis, Italy. Emerg. Infect. Dis. 2019, 25, 1574–1576. [Google Scholar] [CrossRef]
- Macfarlane, C.L.; Budhathoki, S.S.; Johnson, S.; Richardson, M.; Garner, P. Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis. Cochrane. Db. Syst. Rev. 2019, 1, CD003753. [Google Scholar] [CrossRef]
- Budge, P.J.; Herbert, C.; Andersen, B.J.; Weil, G.J. Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature. PLoS Negl. Trop. Dis. 2018, 12, e0006454. [Google Scholar] [CrossRef]
- Chami, G.F.; Bundy, D.A.P. More medicines alone cannot ensure the treatment of neglected tropical diseases. Lancet Infect. Dis. 2019, 19, 330–336. [Google Scholar] [CrossRef]
- Pullan, R.L.; Halliday, K.E.; Oswald, W.E.; Mcharo, C.; Beaumont, E.; Kepha, S.; Witek-McManus, S.; Gichuki, P.M.; Allen, E.; Drake, T.; et al. Effects, equity, and cost of school-based and community-wide treatment strategies for soil-transmitted helminths in Kenya: A cluster-randomised controlled trial. Lancet 2019, 393, 2039–2050. [Google Scholar] [CrossRef] [Green Version]
- Danaher, M.; De Ruyck, H.; Crooks, S.R.; Dowling, G.; O’Keeffe, M. Review of methodology for the determination of benzimidazole residues in biological matrices. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 845, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.D.; Neodo, A.; Coulibaly, J.T.; Keiser, J. Pharmacokinetics of albendazole, Albendazole sulfoxide, and albendazole sulfone determined from plasma, Blood, Dried-blood spots, And mitra samples of hookworm-infected adolescents. Antimicrob. Agents Chemother. 2019, 63, e02489-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, E.K.; Sanuku, N.; Baea, M.; Satofan, S.; Maki, E.; Lombore, B.; Schmidt, M.S.; Siba, P.M.; Weil, G.J.; Kazura, J.W.; et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin. Infect. Dis. 2016, 62, 334–341. [Google Scholar] [CrossRef]
- Ricken, F.J.; Nell, J.; Grüner, B.; Schmidberger, J.; Kaltenbach, T.; Kratzer, W.; Hillenbrand, A.; Henne-Bruns, D.; Deplazes, P.; Moller, P.; et al. Albendazole increases the inflammatory response and the amount of Em2-positive small particles of Echinococcus multilocularis (spems) in human hepatic alveolar echinococcosis lesions. PLoS Negl. Trop. Dis. 2017, 11, e0005636. [Google Scholar] [CrossRef] [PubMed]
- The European Union. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; Official Journal of the European Union: Brussels, Belgium, 2010. [Google Scholar]
- The, U.S. Food and Drug Administration. 21CFR556.34. Specific Tolerances for Residues of Approved and Conditionally Approved New Animal Drugs. Sec. 556.34 Albendazole. In The Code of Federal Regulations; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021. [Google Scholar]
- The Ministry of Agriculture and Rural Affairs of the People’s Republic of China. National Food Safety Standard-Maximum Residue Limits for Veterinary Drugs in Foods; GB 31650-2019; Standards Press of China: Beijing, China, 2019.
- Li, S.; Liang, Q.; Ahmed, S.A.H. Simultaneous determination of five benzimidazoles in agricultural foods by core-shell magnetic covalent organic framework nanoparticle-based solid-phase extraction coupled with high-performance liquid chromatography. Food Anal. Methods 2020, 13, 1111–1118. [Google Scholar] [CrossRef]
- Permana, A.D.; Tekko, I.A.; McCarthy, H.O.; Donnelly, R.F. New HPLC-MS method for rapid and simultaneous quantification of doxycycline, diethylcarbamazine and albendazole metabolites in rat plasma and organs after concomitant oral administration. J. Pharm. Biomed. Anal. 2019, 170, 243–253. [Google Scholar] [CrossRef]
- Bach, T.; Bae, S.; D’Cunha, R.; Winokur, P.; An, G. Development and validation of a simple, fast, and sensitive LC/MS/MS method for the quantification of oxfendazole in human plasma and its application to clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2019, 171, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Dong, J.; Yang, Y.; Liu, Y.; Yang, Q.; Ai, X. Development of a liquid chromatography-tandem mass spectrometry method with modified QuEChERS extraction for the quantification of mebendazole and its metabolites, albendazole and its metabolites, and levamisole in edible tissues of aquatic animals. Food Chem. 2018, 269, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Casado, C.; Moreno-Gonzalez, D.; Lara, F.J.; Garcia-Campana, A.M.; Del Olmo-Iruela, M. Determination of benzimidazoles in meat samples by capillary zone electrophoresis tandem mass spectrometry following dispersive liquid-liquid microextraction. J. Chromatogr. A 2017, 1490, 212–219. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Wang, X.; Li, Y.; Zhong, F.; Li, X.; Huang, Y.; Ding, S.; Shen, J. Validation of a method for simultaneous determination of nitroimidazoles, benzimidazoles and chloramphenicols in swine tissues by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 96–103. [Google Scholar] [CrossRef]
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold nanoparticle-based paper sensor for simultaneous detection of 11 benzimidazoles by one monoclonal antibody. Small 2018, 14, 1701782. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Jiang, N.; Wang, Y.; Chen, D.; Liu, Z.; Yuan, Z. Development and validation of an indirect competitive enzyme-linked immunosorbent assay for the detection of albendazole 2-aminosulfone residues in animal tissues. Food Agric. Immunol. 2015, 27, 273–287. [Google Scholar] [CrossRef]
- Brandon, D.L.; Binder, R.G.; Bates, A.H.; Montague, W.C., Jr. Monoclonal antibody for multiresidue ELISA of benzimidazole anthelmintics in liver. J. Agric. Food Chem. 1994, 42, 1588–1594. [Google Scholar] [CrossRef]
- Brandon, D.L.; Holland, K.P.; Dreas, J.S.; Henry, A.C. Rapid screening for benzimidazole residues in bovine liver. J. Agric. Food Chem. 1998, 46, 3653–3656. [Google Scholar] [CrossRef]
- Eyer, K.; Doineau, R.C.L.; Castrillon, C.E.; Briseño-Roa, L.; Menrath, V.; Mottet, G.; England, P.; Godina, A.; Brient-Litzler, E.; Nizak, C.; et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat. Biotechnol. 2017, 35, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Dong, X.; Zhang, X.; Li, C.; Wang, J.; Mujtaba, M.G.; Zhang, S.; Wen, K.; Yu, X.; Wang, Z. Novel hapten design, antibody recognition mechanism study, and a highly sensitive immunoassay for diethylstilbestrol in shrimp. Anal. Bioanal. Chem. 2019, 411, 5255–5265. [Google Scholar] [CrossRef]
- Li, H.; Ma, S.; Zhang, X.; Li, C.; Dong, B.; Mujtaba, M.G.; Wei, Y.; Liang, X.; Yu, X.; Wen, K.; et al. Generic hapten synthesis, broad-specificity monoclonal antibodies preparation, and ultrasensitive ELISA for five antibacterial synergists in chicken and milk. J. Agric. Food Chem. 2018, 66, 11170–11179. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn, A multifunctional wavefunction analyzer. J Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved marching tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- Wen, K.; Bai, Y.; Wei, Y.; Li, C.; Shen, J.; Wang, Z. Influence of small molecular property on antibody response. J. Agric. Food Chem. 2020, 68, 10944–10950. [Google Scholar] [CrossRef]
- Xu, Z.L.; Shen, Y.D.; Beier, R.C.; Yang, J.Y.; Lei, H.; Wang, H.; Sun, Y.M. Application of computer-assisted molecular modeling for immunoassay of low molecular weight food contaminants: A review. Anal. Chim. Acta. 2009, 647, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, X.; Wen, K.; Li, Y.; Zhang, X.; Ma, M.; Yu, X.; Yu, W.; Shen, J.; Wang, Z. Class-specific monoclonal antibodies and dihydropteroate synthase in bioassays used for the detection of sulfonamides: Structural insights into recognition diversity. Anal. Chem. 2019, 91, 2392–2400. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Ding, S.; He, F.; Beier, R.C.; Li, J.; Jiang, H.; Feng, C.; Wan, Y.; Zhang, S.; et al. Development of a monoclonal antibody-based broad-specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds. Anal. Chem. 2007, 79, 4471–4483. [Google Scholar] [CrossRef]
- Newman, D.J.; Price, C.P. Molecular aspects of design of immunoassays for drugs. Ther. Drug Monit. 1996, 18, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, P.; Cheng, L.; Zhang, S.; Shen, J. Hapten-antibody recognition studies in competitive immunoassay of α-zearalanol analogs by computational chemistry and pearson correlation analysis. J. Mol. Recognit. 2011, 24, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Jiang, H.; Zhang, Y.; Dou, L.; Liu, M.; Yu, W.; Wen, K.; Shen, J.; Ke, Y.; Yu, X.; et al. Hydrophobic moiety of capsaicinoids haptens enhancing antibody performance in immunoassay: Evidence from computational chemistry and molecular recognition. J. Agric. Food Chem. 2021, 69, 9957–9967. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Haptens&IC50 (μg/L) | |||
|---|---|---|---|---|
| [22] | [21] | [20] | This study | |
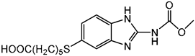 | 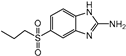 |  | 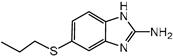 | |
| ABZ | 1.4 | >10,000 | 0.66 | 0.2 |
| ABZSO2 | 1.8 | 1253.2 | 5.34 | 0.26 |
| ABZSO | 1.5 | 2241.4 | 2.91 | 0.77 |
| ABZNH2SO2 | >10,000 | 85.2 | >1000 | 10.5 |
| Carbendazim | - 1 | - | 14.84 | >312.5 |
| Fenbendazole | 3.8 | >10,000 | 0.75 | 1.68 |
| Fenbendazole sulfone | 8.3 | - | 6.27 | - |
| Flubendazole | 0.63 | >10,000 | 0.37 | 3.68 |
| Mebendazole | 2.4 | >10,000 | 0.3 | 4.14 |
| Oxfendazole | 0.62 | >10,000 | 19.99 | >312.5 |
| Oxibendazole | 1.4 | >10,000 | 0.64 | 2.29 |
| Parbendazole | - | - | 1.13 | - |
| Cambendazole | >100 | - | >1000 | - |
| Thiabendazole | >100 | >10,000 | - | >312.5 |
| Analytes | IC50 (μg/L) | CR (%) | |
|---|---|---|---|
| ABZ | 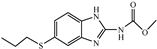 | 0.20 | 100 |
| ABZSO2 | 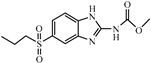 | 0.26 | 76.9 |
| ABZSO | 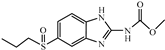 | 0.77 | 26.0 |
| ABZNH2SO2 | 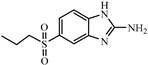 | 10.50 | 1.9 |
| Fenbendazole | 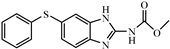 | 1.68 | 11.9 |
| Flubendazole | 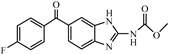 | 3.68 | 5.4 |
| Mebendazole | 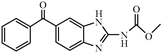 | 4.14 | 4.8 |
| Oxibendazole |  | 2.29 | 8.7 |
| Oxfendazole | 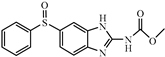 | >312.5 | <0.01 |
| Triclabendazole | 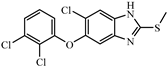 | >312.5 | <0.01 |
| Carbendazim | 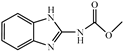 | >312.5 | <0.01 |
| Thiabendazole | 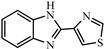 | >312.5 | <0.01 |
| Items | ABZ | ABZSO2 | ABZSO | ABZNH2SO2 | Fenbendazole | Flubendazole | Mebendazole | Oxibendazole |
|---|---|---|---|---|---|---|---|---|
| CR (%) | 100 | 76.9 | 26.0 | 1.9 | 11.9 | 5.4 | 4.8 | 8.7 |
| MW | 265.1 | 297.3 | 281.3 | 239.3 | 299.3 | 313.3 | 295.3 | 249.3 |
| volume(Å3) | 320.9 | 336.4 | 328.5 | 201.8 | 255.3 | 261.1 | 256.2 | 225.0 |
| Log P | 2.8 | 1.2 | 1.2 | 1.0 | 3.4 | 3.1 | 2.9 | 2.4 |
| μ | 4.1 | 7.6 | 4.7 | 6.5 | 4.8 | 6.6 | 6.8 | 1.8 |
| TPSA (Å2) | 67.0 | 101.2 | 84.1 | 88.9 | 67.0 | 84.1 | 84.1 | 76.2 |
| alignment RMSD | 0 | 1.3 × 10−2 | 1.1 × 10−2 | 2.3 × 10−3 | 1.3 × 10−2 | 8.5 × 10−3 | 8.4 × 10−3 | 5.4 × 10−3 |
| ABZs in Milk Samples | |||||
|---|---|---|---|---|---|
| Spiked Level (μg/L) | Recovery (%) | CV (%) | LOD (μg/L) | Linearity Range (μg/L) | |
| ABZ | 0.5 | 87.5% | 1.0% | 0.05 | 0.08–0.4 |
| 2 | 82.1% | 7.7% | |||
| 10 | 78.7% | 6.9% | |||
| ABZSO2 | 0.5 | 94.2% | 4.1% | 0.05 | 0.08–0.5 |
| 2 | 71.4% | 10.5% | |||
| 10 | 60.0% | 6.8% | |||
| ABZSO | 0.5 | 105.1% | 11.9% | 0.05 | 0.1–2.5 |
| 2 | 98.7% | 9.9% | |||
| 10 | 74.5% | 7.9% | |||
| ABZNH2SO2 | 10 | 108.0% | 10.3% | 0.50 | 1.5–73.5 |
| 30 | 76.6% | 12.3% | |||
| 90 | 75.2% | 2.9% | |||
| ABZNH2SO2 in Tissue Samples | |||||
|---|---|---|---|---|---|
| Spiked Level (μg/kg) | Recovery (%) | CV (%) | LOD (μg/kg) | Linearity Range (μg/kg) | |
| Beef | 10 | 84.1% | 2.4% | 1.12 | 2.3–25.9 |
| 30 | 91.6% | 4.9% | |||
| 90 | 74.2% | 6.6% | |||
| Liver | 10 | 87.8% | 4.5% | 0.56 | 1.3–23.7 |
| 30 | 106.1% | 9.1% | |||
| 90 | 108.8% | 15.9% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, S.; Zhou, X.; Dou, L.; Bai, Y.; Mi, J.; Yu, W.; Zhang, S.; Wang, Z.; Wen, K. Hapten Synthesis and Monoclonal Antibody Preparation for Simultaneous Detection of Albendazole and Its Metabolites in Animal-Origin Food. Foods 2021, 10, 3106. https://doi.org/10.3390/foods10123106
Shao S, Zhou X, Dou L, Bai Y, Mi J, Yu W, Zhang S, Wang Z, Wen K. Hapten Synthesis and Monoclonal Antibody Preparation for Simultaneous Detection of Albendazole and Its Metabolites in Animal-Origin Food. Foods. 2021; 10(12):3106. https://doi.org/10.3390/foods10123106
Chicago/Turabian StyleShao, Shibei, Xuping Zhou, Leina Dou, Yuchen Bai, Jiafei Mi, Wenbo Yu, Suxia Zhang, Zhanhui Wang, and Kai Wen. 2021. "Hapten Synthesis and Monoclonal Antibody Preparation for Simultaneous Detection of Albendazole and Its Metabolites in Animal-Origin Food" Foods 10, no. 12: 3106. https://doi.org/10.3390/foods10123106
APA StyleShao, S., Zhou, X., Dou, L., Bai, Y., Mi, J., Yu, W., Zhang, S., Wang, Z., & Wen, K. (2021). Hapten Synthesis and Monoclonal Antibody Preparation for Simultaneous Detection of Albendazole and Its Metabolites in Animal-Origin Food. Foods, 10(12), 3106. https://doi.org/10.3390/foods10123106








