Safety of Vitamin D Food Fortification and Supplementation: Evidence from Randomized Controlled Trials and Observational Studies
Abstract
:1. Introduction
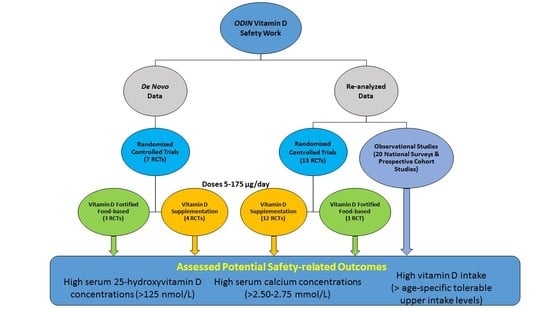
2. Methods, including Safety Approach, within the ODIN Project
2.1. Specification of Safety and the Adverse Effects
2.1.1. High Serum 25-Hydroxyvitamin D
2.1.2. High Serum Calcium Concentration
2.1.3. Dietary Intakes of Vitamin D Exceeding the Tolerable Upper Level
2.2. Studies Contributing Data on Safety and Adverse Effects
2.2.1. Randomized Controlled Trials
2.2.2. Observational Studies
2.2.3. Adverse Health Effects of High Vitamin D Intake or Serum 25(OH)D Concentrations
2.3. Collection of Safety Data
2.4. Ethical Considerations
3. Results
3.1. Study Characteristics
3.1.1. Observational Setting
3.1.2. Interventional Setting
3.2. Dietary Intakes of Vitamin D Exceeding the Tolerable Upper Level: Interventional Setting
3.3. Prevalence of High Serum 25(OH)D Concentrations (>125 nmol/L): Observational and Interventional Setting
3.3.1. Observational Studies in Children, Adolescents, and Older Adults
3.3.2. Interventional Setting: Vitamin D Supplementation RCTs
3.3.3. Interventional Setting: Vitamin D-Fortified Food-Based RCTs
3.4. Prevalence of High Serum Calcium Concentrations in Relation to Vitamin D-Fortified Foods and Dietary Supplements in Randomized Controlled Trials
3.4.1. Vitamin D supplementation RCTs
3.4.2. Vitamin D-Fortified Food-Based RCTs
3.5. Potential Additional Adverse Health Effects of High Vitamin D Intake or Serum 25(OH)D Concentrations
3.5.1. Observational Studies: Serum 25(OH)D and Mortality
3.5.2. Randomized Controlled Trials of Vitamin D Supplementation and Vitamin D-Enriched Foods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rejnmark, L.; Bislev, L.S.; Cashman, K.D.; Eiríksdottir, G.; Gaksch, M.; Grübler, M.; Grimnes, G.; Gudnason, V.; Lips, P.; Pilz, S.; et al. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PLoS ONE 2017, 12, e0180512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberg-Allardt, C.; Brustad, M.; Meyer, H.E.; Steingrimsdottir, L. Vitamin D—A systematic literature review for the 5th edition of the Nordic Nutrition Recommendations. Food Nutr. Res. 2013, 57, 22671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and plan for vitamin D food fortification: A review and guidance paper. Front. Endocrinol. (Lausanne) 2018, 9, 373. [Google Scholar] [CrossRef]
- O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal changes in vitamin D-effective UVB availability in Europe and associations with population serum 25-hydroxyvitamin D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Kiely, M.; Seamans, K.M.; Urbain, P. Effect of Ultraviolet Light-Exposed Mushrooms on Vitamin D Status: Liquid Chromatography-Tandem Mass Spectrometry Reanalysis of Biobanked Sera from a Randomized Controlled Trial and a Systematic Review plus Meta-Analysis. J. Nutr. 2016, 146, 565–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, A.; Duffy, S.; O’Grady, M.; Jakobsen, J.; Galvin, K.; Teahan-Dillon, J.; Kerry, J.; Kelly, A.; O’Doherty, J.; Higgins, S.; et al. Vitamin D-enhanced eggs are protective of wintertime serum 25-hydroxyvitamin D in a randomized controlled trial of adults. Am. J. Clin. Nutr. 2016, 104, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.; Cashman, K.D. Food-based solutions for vitamin D deficiency: Putting policy into practice and the key role for research. Proc. Nutr. Soc. 2017, 76, 54–63. [Google Scholar] [CrossRef]
- Seamans, K.M.; Cashman, K.D. Existing and potentially novel functional markers of vitamin D status: A systematic review. Am. J. Clin. Nutr. 2009, 89, 1997S–2008S. [Google Scholar] [CrossRef] [Green Version]
- Autier, P.; Gandini, S.; Mullie, P. A systematic review: Influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J. Clin. Endocrinol. Metab. 2012, 97, 2606–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, S.J.; Bonjour, J.-P.; Payen, F.D.; Rousseau, B. Moderate amounts of vitamin D3 in supplements are effective in raising serum 25-hydroxyvitamin D from low baseline levels in adults: A systematic review. Nutrients 2015, 7, 2311–2323. [Google Scholar] [CrossRef] [Green Version]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A. Vitamin D3 is more potent than vitamin D2 in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balachandar, R.; Pullakhandam, R.; Kulkarni, B.; Sachdev, H.S. Relative Efficacy of Vitamin D2 and Vitamin D3 in Improving Vitamin D Status: Systematic Review and Meta-Analysis. Nutrients 2012, 13, 3328. [Google Scholar] [CrossRef]
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R. (Eds.) Guidelines on Food Fortification with Micronutrients; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2006; Available online: http://apps.who.int/iris/bitstream/10665/43412/1/9241594012_eng.pdf (accessed on 21 June 2021).
- Institute of Medicine (US) Committee to Review. Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the Tolerable Upper Intake Level of vitamin D. EFSA J. 2012, 10, 2813. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Update of the tolerable upper intake level for vitamin D for infants. EFSA J. 2018, 16, 5365. [Google Scholar]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [CrossRef] [Green Version]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbanska, M.; Lukaszkiewicz, J.; Pludowski, P.; Jones, G. Vitamin D Toxicity-A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC (International Agency for Research on Cancer). Vitamin D and Cancer; IARC Working Group Reports; International Agency for Research on Cancer: Lyon, France, 2008; Volume 5. [Google Scholar]
- Cashman, K.D.; Kiely, M. Towards prevention of vitamin D deficiency and beyond: Knowledge gaps and research needs in vitamin D nutrition and public health. Br. J. Nutr. 2011, 106, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Brannon, P.M. Key questions in vitamin D research. Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 154–162. [Google Scholar]
- Kiely, M.; Cashman, K.D. Summary Outcomes of the ODIN Project on Food Fortification for Vitamin D Deficiency Prevention. Int. J. Environ Res. Public Health 2018, 15, 2342. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Kiely, M. Tackling inadequate vitamin D intakes within the population: Fortification of dairy products with vitamin D may not be enough. Endocrine 2016, 51, 38–46. [Google Scholar] [CrossRef]
- Sempos, C.T.; Vesper, H.W.; Phinney, K.W.; Thienpont, L.M.; Coates, P.M.; Vitamin D Standardization Program (VDSP). Vitamin D status as an international issue: National surveys and the problem of standardization. Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 32–40. [Google Scholar]
- Cashman, K.D.; Kiely, M.; Kinsella, M.; Durazo-Arvizu, R.A.; Tian, L.; Zhang, Y.; Lucey, A.; Flynn, A.; Gibney, M.J.; Vesper, H.W.; et al. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: A case study of the program’s potential for national nutrition and health surveys. Am. J. Clin. Nutr. 2013, 97, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashman, K.D.; Hayes, A.; Galvin, K.; Merkel, J.; Jones, G.; Kaufmann, M.; Hoofnagle, A.N.; Carter, G.D.; Durazo-Arvizu, R.; Sempos, C.T. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: A double-edged sword? Clin. Chem. 2015, 61, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography-Tandem Mass Spectrometry in the US Population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Swart, K.M.; Lips, P.; Brouwer, I.A.; Jorde, R.; Heymans, M.W.; Grimnes, G.; Grubler, M.R.; Gaksch, M.; Tomaschitz, A.; Pilz, S.; et al. Effects of vitamin D supplementation on markers for cardiovascular disease and type 2 diabetes: An individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018, 107, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Wallace, J.M.W.; Muldowney, S.; Fitzgerald, A.P.; Flynn, A.; Strain, J.J.; Kiely, M.; Horigan, G.; Hill, T.R.; Barnes, M.S.; et al. Estimation of the dietary requirement for vitamin D in free-living adults > = 64 y of age. Am. J. Clin. Nutr. 2009, 89, 1366–1374. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Hill, T.R.; Bonham, M.P.; Duffy, E.M.; Strain, J.J.; Wallace, J.M.W.; Kiely, M.; Lucey, A.J.; Taylor, N.; Seamans, K.M.; et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am. J. Clin. Nutr. 2008, 88, 1535–1542. [Google Scholar] [CrossRef] [Green Version]
- Muldowney, S.; Lucey, A.J.; Hill, T.R.; Seamans, K.M.; Taylor, N.; Wallace, J.M.; Horigan, G.; Barnes, M.S.; Bonham, M.P.; Duffy, E.M.; et al. Incremental cholecalciferol supplementation up to 15 mug/d throughout winter at 51-55o N has no effect on biomarkers of cardiovascular risk in healthy young and older adults. J. Nutr. 2012, 142, 1519–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, C.; Damsgaard, C.T.; Hauger, H.; Ritz, C.; Lanham-New, S.A.; Smith, T.J.; Hennessy, Á.; Dowling, K.; Cashman, K.D.; Kiely, M.; et al. Estimation of the dietary requirement for vitamin D in white children aged 4–8 y: A randomized, controlled, dose-response trial. Am. J. Clin. Nutr. 2016, 104, 1310–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.J.; Tripkovic, L.; Damsgaard, C.T.; Molgaard, C.; Ritz, C.; Wilson-Barnes, S.L.; Dowling, K.G.; Hennessy, Á.; Cashman, K.D.; Kiely, M.; et al. Estimation of the dietary requirement for vitamin D in adolescents aged 14–18 y: A dose-response, double-blind, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2016, 104, 1301–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, K.M.; Hennessy, Á.; Hull, G.L.; Healy, K.; Ritz, C.; Kenny, L.C.; Cashman, K.D.; Kiely, M.E. Estimation of the maternal vitamin D intake that maintains circulating 25-hydroxyvitamin D in late gestation at a concentration sufficient to keep umbilical cord sera 25–30 nmol/L: A dose-response, double-blind, randomized placebo-controlled trial in pregnant women at northern latitude. Am. J. Clin. Nutr. 2018, 108, 77–91. [Google Scholar] [PubMed] [Green Version]
- Adebayo, F.A.; Itkonen, S.T.; Öhman, T.; Skaffari, E.; Saarnio, E.M.; Erkkola, M.; Cashman, K.D.; Lamberg-Allardt, C. Vitamin D intake, serum 25-hydroxyvitamin D status and response to moderate vitamin D3 supplementation: A randomised controlled trial in East African and Finnish women. Br. J. Nutr. 2018, 119, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Chel, V.; Wijnhoven, H.A.; Smit, J.H.; Ooms, M.; Lips, P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos. Int. 2008, 19, 663–671. [Google Scholar]
- Wicherts, I.S.; Boeke, A.J.; van der Meer, I.M.; van Schoor, N.M.; Knol, D.L.; Lips, P. Sunlight exposure or vitamin D supplementation for vitamin D-deficient non-western immigrants: A randomized clinical trial. Osteoporos. Int. 2011, 22, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Oosterwerff, M.M.; Eekhoff, E.M.; Van Schoor, N.M.; Boeke, A.J.; Nanayakkara, P.; Meijnen, R.; Knol, D.L.; Kramer, M.H.; Lips, P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 152–160. [Google Scholar] [CrossRef]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grubler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; O Hartaigh, B.; Obermayer-Pietsch, B.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar]
- Sollid, S.T.; Hutchinson, M.Y.; Fuskevag, O.M.; Figenschau, Y.; Joakimsen, R.M.; Schirmer, H.; Njolstad, I.; Svartberg, J.; Kamycheva, E.; Jorde, R. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care 2014, 37, 2123–2131. [Google Scholar] [CrossRef] [Green Version]
- Sneve, M.; Figenschau, Y.; Jorde, R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur. J. Endocrinol. 2008, 159, 675–684. [Google Scholar] [CrossRef]
- Jorde, R.; Sneve, M.; Torjesen, P.; Figenschau, Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J. Intern Med. 2010, 267, 462–4672. [Google Scholar] [CrossRef]
- Beilfuss, J.; Berg, V.; Sneve, M.; Jorde, R.; Kamycheva, E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine 2012, 60, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Grimnes, G.; Figenschau, Y.; Almas, B.; Jorde, R. Vitamin D, insulin secretion, sensitivity, and lipids: Results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes 2011, 60, 2748–2757. [Google Scholar] [CrossRef] [Green Version]
- Kjaergaard, M.; Waterloo, K.; Wang, C.E.; Almas, B.; Figenschau, Y.; Hutchinson, M.S.; Svartberg, J.; Jorde, R. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: Nested case-control study and randomised clinical trial. Br. J. Psychiatry 2012, 201, 360–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimnes, G.; Joakimsen, R.; Figenschau, Y.; Torjesen, P.A.; Almas, B.; Jorde, R. The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass—A randomized controlled 1-year trial. Osteoporos. Int. 2012, 23, 201–211. [Google Scholar] [CrossRef]
- Wamberg, L.; Kampmann, U.; Stodkilde-Jorgensen, H.; Rejnmark, L.; Pedersen, S.B.; Richelsen, B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—Results from a randomized trial. Eur. J. Intern Med. 2013, 24, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Wamberg, L.; Pedersen, S.B.; Richelsen, B.; Rejnmark, L. The effect of high-dose vitamin D supplementation on calciotropic hormones and bone mineral density in obese subjects with low levels of circulating 25-hydroxyvitamin d: Results from a randomized controlled study. Calcif. Tissue Int. 2013, 93, 69–77. [Google Scholar] [CrossRef]
- Urbain, P.; Singler, F.; Ihorst, G.; Biesalski, H.K.; Bertz, H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Itkonen, S.T.; Skaffari, E.; Saaristo, P.; Saarnio, E.M.; Erkkola, M.; Jakobsen, J.; Cashman, K.D.; Lamberg-Allardt, C. Effects of vitamin D2-fortified bread v. supplementation with vitamin D2 or D3 on serum 25-hydroxyvitamin D metabolites: An 8-week randomised-controlled trial in young adult Finnish women. Br. J. Nutr. 2016, 115, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Manios, Y.; Moschonis, G.; Mavrogianni, C.; van den Heuvel, E.; Singh-Povel, C.M.; Kiely, M.; Cashman, K.D. Reduced-fat Gouda-type cheese enriched with vitamin D3 effectively prevents vitamin D deficiency during winter months in postmenopausal women in Greece. Eur. J. Nutr. 2017, 56, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Grønborg, I.M.; Tetens, I.; Christensen, T.; Andersen, E.W.; Jakobsen, J.; Kiely, M.; Cashman, K.D.; Andersen, R. Vitamin D-fortified foods improve wintertime vitamin D status in women of Danish and Pakistani origin living in Denmark: A randomized controlled trial. Eur. J. Nutr. 2020, 59, 741–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, L.A.; De Henauw, S.; González-Gross, M.; Kersting, M.; Molnár, D.; Gottrand, F.; Barrios, L.; Sjöström, M.; Manios, Y.; Gilbert, C.C.; et al. Design and implementation of the Healthy Lifestyle in Europe by Nutrition in Adolescence Cross-Sectional Study. Int. J. Obes. (Lond.) 2008, 32, S4–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Gross, M.; Valtueña, J.; Breidenassel, C.; Moreno, L.A.; Ferrari, M.; Kersting, M.; De Henauw, S.; Gottrand, F.; Azzini, E.; Widhalm, K.; et al. Vitamin D status among adolescents in Europe: The Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br. J. Nutr. 2012, 107, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Damsgaard, C.T.; Dalskov, S.M.; Petersen, R.A.; Sørensen, L.B.; Mølgaard, C.; Biltoft-Jensen, A.; Andersen, R.; Thorsen, A.V.; Tetens, I.; Sjödin, A.; et al. Design of the OPUS School Meal Study: A randomised controlled trial assessing the impact of serving school meals based on the New Nordic Diet. Scand. J. Public Health 2012, 40, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Damsgaard, C.T.; Dalskov, S.-M.; Laursen, R.P.; Ritz, C.; Hjorth, M.F.; Lauritzen, L.; Louise, B.S.; Petersen, R.A.; Andersen, M.R.; Stender, S.; et al. Provision of healthy school meals does not affect the metabolic syndrome score in 8-11-year-old children, but reduces cardiometabolic risk markers despite increasing waist circumference. Br. J. Nutr. 2014, 112, 1826–1836. [Google Scholar]
- Oberg, J.; Jorde, R.; Almas, B.; Emaus, N.; Grimnes, G. Vitamin D deficiency and lifestyle risk factors in a Norwegian adolescent population. Scand. J. Public Health 2014, 42, 593–602. [Google Scholar] [CrossRef]
- Winther, A.; Dennison, E.; Ahmed, L.A.; Furberg, A.S.; Grimnes, G.; Jorde, R.; Gjesdal, C.G.; Emaus, N. The Tromsø Study: Fit Futures: A study of Norwegian adolescents’ lifestyle and bone health. Arch. Osteoporos. 2014, 9, 185. [Google Scholar]
- The Tromsø Study: UiT the Artic University of Norway. Available online: https://uit.no/research/tromsostudy (accessed on 29 April 2021).
- Moschonis, G.; Tanagra, S.; Vandorou, A.; Kyriakou, A.E.; Dede, V.; Siatitsa, P.E.; Koumpitski, A.; Androutsos, O.; Grammatikaki, E.; Kantilafti, M.; et al. Social, economic and demographic correlates of overweight and obesity in primary-school children: Preliminary data from the Healthy Growth Study. Public Health Nutr. 2010, 13, 1693–1700. [Google Scholar] [CrossRef] [Green Version]
- McBride, D.; Keil, T.; Grabenhenrich, L.; Dubakiene, R.; Drasutiene, G.; Fiocchi, A.; Dahdah, L.; Sprikkelman, A.B.; Schoemaker, A.A.; Roberts, G.; et al. The EuroPrevall birth cohort study on food allergy: Baseline characteristics of 12,000 newborns and their families from nine European countries. Pediatr. Allergy Immunol. 2012, 23, 230–239. [Google Scholar] [CrossRef]
- Kiely, M.; O’Donovan, S.M.; Kenny, L.C.; Hourihane, J.O.; Irvine, A.D.; Murray, D.M. Vitamin D metabolite concentrations in umbilical cord blood serum and associations with clinical characteristics in a large prospective mother-infant cohort in Ireland. J. Steroid Biochem. Mol. Biol. 2017, 167, 162–168. [Google Scholar] [CrossRef]
- Ní Chaoimh, C.; McCarthy, E.K.; Hourihane, J.O.; Kenny, L.C.; Irvine, A.D.; Murray, D.M.; Kiely, M.E. Low vitamin D deficiency in Irish toddlers despite northerly latitude and a high prevalence of inadequate intakes. Eur. J. Nutr. 2018, 57, 783–794. [Google Scholar] [CrossRef]
- Kurth, B.-M.; Kamtsiuris, P.; Hölling, H.; Schlaud, M.; Dölle, R.; Ellert, U.; Kahl, H.; Knopf, H.; Lange, M.; Mensink, G.B.; et al. The challenge of comprehensively mapping children’s health in a nation-wide health survey: Design of the German KiGGS-Study. BMC Public Health 2008, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Public Health England and Food Standards Agency. National Diet and Nutrition Survey Results from Years 1 to 4 (combined) of the Rolling Programme (2008/2009–2011/2012). 2014. Available online: https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (accessed on 29 April 2021).
- Scheidt-Nave, C.; Kamtsiuris, P.; Gößwald, A.; Hölling, H.; Lange, M.; Busch, M.A.; Dahm, S.; Dölle, R.; Ellert, U.; Fuchs, J.; et al. German health interview and examination survey for adults (DEGS)—design, objectives and implementation of the first data collection wave. BMC Public Health 2012, 12, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamtsiuris, P.; Lange, M.; Hoffmann, R.; Schaffrath Rosario, A.; Dahm, S.; Kuhnert, R.; Kurth, B.M. The first wave of the German Health Interview and Examination Survey for Adults (DEGS1): Sample design, response, weighting and representativeness. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Grimnes, G.; Almaas, B.; Eggen, A.E.; Emaus, N.; Figenschau, Y.; Hopstock, L.A.; Hutchinson, M.S.; Methlie, P.; Mihailova, A.; Sneve, M.; et al. Effect of smoking on the serum levels of 25-hydroxyvitamin D depends on the assay employed. Eur. J. Endocrinol. 2010, 163, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, B.K.; Eggen, A.E.; Mathiesen, E.B.; Wilsgaard, T.; Njølstad, I. Cohort profile: The Tromsø Study. Int. J. Epidemiol. 2012, 41, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Eggen, A.E.; Mathiesen, E.B.; Wilsgaard, T.; Jacobsen, B.K.; Njølstad, I. The sixth survey of the Tromsø Study (Tromsø 6) in 2007–08: Collaborative research in the interface between clinical medicine and epidemiology: Study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand. J. Public Health 2013, 41, 65–80. [Google Scholar]
- van ’t Riet, E.; Alssema, M.J.; Rijkelijkhuizen, J.M.; Kostense, P.J.; Nijpels, G.; Dekker, J.M. Relationship between A1C and glucose levels in the general Dutch population: The new Hoorn study. Diabetes Care 2010, 33, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huisman, M.; Poppelaars, J.L.; van der Horst, M.H.L.; Beekman, A.T.F.; Brug, J.; van Tilburg, T.G.; Deeg, D.J.H. Cohort profile: The Longitudinal Aging Study Amsterdam. Int. J. Epidemiol. 2011, 40, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Harris, T.B.; Launer, L.J.; Eiriksdottir, G.; Kjartansson, O.; Jonsson, P.V.; Sigurdsson, G.; Thorgeirsson, G.; Aspelund, T.; Garcia, M.E.; Cotch, M.F.; et al. Age, Gene/Environment Susceptibility–Reykjavik Study: Multidisciplinary applied phenomics. Am. J. Epidemiol. 2007, 165, 1076–1087. [Google Scholar] [CrossRef] [Green Version]
- Tiittala, P.J.; Kivelä, P.S.; Ristola, M.A.; Surcel, H.M.; Koponen, P.M.; Mölsä, M.; Ollgren, J.; Liitsola, K. Achieving high acceptability of HIV testing in a population-based survey among immigrants in Finland. Scand. J. Public Health 2015, 43, 393–398. [Google Scholar] [CrossRef]
- Finnish Institute for Health and Welfare. Migrant Health and Wellbeing Study (Maamu). 2021. Available online: https://www.thl.fi/fi/web/thlfi-en/research-and-expertwork/population-studies/migrant-health-and-wellbeing-study-maamu- (accessed on 29 April 2021).
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe—Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiely, M.E.; Zhang, J.Y.; Kinsella, M.; Khashan, A.S.; Kenny, L.C. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am. J. Clin. Nutr. 2016, 104, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bärebring, L.; Schoenmakers, I.; Glantz, A.; Hulthén, L.; Jagner, Å.; Ellis, J.; Bärebring, M.; Bullarbo, M.; Augustin, H. Vitamin D Status during Pregnancy in a Multi-Ethnic Population-Representative Swedish Cohort. Nutrients 2016, 22, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njolstad, I.; Lochen, M.L.; Marz, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar]
- Appel, L.J.; Michos, E.D.; Mitchell, C.M.; Blackford, A.L.; Sternberg, A.L.; Miller, E.R., 3rd; Juraschek, S.P.; Schrack, J.A.; Szanton, S.L.; Charleston, J.; et al. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial. Ann. Intern Med. 2021, 174, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.L.; Kroger, H.; Karppinen, J.; et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population—Evidence from an 11-year follow-up based on standardized 25-hydroxyvitamin D data. Am. J. Clin. Nutr. 2017, 105, 1512–1520. [Google Scholar] [CrossRef] [Green Version]
- Rippin, H.L.; Hutchinson, J.; Evans, C.E.L.; Jewell, J.; Breda, J.J.; Cade, J.E. National nutrition surveys in Europe: A review on the current status in the 53 countries of the WHO European region. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Durazo-Arvizu, R.A.; Dawson-Hughes, B.; Kramer, H.; Cao, G.; Merkel, J.; Coates, P.M.; Sempos, C.T. The Reverse J-Shaped Association Between Serum Total 25-Hydroxyvitamin D Concentration and All-Cause Mortality: The Impact of Assay Standardization. Am. J. Epidemiol. 2017, 185, 720–726. [Google Scholar] [CrossRef]
- Grant, W.B.; Karras, S.N.; Bischoff-Ferrari, H.A.; Annweiler, C.; Boucher, B.J.; Juzeniene, A.; Garland, C.F.; Holick, M.F. Do studies reporting ‘U’-shaped serum 25-hydroxyvitamin D-health outcome relationships reflect adverse effects? Dermato-Endocrinol. 2016, 8, e1187349. [Google Scholar] [CrossRef] [Green Version]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal Relationship between Vitamin D Status and Parathyroid Hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef] [PubMed]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA 2019, 322, 736–745. [Google Scholar] [CrossRef]
- Mo, M.; Wang, S.; Chen, Z.; Muyiduli, X.; Wang, S.; Shen, Y.; Shao, B.; Li, M.; Chen, D.; Chen, Z.; et al. A systematic review and meta-analysis of the response of serum 25-hydroxyvitamin D concentration to vitamin D supplementation from RCTs from around the globe. Eur. J. Clin. Nutr. 2019, 73, 816–834. [Google Scholar] [CrossRef]
- Malihi, Z.; Wu, Z.; Stewart, A.W.; Lawes, C.M.; Scragg, R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1039–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itkonen, S.T.; Erkkola, M.; Lamberg-Allardt, C.J.E. Vitamin D fortification of fluid milk products and their contribution to vitamin D intake and vitamin D status in observational studies—A review. Nutrients 2018, 10, 1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, L.J.; Walton, J.; Flynn, A.; Cashman, K.D.; Kiely, M. Small Increments in Vitamin D Intake by Irish Adults over a Decade Show That Strategic Initiatives to Fortify the Food Supply Are Needed. J. Nutr. 2015, 145, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| References | Country | Total n | Age (Yrs) | Sex: % Female | Population Group | Duration of Intervention | Intervention Groups |
|---|---|---|---|---|---|---|---|
| Cashman et al., 2009 *, Muldowney et al., 2012 * | Ireland | 200 | ≥64 | 59.2 | Adults | 22 wk | Placebo-controlled |
| Vitamin D3 Supplements (5 or 10 or 15 µg/d) | |||||||
| Cashman et al., 2008 *, Muldowney et al., 2012 * | Ireland | 214 | 20–40 | 50.0 | Adults | 22 wk | Placebo-controlled |
| Vitamin D3 Supplements (5 or 10 or 15 µg/d) | |||||||
| Mortensen et al., 2016 ** | Denmark | 119 | 4–8 | 53.1 | Children | 20 wk | Placebo-controlled |
| Vitamin D3 Supplements (10 or 20 µg/d) | |||||||
| Smith et al., 2016 ** | UK | 105 | 14–18 | 57.3 | Adolescents | 20 wk | Placebo-controlled |
| Vitamin D3 Supplements (10 or 20 µg/d) | |||||||
| O’Callaghan et al., 2018 ** | Ireland | 144 | 21–41 | 100 | Pregnant women | 25 wk | Placebo-controlled |
| Vitamin D3 Supplements (10 or 20 µg/d) | |||||||
| Adebayo et al., 2018 ** | Finland | 125 | 21–64 | 100 | Ethnic women | 5 mo | Placebo-controlled |
| Vitamin D3 Supplements (10 or 20 µg/d) | |||||||
| Chel et al., 2008 * | Netherlands | 273 | >70 years | 77.4 | Nursing home residents | 4 mo | Placebo-controlled |
| Vitamin D3 Supplements (15 µg/d) | |||||||
| Wicherts et al., 2011 * | Netherlands | 148 | 18–65 | 74.8 | Non-western immigrants, 25(OH)D <25 nmol/L | 6 mo | Placebo-controlled |
| Vitamin D3 Supplements (20 µg/d) | |||||||
| Oosterwerff et al., 2014 * | Netherlands | 110 | 20–65 | 60.0 | Non-western immigrants, prediabetic, with 25(OH)D <50 nmol/L | 16 wk | Placebo-controlled |
| Vitamin D3 Supplements (30 µg/d) | |||||||
| Pilz et al., 2015 * | Austria | 187 | ≥18 | 47.0 | Persons with history of arterial hypertension, 25(OH)D <75 nmol/L | 8 wk | Placebo-controlled |
| Vitamin D3 Supplements (70 µg/d) | |||||||
| Sollid et al., 2014 * | Norway | 484 | 21–80 | 38.6 | Persons with IGT and/or IFG | 1yr | Placebo-controlled |
| Vitamin D3 Supplements (71 µg/d) | |||||||
| Sneve et al., 2008 *, Jorde et al., 2010 *, Beilfuss et al., 2012 * | Norway | 334 | 21-70 | 64.2 | Persons with high BMI | 1 yr | Placebo-controlled |
| Vitamin D3 Supplements (71 or 143 µg/d) | |||||||
| Grimnes et al., 2011 * | Norway | 93 | 30–75 | 49.5 | Persons with 25(OH)D <42 nmol/L | 6 mo | Placebo-controlled |
| Vitamin D3 Supplements (143 µg/d) | |||||||
| Kjaergaard et al., 2012 * | Norway | 230 | 30–75 | 54.7 | Persons with 25(OH)D <55 nmol/L | 6 mo | Placebo-controlled |
| Vitamin D3 Supplements (143 µg/d) | |||||||
| Grimnes et al., 2012 * | Norway | 275 | 50–80 | 100 | Women with low BMD | 1 yr | Placebo-controlled |
| Vitamin D3 Supplements (163 µg/d) | |||||||
| Wamberg et al., 2013 * Wamberg et al., 2013 * | Denmark | 43 | 18–50 | 71.2 | Persons with high BMI, 25(OH)D <50 nmol/L | 6 mo | Placebo-controlled |
| Vitamin D3 Supplements (175 µg/d) | |||||||
| Urbain et al., 2011 * | Germany | 26 | ≤45 | 65 | Adults | 4 wk | Placebo-controlled |
| Vitamin D2-enriched mushrooms or D2 supplement (700 µg/wk) | |||||||
| Itkonen et al., 2016 ** | Finland | 37 | 20–37 | 100 | Adults | 8 wk | Placebo-controlled |
| Vitamin D2-enriched bread or D2 supplement (25 µg/d) or D3 (25 µg/d) | |||||||
| Manios et al., 2017 ** | Greece | 79 | 55–75 | 100 | Adults | 20 wk | Placebo-controlled |
| Vitamin D3-enriched Gouda cheese (5.7 µg/d) | |||||||
| Grønborg et al., 2020 ** | Denmark | 127 | 18–50 | 100 | Ethnic women | 3 mo | Placebo-controlled |
| Vitamin D3-enriched food (20 µg/d) |
| References | Population Group | Duration of Intervention | Intervention Groups | Prevalence (%) of S-25(OH)D >125 nmol/L (n/Total n) | |
|---|---|---|---|---|---|
| Baseline | Endpoint | ||||
| Vitamin D supplementation RCTs—Supplemental vitamin D3 dose ≤70 µg/d | |||||
| Cashman et al., 2009 *, Muldowney et al., 2012 * | Persons >63 years of age | 22 wk | 0 µg of vitamin D3 | 0 | 0 |
| 5 µg of vitamin D3 | 0 | 0 | |||
| 10 µg of vitamin D3 | 0 | 0 | |||
| 15 µg of vitamin D3 | 0 | 0 | |||
| Cashman et al., 2008 *, Muldowney et al., 2012 * | Persons 20–40 years of age | 22 wk | 0 µg of vitamin D3 | 1.8 (1/56) | 0 |
| 5 µg of vitamin D3 | 0 | 0 | |||
| 10 µg of vitamin D3 | 1.8 (1/57) | 0 | |||
| 15 µg of vitamin D3 | 1.9 (1/52) | 0 | |||
| Mortensen et al., 2016 ** | Children | 20 wk | 0 µg of vitamin D3 | 0 | 0 |
| 10 µg of vitamin D3 | 0 | 0 | |||
| 20 µg of vitamin D3 | 0 | 0 | |||
| Smith et al., 2016 ** | Adolescents | 20 wk | 0 µg of vitamin D3 | 0 | 0 |
| 10 µg of vitamin D3 | 0 | 0 | |||
| 20 µg of vitamin D3 | 0 | 2.6 (1/38) | |||
| O’Callaghan et al., 2018 ** | Pregnant women | 25 wk | 0 µg of vitamin D3 | 0 | 0 |
| 10 µg of vitamin D3 | 0 | 13.5 (5/37) | |||
| 20 µg of vitamin D3 | 0 | 13.6 (6/44) | |||
| Adebayo et al., 2018 ** | Women of East African descent | 5 mo | 0 µg of vitamin D3 | 0 | 0 |
| 10 µg of vitamin D3 | 0 | 0 | |||
| 20 µg of vitamin D3 | 0 | 8.3 (1/12) | |||
| Women of Finnish descent | 0 µg of vitamin D3 | 0 | 0 | ||
| 10 µg of vitamin D3 | 0 | 0 | |||
| 20 µg of vitamin D3 | 0 | 0 | |||
| Chel et al., 2008 * | Nursing home residents >70 years of age | 4 mo | 0 µg of vitamin D3 | 0 | 0 |
| 15 µg of vitamin D3 | 0 | 0 | |||
| Wicherts et al., 2011 * | Non-western immigrants with 25(OH)D values <25 nmol/L | 6 mo | 0 µg of vitamin D3 | 0 | 0 |
| 20 µg of vitamin D3 | 0 | 0 | |||
| Oosterwerff et al., 2014 * | Non-western immigrants with pre-diabetes and 25(OH)D values <50 nmol/L | 16 wk | 0 µg of vitamin D3 | 0 | 0 |
| 30 µg of vitamin D3 | 0 | 0 | |||
| Pilz et al., 2015 * | Persons with a history of arterial hypertension and 25(OH)D values <75 nmol/L | 8 wk | 0 µg of vitamin D3 | 0 | 0 |
| 70 µg of vitamin D3 | 0 | 0 | |||
| Vitamin D supplementation RCTs—Supplemental vitamin D3 dose ≥71 µg/d | |||||
| Sollid et al., 2014 * | Persons with IGT and/or IFG | 1 yr | 0 µg of vitamin D3 | 0.4 (1/255) | 0.8 (2/242) |
| 71 µg of vitamin D3 | 0.4 (1/256) | 7.0 (17/242) | |||
| Sneve et al., 2008 *, Jorde et al., 2010 *, Beilfuss et al., 2012 * | Persons with a high BMI | 1 yr | 0 µg of vitamin D3 | 0 | 0 |
| 71 µg of vitamin D3 | 0 | 11.3 (12/106) | |||
| 143 µg of vitamin D3 | 0 | 53.4 (62/116) | |||
| Grimnes et al., 2011 * | Persons with 25(OH)D values <42 nmol/L | 6 mo | 0 µg of vitamin D3 | 0 | 0 |
| 143 µg of vitamin D3 | 0 | 46.9 (23/49) | |||
| Kjaergaard et al., 2012 * | Persons with 25(OH)D values <55 nmol/L | 6 mo | 0 µg of vitamin D3 | 0 | 0 |
| 143 µg of vitamin D3 | 0 | 49.2 (59/120) | |||
| Grimnes et al., 2012 * | Women with a low BMD | 1 yr | 0 µg of vitamin D3 | 0.7 (1/148) | 0 |
| 163 µg of vitamin D3 | 1.3 (2/149) | 91.9 (125/136) | |||
| Wamberg et al., 2013 * Wamberg et al., 2013 * | Persons with a high BMI and 25(OH)D values <50 nmol/L | 6 mo | 0 µg of vitamin D3 | 3.8 (1/26) | 0 |
| 175 µg of vitamin D3 | 0 | 31.8 (7/22) | |||
| Vitamin D fortified food based RCTs | |||||
| Urbain et al., 2011 * | Adults | 4 wk | Placebo-controlled | 0 | 0 |
| D2-enriched mushrooms providing 700 µg of vitamin D2 weekly | 0 | 0 | |||
| D2 supplement providing 700 µg of vitamin D2 weekly | 0 | 0 | |||
| Itkonen et al., 2016 ** | Adults | 8 wk | Placebo-controlled | 0 | 0 |
| D2-enriched bread providing 25 µg of vitamin D2 daily | 0 | 0 | |||
| D2 supplement providing 25 µg of vitamin D2 daily | 0 | 0 | |||
| D3 supplement providing 25 µg of vitamin D3 daily | 0 | 0 | |||
| Manios et al., 2017 ** | Adults | 20 wk | Placebo-controlled | 0 | 0 |
| D3-enriched Gouda cheese providing 5.7 µg of vitamin D3 daily | 0 | 0 | |||
| Grønborg et al., 2020 ** | Women of Pakistani descent | 3 mo | Placebo-controlled | 0 | 0 |
| D3-enriched food providing 20 µg of vitamin D3 daily | 2.9 (1/35) | 2.9 (1/35) | |||
| Women of Danish descent | Placebo-controlled | 0 | 0 | ||
| D3-enriched food providing 20 µg of vitamin D3 daily | 0 | 2.7 (1/37) | |||
| References | Population Group | Type of Intervention | Upper Limit (UL) of Reference Range for S-Ca (mmol/L) | Number of Subjects with S-Ca >UL Out of Total Number of Subjects | Prevalence (%) of Subjects Exceeding Upper Limit of Reference Range of S-Ca | Highest S-Ca Concentration mmol/L if >UL |
|---|---|---|---|---|---|---|
| Vitamin D supplementation RCTs—Supplemental vitamin D3 dose ≤70 µg/d | ||||||
| Cashman et al., 2009 *, Muldowney et al., 2012 * | Persons of age > 63 yrs | Vitamin D3 | 2.60 | 1/200 | 0.5 | NA |
| Cashman et al., 2008 *, Muldowney et al., 2012 * | Persons of age 20–40 yrs | Vitamin D3 | 2.60 | 0/214 | 0 | NA |
| Mortensen et al., 2016 ** | Children | Vitamin D3 | >2.70 | 0 | 0 | |
| Smith et al., 2016 ** | Adolescents | Vitamin D3 | 2.50 | 0 | 0 | |
| O’Callaghan et al., 2018 ** | Pregnant women | Vitamin D3 | 2.63 | 0 | 0 | 0 |
| Adebayo et al., 2018 ** | Ethnic women | Vitamin D3 | 2.65 | 9/147 (baseline) 2/125 (endpoint) | 6.1 (baseline) 1.6 (endpoint) | 2.82 (baseline) 2.74 (endpoint) |
| Chel et al., 2008 * | Nursing home residents, age >70 years | Vitamin D3 | NA | NA | NA | NA |
| Wicherts et al., 2011 * | Non-western immigrants, 25(OH)D <25 nmol/L | Vitamin D3 | 2.60 | 1/112 | 0.9 | NA |
| Oosterwerff et al., 2014 * | Non-western immigrants, prediabetic, with 25(OH)D <50 nmol/L | Vitamin D3 *** | 2.60 | 0/110 | 0 | NA |
| Pilz et al., 2015 * | Persons with history of arterial hypertension, 25(OH)D <75 nmol/L | Vitamin D3 | 2.55 | 3/188 | 1.6 | NA |
| Vitamin D supplementation RCTs—Supplemental vitamin D3 dose ≥71 µg/d | ||||||
| Sollid et al., 2014 * | Persons with IGT and/or IFG | Vitamin D3 *** | 2.60 | 0/484 | 0 | NA |
| Sneve et al., 2008 *, Jorde et al., 2010 *, Beilfuss et al., 2012 * | Persons with high BMI | Vitamin D3 | 2.60 | 0/334 | 0 | NA |
| Grimnes et al., 2011 * | Persons with 25(OH)D <42 nmol/L | Vitamin D3 | 2.60 | 0/94 | 0 | NA |
| Kjaergaard et al., 2012 * | Persons with 25(OH)D <55 nmol/L | Vitamin D3 | 2.55 | 1/230 | 0.4 | NA |
| Grimnes et al., 2012 * | Women with low BMD | Vitamin D3 *** | 2.55 | 8/275 | 2.9 | NA |
| Wamberg et al., 2013 * Wamberg et al., 2013 * | Persons with high BMI, 25(OH)D <50 nmol/L | Vitamin D3 | NA | NA | NA | NA |
| Vitamin D fortified food based RCTs | ||||||
| Urbain et al., 2011 * | Adults | D2-enriched mushrooms | >2.70 | 0 | 0 | |
| Itkonen et al., 2016 ** | Adults | D2-enriched bread, vitamin D2 and D3 supplements | 2.65 | 1/37 (endpoint) | 2.7 (endpoint) | 2.86 (endpoint) |
| Manios et al., 2017 ** | Adults | D3-enriched Gouda cheese | NA | NA | NA | NA |
| Grønborg et al., 2020 ** | Ethnic women | D3-enriched food | 2.55 | 9/127 (Baseline) 13/127 (endpoint) | 7.1 (baseline) 10.2 (endpoint) | 2.67 (Baseline) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adebayo, F.A.; Itkonen, S.T.; Öhman, T.; Kiely, M.; Cashman, K.D.; Lamberg-Allardt, C.; on behalf of the ODIN Consortium. Safety of Vitamin D Food Fortification and Supplementation: Evidence from Randomized Controlled Trials and Observational Studies. Foods 2021, 10, 3065. https://doi.org/10.3390/foods10123065
Adebayo FA, Itkonen ST, Öhman T, Kiely M, Cashman KD, Lamberg-Allardt C, on behalf of the ODIN Consortium. Safety of Vitamin D Food Fortification and Supplementation: Evidence from Randomized Controlled Trials and Observational Studies. Foods. 2021; 10(12):3065. https://doi.org/10.3390/foods10123065
Chicago/Turabian StyleAdebayo, Folasade A., Suvi T. Itkonen, Taina Öhman, Mairead Kiely, Kevin D. Cashman, Christel Lamberg-Allardt, and on behalf of the ODIN Consortium. 2021. "Safety of Vitamin D Food Fortification and Supplementation: Evidence from Randomized Controlled Trials and Observational Studies" Foods 10, no. 12: 3065. https://doi.org/10.3390/foods10123065
APA StyleAdebayo, F. A., Itkonen, S. T., Öhman, T., Kiely, M., Cashman, K. D., Lamberg-Allardt, C., & on behalf of the ODIN Consortium. (2021). Safety of Vitamin D Food Fortification and Supplementation: Evidence from Randomized Controlled Trials and Observational Studies. Foods, 10(12), 3065. https://doi.org/10.3390/foods10123065