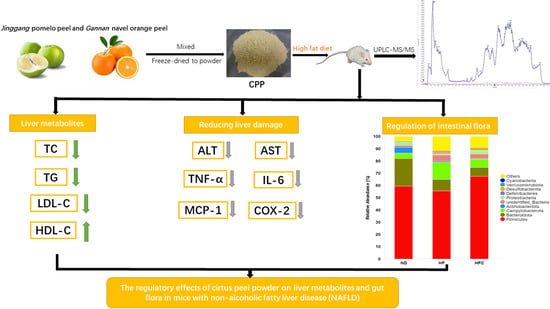
The Regulatory Effects of Citrus Peel Powder on Liver Metabolites and Gut Flora in Mice with Non-Alcoholic Fatty Liver Disease (NAFLD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Determination of the Bioactive Components of CPP
2.3. Animals, Diet, and Experimental Design
2.4. Histological Analysis
2.5. Serum and Liver Biochemical Analysis
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. The Bioactive Components of CPP
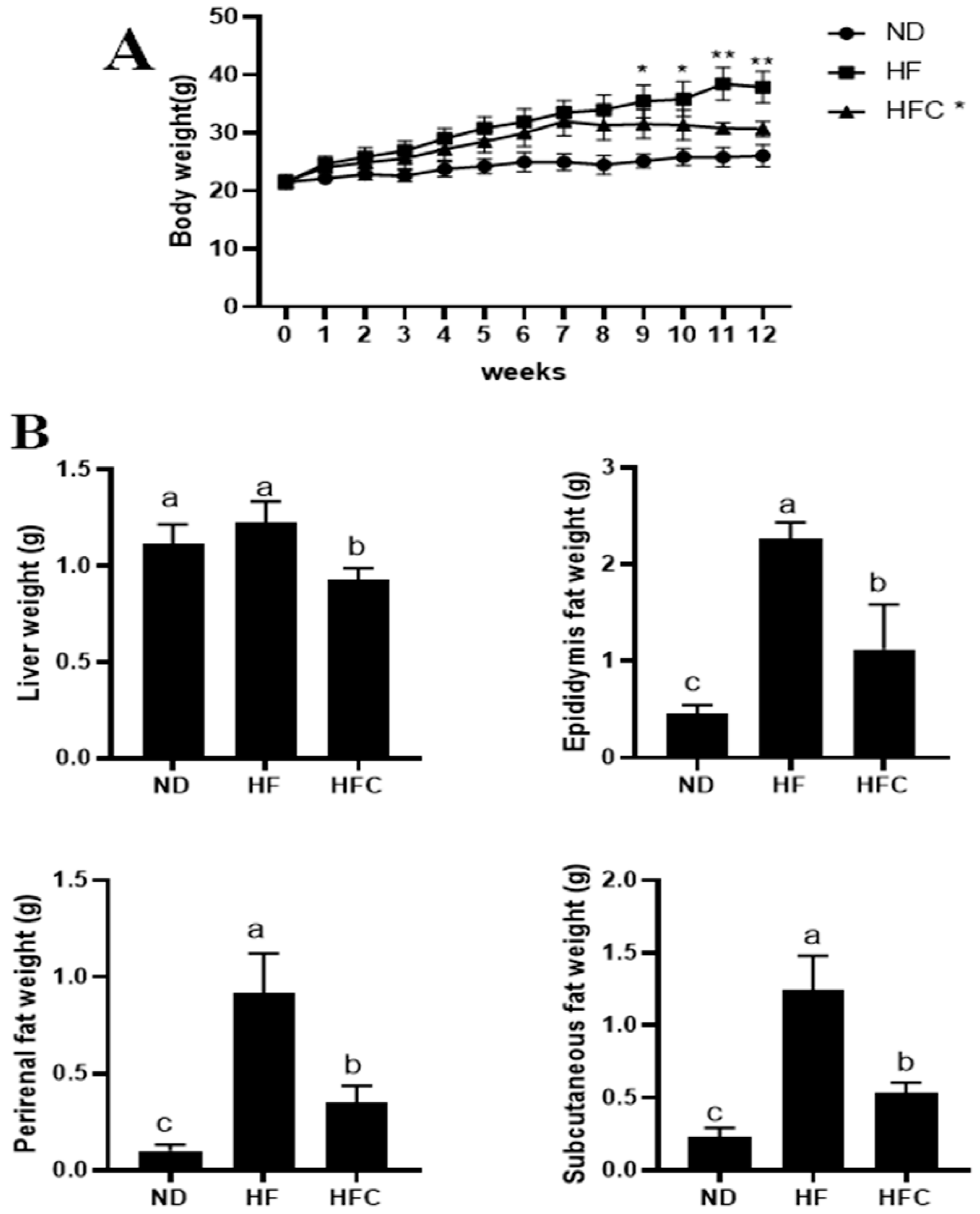
3.2. CPP Prevents Obesity Caused by High-Fat Diet
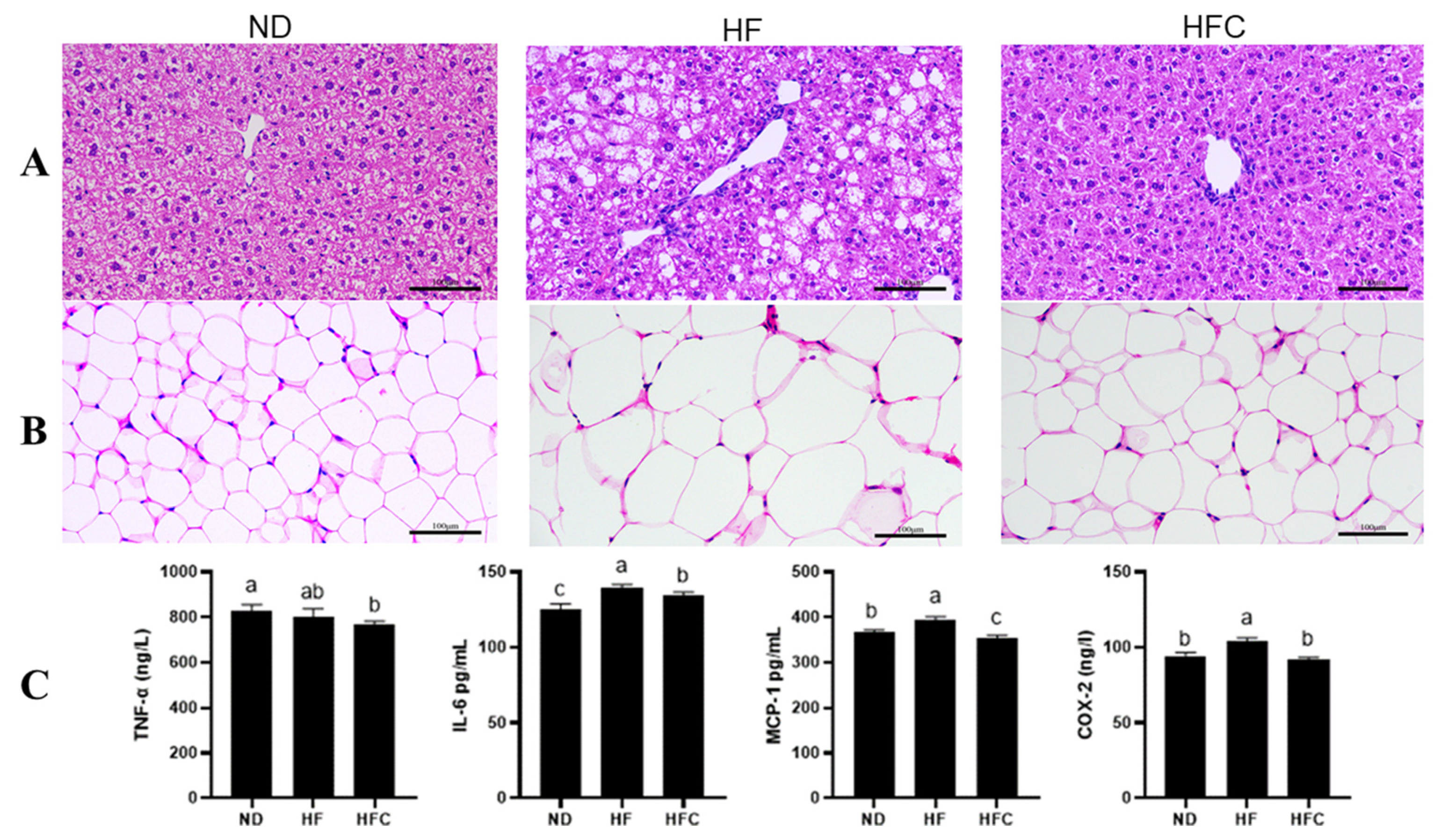
3.3. Histological Analysis
3.4. The Anti-Inflammatory Effect of CPP
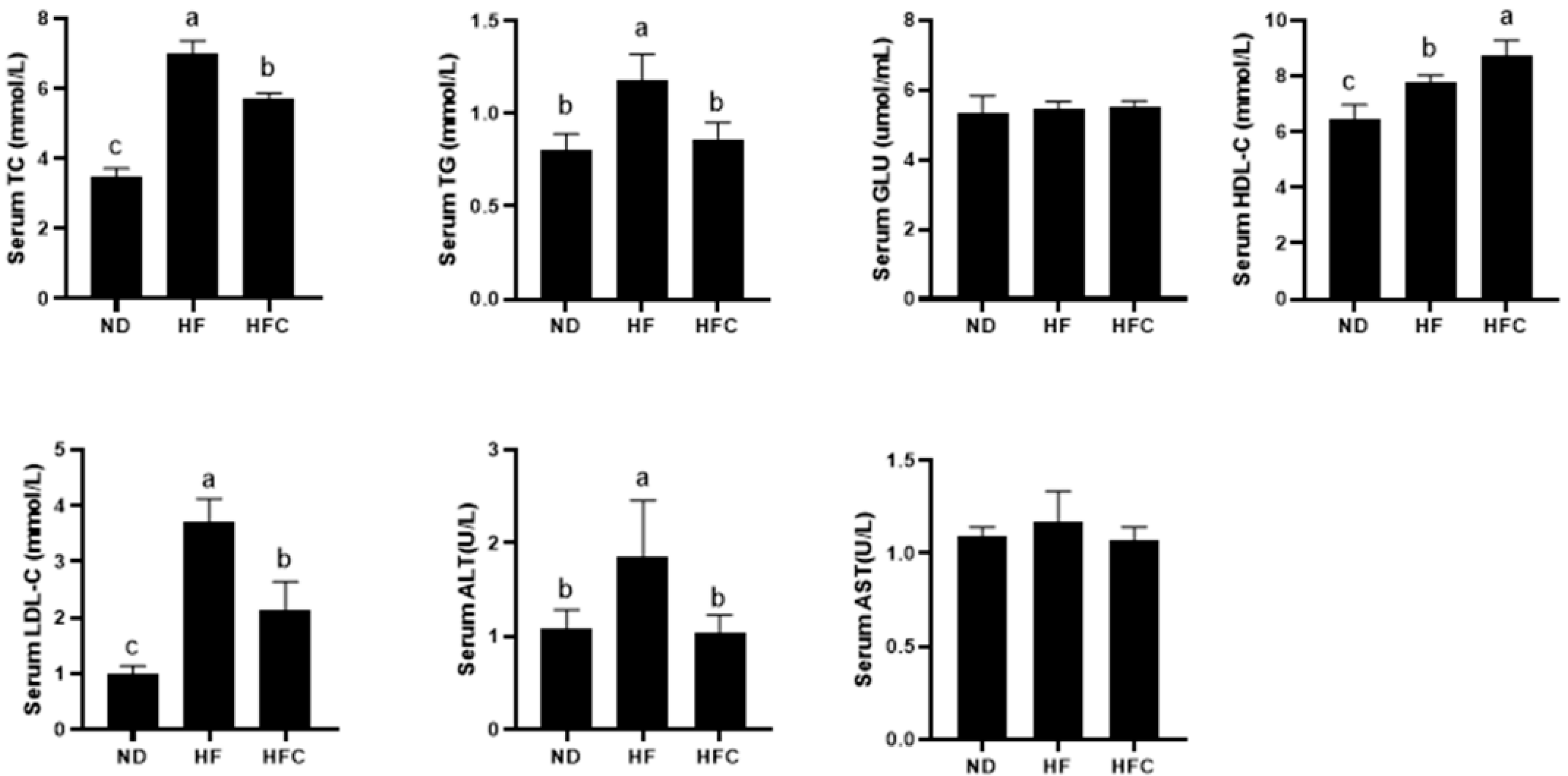
3.5. Effect of CPP on Serum Biochemical Parameters
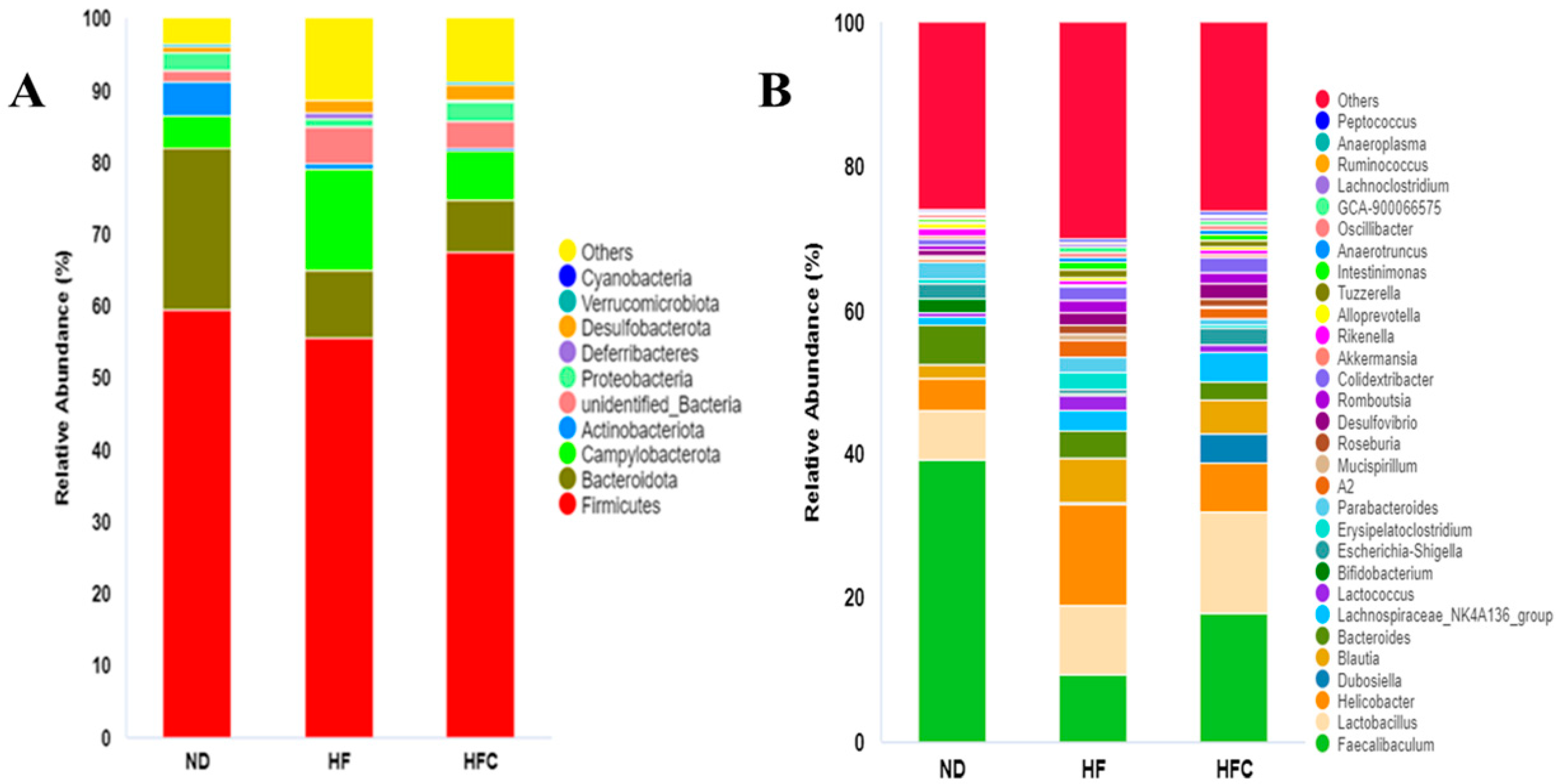
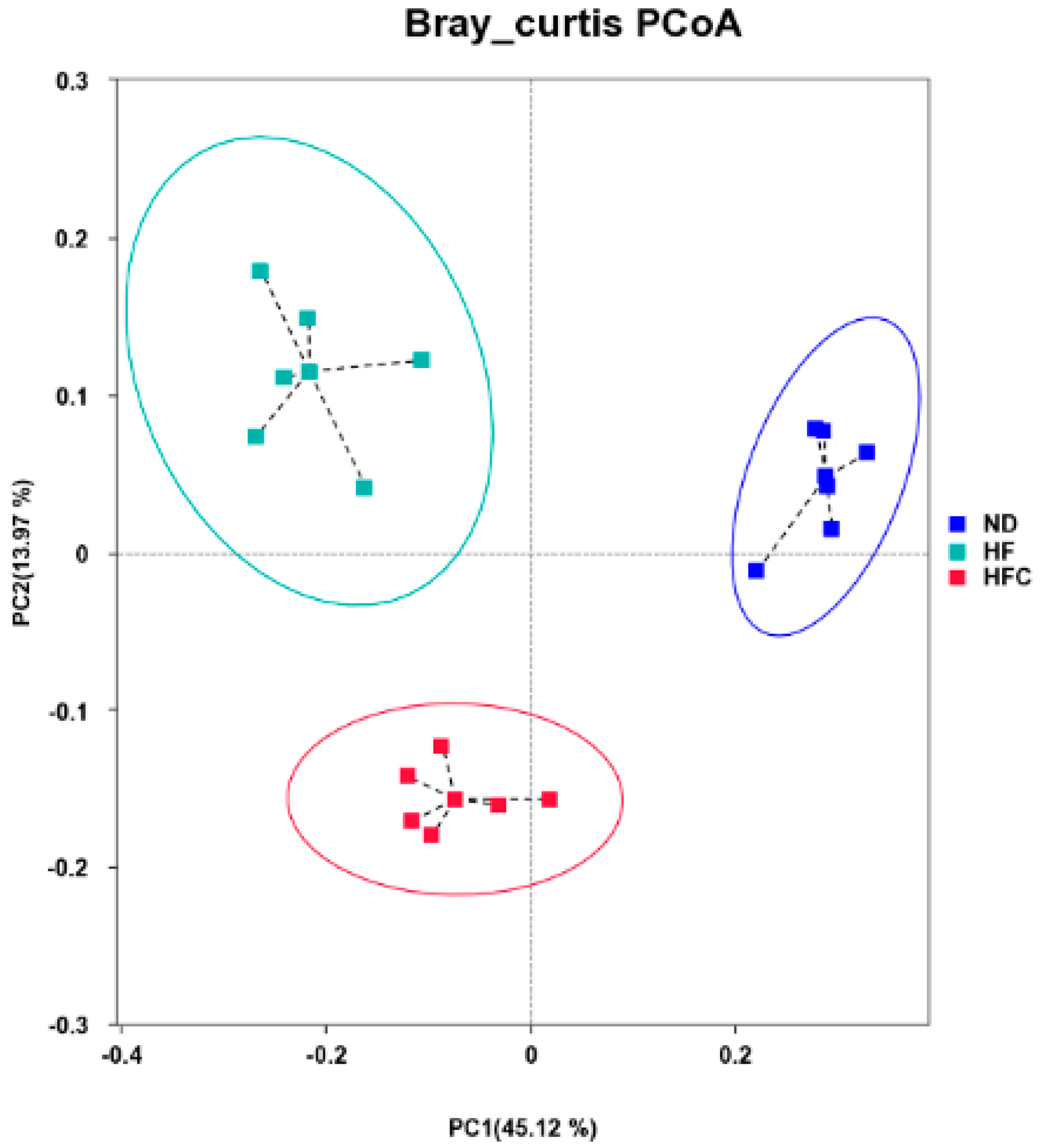
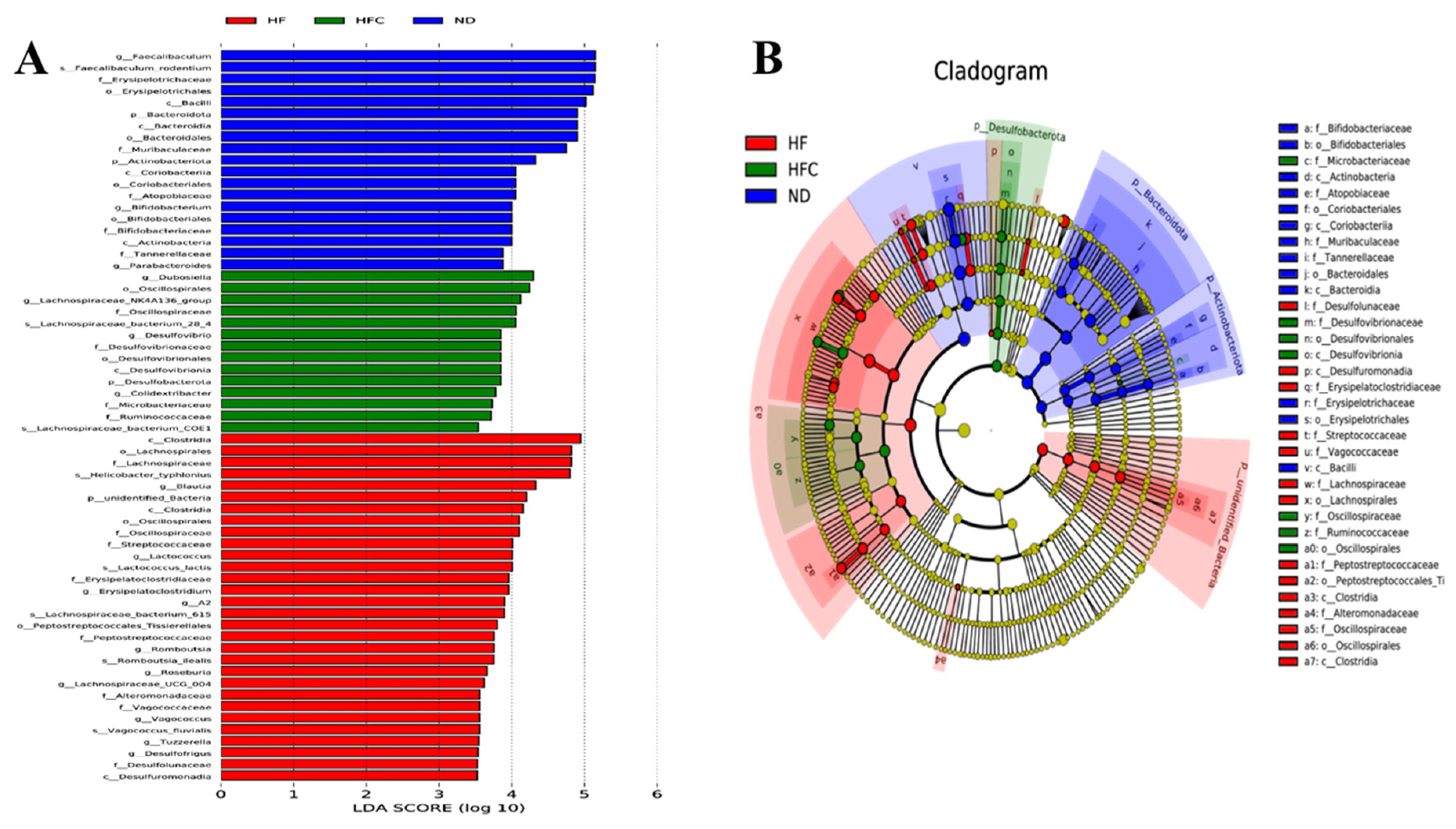
3.6. CPP Modulated Gut Microbiota Diversity Induced by NAFLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Chen, K.; Ma, J.; Jia, X.; Ai, W.; Ma, Z.; Pan, Q. Advancing the understanding of NAFLD to hepatocellular carcinoma development: From experimental models to humans. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 117–125. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.M.; Zhang, Q.Z.; Chen, M.L.; Jiang, M.; Zhou, Y.; Xu, X.J.; Wang, D.M.; Pan, Y.N.; Liu, X.Q. Anti-NAFLD effect of defatted walnut powder extract in high fat diet-induced C57BL/6 mice by modulating the gut microbiota. J. Ethnopharmacol. 2021, 270, 113814. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Petrides, J.; Collins, P.; Kowalski, A.; Sepede, J.; Vermeulen, M. Lifestyle Changes for Disease Prevention. Prim. Care 2019, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of Citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Saija, A.; Dugo, G.; Rosario, B.L.C.; Faulds, C.B.; Waldron, K.W. Characterization of Flavonoids and Pectins from Bergamot (Citrus bergamia Risso) Peel, a Major Byproduct of Essential Oil Extraction. J. Agric. Food Chem. 2006, 54, 197–203. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Tocmo, R.; Pena-Fronteras, J.; Calumba, K.F.; Mendoza, M.; Johnson, J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1969–2012. [Google Scholar] [CrossRef]
- Razola-Diaz, M.D.C.; Guerra-Hernandez, E.J.; Rodriguez-Perez, C.; Gomez-Caravaca, A.M.; Garcia-Villanova, B.; Verardo, V. Optimization of Ultrasound-Assisted Extraction via Sonotrode of Phenolic Compounds from Orange By-Products. Foods 2021, 10, 1120. [Google Scholar] [CrossRef]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef]
- Simon, J.; Casado-Andres, M.; Goikoetxea-Usandizaga, N.; Serrano-Macia, M.; Martinez-Chantar, M.L. Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients 2020, 12, 3517. [Google Scholar] [CrossRef]
- Bayram, H.M.; Majoo, F.M.; Ozturkcan, A. Polyphenols in the prevention and treatment of non-alcoholic fatty liver disease: An update of preclinical and clinical studies. Clin. Nutr. ESPEN 2021, 44, 1–14. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Perez-Jimenez, F.; Camargo, A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem. 2019, 70, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xie, M.; Dai, Z.; Wan, P.; Ye, H.; Zeng, X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1700485. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Fang, X.; Guo, A.; Li, E. Inhibitory effects of fermented Ougan (Citrus reticulata cv. Suavissima) juice on high-fat diet-induced obesity associated with white adipose tissue browning and gut microbiota modulation in mice. Food Funct. 2021, 12, 9300–9314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Wu, Q.X.; Qin, X.M. Camellia nitidissima Chi flower extract alleviates obesity and related complications and modulates gut microbiota composition in rats with high-fat-diet-induced obesity. J. Sci. Food Agric. 2020, 100, 4378–4389. [Google Scholar] [CrossRef]
- Tang, R.; Yu, H.; Ruan, Z.; Zhang, L.; Xue, Y.; Yuan, X.; Qi, M.; Yao, Y. Effects of food matrix elements (dietary fibres) on grapefruit peel flavanone profile and on faecal microbiota during in vitro fermentation. Food Chem. 2021, 371, 131065. [Google Scholar] [CrossRef]
- Liu, K.; Deng, W.; Hu, W.; Cao, S.; Zhong, B.; Chun, J. Extraction of ‘Gannanzao’ Orange Peel Essential Oil by Response Surface Methodology and its Effect on Cancer Cell Proliferation and Migration. Molecules 2019, 24, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandee, Y.; Uttapap, D.; Mischnick, P. Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocoll. 2019, 87, 237–244. [Google Scholar] [CrossRef]
- Yuan, G.; Tan, M.; Chen, X. Punicic acid ameliorates obesity and liver steatosis by regulating gut microbiota composition in mice. Food Funct. 2021, 12, 7897–7908. [Google Scholar] [CrossRef]
- Guo, C.; Han, L.; Li, M.; Yu, L. Seabuckthorn (Hippophae rhamnoides) Freeze-Dried Powder Protects against High-Fat Diet-Induced Obesity, Lipid Metabolism Disorders by Modulating the Gut Microbiota of Mice. Nutrients 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Ge, J.; He, X.; Sheng, Y.; Zheng, S.; Zhang, C.; Xu, W.; Huang, K. Caffeic acid reduces body weight by regulating gut microbiota in diet-induced-obese mice. J. Funct. Foods 2020, 74, 104061. [Google Scholar] [CrossRef]
- Ren, F.; Meng, C.; Chen, W.; Chen, H.; Chen, W. Ganoderma amboinense polysaccharide prevents obesity by regulating gut microbiota in high-fat-diet mice. Food Biosci. 2021, 42, 101107. [Google Scholar] [CrossRef]
- Han, Y.; Song, M.; Gu, M.; Ren, D.; Zhu, X.; Cao, X.; Li, F.; Wang, W.; Cai, X.; Yuan, B.; et al. Dietary Intake of Whole Strawberry Inhibited Colonic Inflammation in Dextran-Sulfate-Sodium-Treated Mice via Restoring Immune Homeostasis and Alleviating Gut Microbiota Dysbiosis. J. Agric. Food Chem. 2019, 67, 9168–9177. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldana, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Unraveling the selective antibacterial activity and chemical composition of citrus essential oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef] [Green Version]
- Weimer, P.; Lisbôa Moura, J.G.; Mossmann, V.; Immig, M.L.; de Castilhos, J.; Rossi, R.C. Citrus aurantiifolia (Christm) Swingle: Biological potential and safety profile of essential oils from leaves and fruit peels. Food Biosci. 2021, 40, 100905. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Walker, R.E.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q.; et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019, 68, 1801–1812. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, L.; Shi, Z.; Wang, L.; Shan, L.; Efferth, T. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-kappaB and MAPKs. Phytomedicine 2019, 64, 153082. [Google Scholar] [CrossRef]
- Park, H.Y.; Ha, S.K.; Eom, H.; Choi, I. Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice. Food Chem. Toxicol. 2013, 55, 637–644. [Google Scholar] [CrossRef]
- Samsudin, R.R.; Kunsah, B.; Widyastuti, R. The effect of pacitan’s sweet orange’s (Citrus sinensis (L.) Osbeck) peel powder on the lipid profile of male dyslipidemia rats (Rattus novergicus). Bali Med. J. 2017, 3, 51–54. [Google Scholar] [CrossRef]
- Lien, D.N.; Quynh, N.T.T.; Phuc, D.V.; Phong, V.C.; Huong, P.T. Effect of pomelo (Citrus grandis (L). Osbeck) peel extract on lipid-carbohydrate metabolic enzymes and blood lipid, glucose parameters in experimental obese and diabetic mice. VNU J. Sci. Nat. Sci. Technol. 2010, 26, 224–232. [Google Scholar]
- Lu, F.B.; Hu, E.D.; Xu, L.M.; Chen, L.; Wu, J.L.; Li, H.; Chen, D.Z.; Chen, Y.P. The relationship between obesity and the severity of non-alcoholic fatty liver disease: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 491–502. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, T.Y.; Yang, S.S.; Tung, C.F.; Yeh, H.Z.; Chang, C.S. Risk factors and metabolic abnormality of patients with non-alcoholic fatty liver disease: Either non-obese or obese Chinese population. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 45–48. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.; Joo, S.K.; Kim, J.H.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Predictors of nonalcoholic steatohepatitis and significant fibrosis in non-obese nonalcoholic fatty liver disease. Liver Int. 2019, 39, 332–341. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Liu, L.; Du, X.; Liu, Y.; Yan, X.; Kombo Osoro, E.; Zhang, F.; Feng, L.; Liang, D.; et al. Anti-toll-like receptor 2 antibody ameliorates hepatic injury, inflammation, fibrosis and steatosis in obesity-related metabolic disorder rats via regulating MAPK and NF-kappaB pathways. Int. Immunopharmacol. 2020, 82, 106368. [Google Scholar] [CrossRef]
- Skelly, M.M.; James, P.D.; Ryder, S.D. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J. Hepatol. 2001, 35, 195–199. [Google Scholar] [CrossRef]
- Caussy, C.; Loomba, R. Gut microbiome, microbial metabolites and the development of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 719–720. [Google Scholar] [CrossRef]
- Xie, C.; Halegoua-DeMarzio, D. Role of Probiotics in Non-alcoholic Fatty Liver Disease: Does Gut Microbiota Matter? Nutrients 2019, 11, 2837. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olbjorn, C.; Cvancarova Smastuen, M.; Thiis-Evensen, E.; Nakstad, B.; Vatn, M.H.; Jahnsen, J.; Ricanek, P.; Vatn, S.; Moen, A.E.F.; Tannaes, T.M.; et al. Fecal microbiota profiles in treatment-naive pediatric inflammatory bowel disease-associations with disease phenotype, treatment, and outcome. Clin. Exp. Gastroenterol. 2019, 12, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.N.; Meng, X.C.; Dong, Y.F.; Zhao, X.H.; Qian, J.M.; Wang, H.Y.; Li, J.N. Effects of probiotics and prebiotics on intestinal microbiota in mice with acute colitis based on 16S rRNA gene sequencing. Chin. Med. J. 2019, 132, 1833–1842. [Google Scholar] [CrossRef]
- Antonissen, G.; Eeckhaut, V.; Van Driessche, K.; Onrust, L.; Haesebrouck, F.; Ducatelle, R.; Moore, R.J.; Van Immerseel, F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016, 45, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Li, D.P.; Cui, M.; Tan, F.; Liu, X.Y.; Yao, P. High Red Meat Intake Exacerbates Dextran Sulfate-Induced Colitis by Altering Gut Microbiota in Mice. Front. Nutr. 2021, 8, 646819. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Chen, Y.R.; Jing, Q.L.; Chen, F.L.; Zheng, H.; Chen, L.D.; Yang, Z.C. Desulfovibrio is not always associated with adverse health effects in the Guangdong Gut Microbiome Project. PeerJ 2021, 9, e12033. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Seebauer, C.T.; Mazagova, M.; Horvath, A.; Wang, L.; Llorente, C.; Varki, N.M.; Brandl, K.; Ho, S.B.; Schnabl, B. Deficiency of intestinal mucin-2 protects mice from diet-induced fatty liver disease and obesity. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G310–G322. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Zhang, L.; Ruan, Z.; Han, P.; Yu, Y. The Regulatory Effects of Citrus Peel Powder on Liver Metabolites and Gut Flora in Mice with Non-Alcoholic Fatty Liver Disease (NAFLD). Foods 2021, 10, 3022. https://doi.org/10.3390/foods10123022
Hu M, Zhang L, Ruan Z, Han P, Yu Y. The Regulatory Effects of Citrus Peel Powder on Liver Metabolites and Gut Flora in Mice with Non-Alcoholic Fatty Liver Disease (NAFLD). Foods. 2021; 10(12):3022. https://doi.org/10.3390/foods10123022
Chicago/Turabian StyleHu, Meiyi, Li Zhang, Zheng Ruan, Peiheng Han, and Yujuan Yu. 2021. "The Regulatory Effects of Citrus Peel Powder on Liver Metabolites and Gut Flora in Mice with Non-Alcoholic Fatty Liver Disease (NAFLD)" Foods 10, no. 12: 3022. https://doi.org/10.3390/foods10123022
APA StyleHu, M., Zhang, L., Ruan, Z., Han, P., & Yu, Y. (2021). The Regulatory Effects of Citrus Peel Powder on Liver Metabolites and Gut Flora in Mice with Non-Alcoholic Fatty Liver Disease (NAFLD). Foods, 10(12), 3022. https://doi.org/10.3390/foods10123022