Epilobiumpyrricholophum Extract Suppresses Porcine Pancreatic Elastase and Cigarette Smoke Extract-Induced Inflammatory response in a Chronic Obstructive Pulmonary Disease Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Material
2.3. Preparation of Cigarette Smoke Extract (CSE)
2.4. Cell Culture
2.5. Animal Study
2.6. RNA Extraction, Real-time Quantitative PCR, and Western Blotting
2.7. UPLC-Q-TOF MS Analysis
2.8. Quantification and Statistical Analysis
3. Results
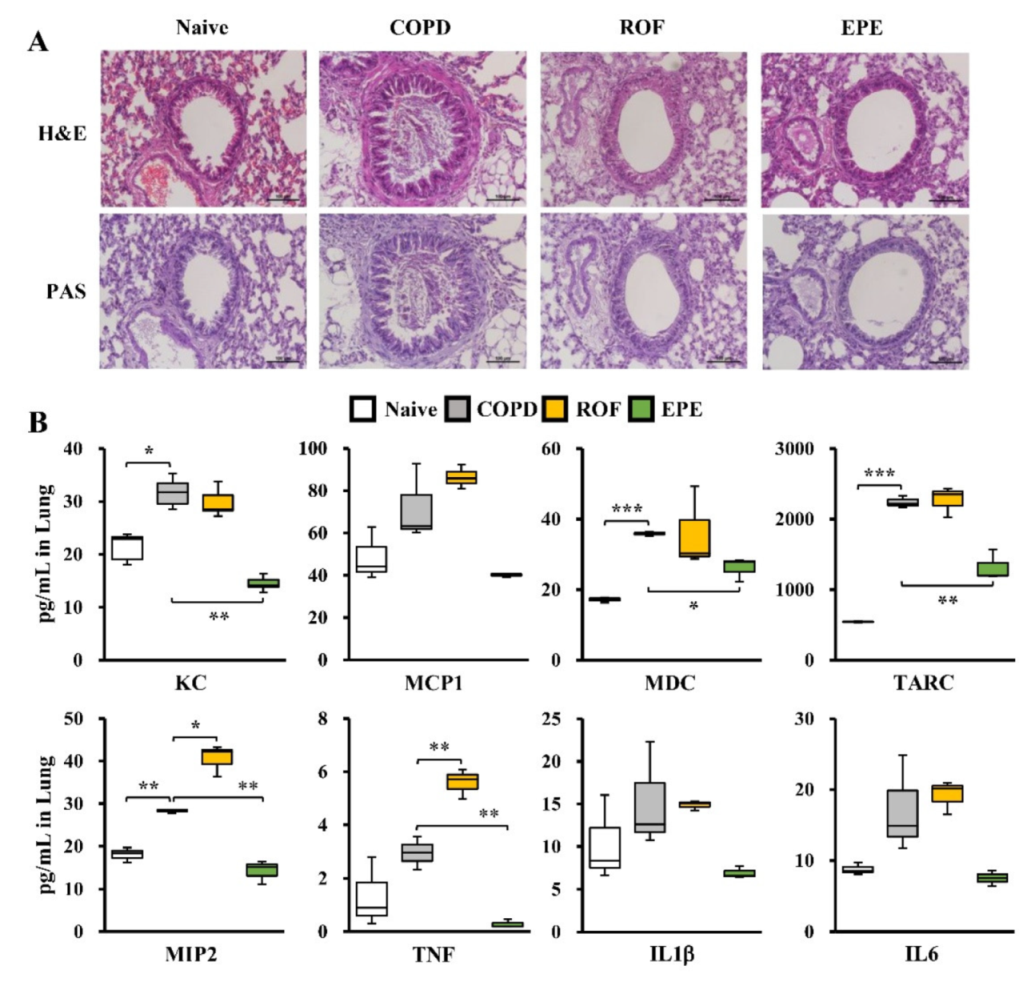
3.1. EPE Attenuates Inflammatory Cell Infiltration and Pro-Inflammatory Gene Expression in COPD Mouse BALF
3.2. EPE Ameliorates PPE and CSE-Induced Lung Inflammation in COPD Mice
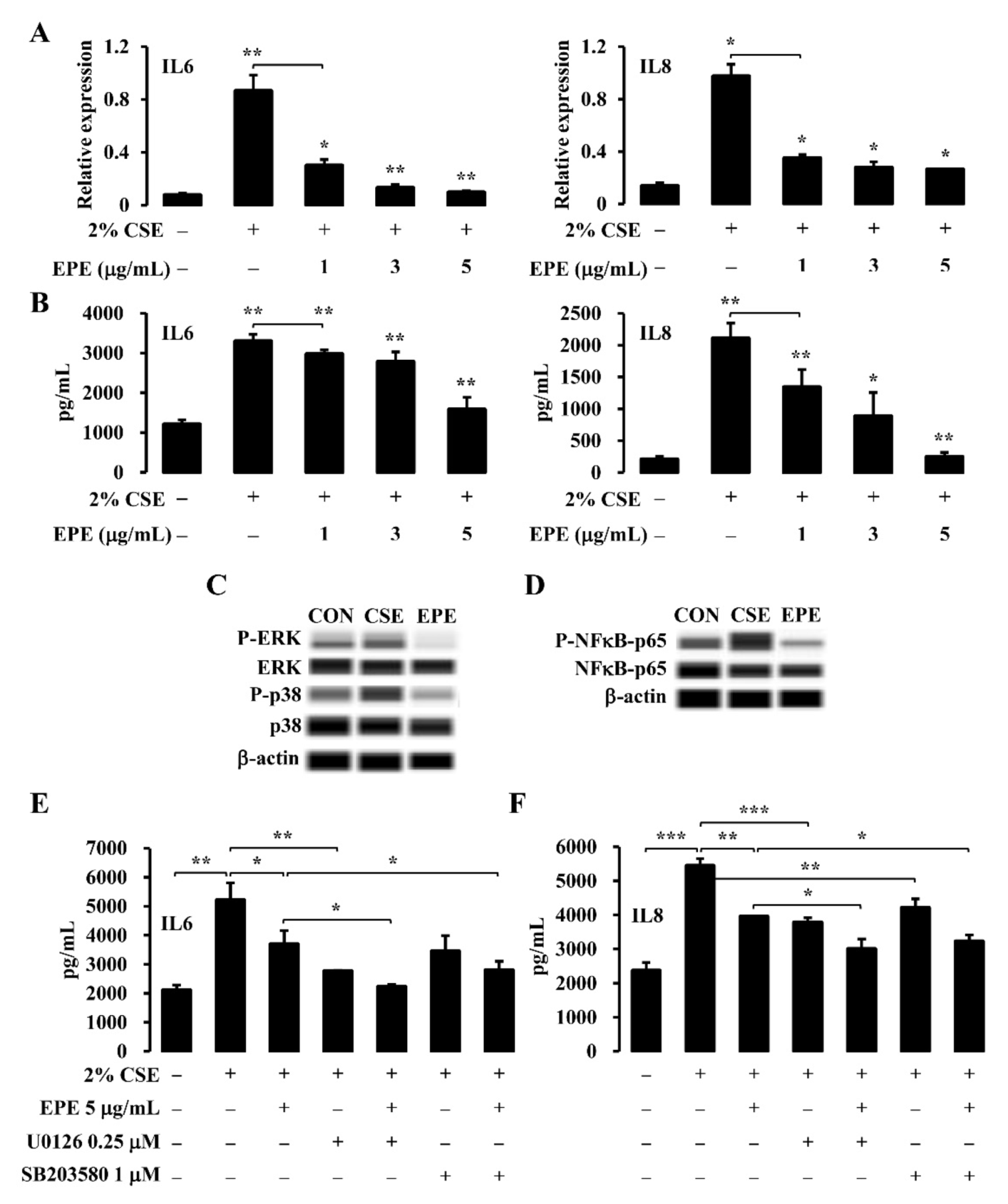
3.3. EPE Suppresses CSE-Induced Inflammatory Gene Expression through the MAPK and NFκB Pathway in NCI-H292 Cells
3.4. Identification of the Active Compounds in EPE That Ameliorates Inflammatory Gene Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Amaral, A.F.S.; Burney, P.G.J.; Patel, J.; Minelli, C.; Mejza, F.; Mannino, D.M.; Seemungal, T.A.R.; Mahesh, P.A.; Lo, L.C.; Janson, C.; et al. Chronic airflow obstruction and ambient particulate air pollution. Thorax 2021, 76, 1236–1241. [Google Scholar] [CrossRef]
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef]
- Russell, D.W.; Gaggar, A.; Solomon, G.M. Neutrophil Fates in Bronchiectasis and Alpha-1 Antitrypsin Deficiency. Ann. Am. Thorac. Soc. 2016, 13, S123–S129. [Google Scholar] [CrossRef]
- Barnes, P.J. Alveolar Macrophages as Orchestrators of COPD. COPD J. Chronic Obstr. Pulm. Dis. 2004, 1, 59–70. [Google Scholar] [CrossRef]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Roda, M.A.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kośmider, A.; Piwowarski, J. Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine 2010, 18, 557–560. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Jakiw, L.; Khlebnikov, A.I.; Blaskovich, C.L.; Jutila, M.A.; Quinn, M.T. Immunomodulatory Activity of Oenothein B Isolated from Epilobium angustifolium. J. Immunol. 2009, 183, 6754–6766. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.; Czerwińska, M.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium spp: Pharmacology and Phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef]
- Lee, S.; Heo, K.-I.; Lee, S.; Yoo, M.; Kim, Y.; Lee, J.S.; Kim, S.-C. Taxonomic studies of tribe Epilobieae Endl. (Onagraceae) in Korea based on morphology and seed microstructure. Korean J. Plant Taxon. 2013, 43, 208–222. [Google Scholar] [CrossRef][Green Version]
- Deng, L.; Zong, W.; Tao, X.; Liu, S.; Feng, Z.; Lin, Y.; Liao, Z.; Chen, M. Evaluation of the therapeutic effect against benign prostatic hyperplasia and the active constituents from Epilobium angustifolium L. J. Ethnopharmacol. 2018, 232, 1–10. [Google Scholar] [CrossRef]
- Tóth, B.H.; Blazics, B.; Kéry, Á. Polyphenol composition and antioxidant capacity of Epilobium species. J. Pharm. Biomed. Anal. 2009, 49, 26–31. [Google Scholar] [CrossRef]
- Kosalec, I.; Kopjar, N.; Kremer, D. Antimicrobial activity of Willowherb (Epilobium angustifolium L.) leaves and flowers. Curr. Drug Targets 2013, 14, 986–991. [Google Scholar] [CrossRef]
- Tita, B.; Abdel-Haq, H.; Vitalone, A.; Mazzanti, G.; Saso, L. Analgesic properties of Epilobium angustifolium, evaluated by the hot plate test and the writhing test. Il Farm. 2001, 56, 341–343. [Google Scholar] [CrossRef]
- Vitalone, A.; Bordi, F.; Baldazzi, C.; Mazzanti, G.; Saso, L.; Tita, B. Anti-proliferative effect on a prostatic epithelial cell line (PZ-HPV-7) by Epilobium angustifolium L. Il Farm. 2001, 56, 483–489. [Google Scholar] [CrossRef]
- Rabe, K.F.; Bateman, E.D.; O’Donnell, D.; Witte, S.; Bredenbröker, D.; Bethke, T.D. Roflumilast—An oral anti-inflammatory treatment for chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2005, 366, 563–571. [Google Scholar] [CrossRef]
- McCluskie, K.; Klein, U.; Linnevers, C.; Ji, Y.-H.; Yang, A.; Husfeld, C.; Thomas, G.R. Phosphodiesterase Type 4 Inhibitors Cause Proinflammatory Effects in Vivo. J. Pharmacol. Exp. Ther. 2006, 319, 468–476. [Google Scholar] [CrossRef]
- Kasetty, G.; Papareddy, P.; Bhongir, R.K.; Egesten, A. Roflumilast Increases Bacterial Load and Dissemination in a Model of Pseudomononas Aeruginosa Airway Infection. J. Pharmacol. Exp. Ther. 2016, 357, 66–72. [Google Scholar] [CrossRef]
- Takeyama, K.; Dabbagh, K.; Lee, H.-M.; Agustí, C.; Lausier, J.A.; Ueki, I.F.; Grattan, K.M.; Nadel, J.A. Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. USA 1999, 96, 3081–3086. [Google Scholar] [CrossRef]
- Shao, M.X.G.; Ueki, I.F.; Nadel, J.A. Tumor necrosis factor -converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11618–11623. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Cao, C.; Wu, Y.-F.; Li, M.; Lai, T.-W.; Zhu, C.; Wang, Y.; Ying, S.-M.; Chen, Z.-H.; Shen, H.-H.; et al. Activating transcription factor 3 represses cigarette smoke-induced IL6 and IL8 expression via suppressing NF-κB activation. Toxicol. Lett. 2017, 270, 17–24. [Google Scholar] [CrossRef]
- Kim, G.-D.; Lee, S.E.; Kim, T.-H.; Jin, Y.-H.; Park, Y.S.; Park, C.-S. Melatonin suppresses acrolein-induced IL-8 production in human pulmonary fibroblasts. J. Pineal Res. 2011, 52, 356–364. [Google Scholar] [CrossRef]
- Li, D.; Hu, J.; Wang, T.; Zhang, X.; Liu, L.; Wang, H.; Wu, Y.; Xu, D.; Wen, F. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells. Sci. Rep. 2016, 6, 37751. [Google Scholar] [CrossRef]
- Pera, T.; Atmaj, C.; Van Der Vegt, M.; Halayko, A.J.; Zaagsma, J.; Meurs, H. Role for TAK1 in cigarette smoke-induced proinflammatory signaling and IL-8 release by human airway smooth muscle cells. Am. J. Physiol. Cell. Mol. Physiol. 2012, 303, L272–L278. [Google Scholar] [CrossRef]
- Raherison, C.; Girodet, P.-O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221. [Google Scholar] [CrossRef]
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L205–L218. [Google Scholar] [CrossRef]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef]
- Olloquequi, J.; Jaime, S.; Parra, V.; Cornejo-Córdova, E.; Valdivia, G.; Agustí, A.; Silva, O.R. Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respir. Res. 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Hoffmann, R.F.; Zarrintan, S.; Brandenburg, S.M.; Kol, A.; De Bruin, H.G.; Jafari, S.; Dijk, F.; Kalicharan, D.; Kelders, M.; Gosker, H.R.; et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 2013, 14, 97. [Google Scholar] [CrossRef]
- Richmond, B.W.; Brucker, R.M.; Han, W.; Du, R.-H.; Zhang, Y.; Cheng, D.-S.; Gleaves, L.; Abdolrasulnia, R.; Polosukhina, D.; Clark, P.E.; et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat. Commun. 2016, 7, 11240. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Goldstein, N.M.; Houghton, A.M.; Kobayashi, D.K.; Kelley, D.; Belaaouaj, A. Neutrophil Elastase Contributes to Cigarette Smoke-Induced Emphysema in Mice. Am. J. Pathol. 2003, 163, 2329–2335. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Laurell, C.-B.; Eriksson, S. The Electrophoretic α1-Globulin Pattern of Serum in α1-Antitrypsin Deficiency. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10 (Suppl. S1), 3–8. [Google Scholar] [CrossRef]
- Brantly, M.L.; Paul, L.D.; Miller, B.H.; Falk, R.T.; Wu, M.; Crystal, R.G. Clinical Features and History of the Destructive Lung Disease Associated with Alpha-1-Antitrypsin Deficiency of Adults with Pulmonary Symptoms. Am. Rev. Respir. Dis. 1988, 138, 327–336. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Lim, S.; Roche, N.; Oliver, B.G.; Mattos, W.; Barnes, P.J.; Chung, K.F. Balance of Matrix Metalloprotease-9 and Tissue Inhibitor of Metalloprotease-1 from Alveolar Macrophages in Cigarette Smokers. Am. J. Respir. Crit. Care Med. 2000, 162, 1355–1360. [Google Scholar] [CrossRef]
- Russell, R.; Culpitt, S.V.; DeMatos, C.; Donnelly, L.; Smith, M.; Wiggins, J.; Barnes, P.J. Release and Activity of Matrix Metalloproteinase-9 and Tissue Inhibitor of Metalloproteinase-1 by Alveolar Macrophages from Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2002, 26, 602–609. [Google Scholar] [CrossRef]
- Omachi, T.A.; Eisner, M.D.; Rames, A.; Markovtsova, L.; Blanc, P.D. Matrix metalloproteinase-9 predicts pulmonary status declines in α1-antitrypsin deficiency. Respir. Res. 2011, 12, 35. [Google Scholar] [CrossRef]
- Culpitt, S.; Rogers, D.; Traves, S.; Barnes, P.; Donnelly, L. Sputum matrix metalloproteases: Comparison between chronic obstructive pulmonary disease and asthma. Respir. Med. 2005, 99, 703–710. [Google Scholar] [CrossRef]
- Hautamaki, R.D.; Kobayashi, D.K.; Senior, R.M.; Shapiro, S.D. Requirement for Macrophage Elastase for Cigarette Smoke-Induced Emphysema in Mice. Science 1997, 277, 2002–2004. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lin, A.-H.; Chen, C.-H.; Huang, W.-C.; Wang, H.-Y.; Liu, M.-H.; Lee, T.-S.; Kou, Y.R. Glucosamine attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Free Radic. Biol. Med. 2014, 69, 208–218. [Google Scholar] [CrossRef]
- Caramori, G.; Romagnoli, M.; Casolari, P.; Bellettato, C.; Casoni, G.L.; Boschetto, P.; Chung, K.F.; Barnes, P.J.; Adcock, I.; Ciaccia, A.; et al. Nuclear localisation of p65 in sputum macrophages but not in sputum neutrophils during COPD exacerbations. Thorax 2003, 58, 348–351. [Google Scholar] [CrossRef]
- Gao, W.; Li, L.; Wang, Y.; Zhang, S.; Adcock, I.; Barnes, P.J.; Huang, M.; Yao, X. Bronchial epithelial cells: The key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology 2015, 20, 722–729. [Google Scholar] [CrossRef]
- Benjamin, J.T.; van der Meer, R.; Im, A.M.; Plosa, E.J.; Zaynagetdinov, R.; Burman, A.; Havrilla, M.E.; Gleaves, L.A.; Polosukhin, V.V.; Deutsch, G.; et al. Epithelial-Derived Inflammation Disrupts Elastin Assembly and Alters Saccular Stage Lung Development. Am. J. Pathol. 2016, 186, 1786–1800. [Google Scholar] [CrossRef] [PubMed]
- Culpitt, S.V.; Maziak, W.; Loukidis, S.; Nightingale, J.; Matthews, J.L.; Barnes, P.J. Effect of High Dose Inhaled Steroid on Cells, Cytokines, and Proteases in Induced Sputum in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1635–1639. [Google Scholar] [CrossRef]
- Loppow, D.; Schleiss, M.; Kanniess, F.; Taube, C.; Jörres, R.; Magnussen, H. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respir. Med. 2001, 95, 115–121. [Google Scholar] [CrossRef]
- Gambari, R.; Borgatti, M.; Lampronti, I.; Fabbri, E.; Brognara, E.; Bianchi, N.; Piccagli, L.; Yuen, M.C.-W.; Kan, C.-W.; Hau, D.K.-P.; et al. Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012, 13, 308–315. [Google Scholar] [CrossRef]
- Wei, Y.; Kim, T.J.; Peng, D.; Duan, D.; Gibbons, D.L.; Yamauchi, M.; Jackson, J.R.; Le Saux, C.J.; Calhoun, C.; Peters, J.; et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J. Clin. Investig. 2017, 127, 3675–3688. [Google Scholar] [CrossRef]
- Chung, S.-K.; Nam, J.-A.; Jeon, S.-Y.; Kim, S.-I.; Lee, H.-J.; Chung, T.H.; Song, K.-S. A Prolyl endopeptidase-Inhibiting antioxidant fromPhyllanthus ussurensis. Arch. Pharmacal Res. 2003, 26, 1024–1028. [Google Scholar] [CrossRef]


| Peak No. | RT (min) | Measured Mass (m/z) (M + H)/(M − H) | Fragment Ions | Tentative Identification | Exact Mass | Formula |
|---|---|---|---|---|---|---|
| 1 | 3.66 | 481.0975 | 153, 303, 319 | Myricetin 3-O-galactoside | 480.09 | C21H20O13 |
| 2 | 4.05 | 447.0926 | 287, 303, 305, 435 | Quercitrin | 448.10 | C21H20O11 |
| 3 | 2.70 | 633.0722 | 231, 301, 633, 634 | Corilagin | 634.08 | C27H22O18 |
| 4 | 3.34 | 291.0135 | 191, 247, 291 | Brevifolin carboxylic acid | 292.02 | C13H8O8 |
| 5 | 3.88 | 463.0877 | 259, 271, 301, 316 | Myricitrin | 464.09 | C21H20O12 |
| 6 | 4.16 | 449.1074 | 153, 303, 287 | Avicularin | 434.08 | C20H18O11 |
| 7 | 4.38 | 431.0978 | 227, 255, 284, 285 | Kaempferol 3-rhamnoside | 432.10 | C21H20O10 |
| Selected Identified Compounds | IL6 (pg/mL) | ||||
|---|---|---|---|---|---|
| Naive | CSE | 10 mM | 30 mM | 50 mM | |
| Myricetin 3-O-galactoside | 4337 ± 540.55 | 7952 ± 294.25 *** | 7241 ± 984.36 | 6170 ± 789.79 * | 5957 ± 475.03 ** |
| Quercitrin | 1910 ± 215.29 | 5566 ± 550.26 ** | 5200 ± 323.57 | 6006 ± 66.02 | 5837 ± 183.59 |
| Corilagin | 4157 ± 314.88 | 8624 ± 1030.07 *** | 4811 ± 207.17 ** | 2733 ± 213.61 ** | 1722 ± 121.74 ** |
| Brevifolin carboxylic acid | 3697 ± 308.24 | 7687 ± 612.90 *** | 6129 ± 1004.49 | 5894 ± 1136.34 | 4856 ± 232.14 ** |
| Myricitrin | 2116 ± 734.62 | 6250 ± 1038.64 ** | 6433 ± 588.96 | 5559 ± 552.10 | 5721 ± 958.47 |
| Avicularin | 4039 ± 86.99 | 8405 ± 721.72 *** | 6996 ± 1113.98 | 6455 ± 1193.42 * | 5048 ± 130.94 ** |
| Kaempferol 3-rhamnoside | 3269 ± 14.01 | 7631 ± 438.60 ** | 7907 ± 582.67 | 8100 ± 196.39 | 8041 ± 731.14 |
| Selected Identified Compounds | IL8 (pg/mL) | ||||
|---|---|---|---|---|---|
| Naive | CSE | 10 mM | 30 mM | 50 mM | |
| Myricetin 3-O-galactoside | 4935 ± 140.33 | 10307 ± 147.22 *** | 10634 ± 304.01 | 9298 ± 67.32 ** | 9505 ± 643.80 |
| Quercitrin | 1632 ± 15.25 | 7140 ± 152.04 *** | 7797 ± 243.46 | 8090 ± 20.78 | 7651 ± 217.29 |
| Corilagin | 4982 ± 449.37 | 11525 ± 572.59 ** | 10315 ± 27.27 * | 5011 ± 98.98 ** | 655 ± 138.62 ** |
| Brevifolin carboxylic acid | 4680 ± 121.96 | 9770 ± 325.39 ** | 10042 ± 115.64 | 9605 ± 545.27 | 10149 ± 182.90 |
| Myricitrin | 1652 ± 311.55 | 7315 ± 376.04 *** | 7769 ± 588.49 | 7213 ± 436.87 | 7404 ± 279.26 |
| Avicularin | 4643 ± 576.67 | 10948 ± 498.87 ** | 10748 ± 898.44 | 10692 ± 537.91 | 11149 ± 512.04 |
| Kaempferol 3-rhamnoside | 2452 ± 218.74 | 8071 ± 177.38 *** | 8793 ± 329.75 | 9093 ± 422.45 | 8930 ± 141.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.Y.; Kim, G.-D.; Choi, D.W.; Shin, D.-U.; Eom, J.-E.; Kim, S.Y.; Chai, O.H.; Kim, H.-J.; Lee, S.-Y.; Shin, H.S. Epilobiumpyrricholophum Extract Suppresses Porcine Pancreatic Elastase and Cigarette Smoke Extract-Induced Inflammatory response in a Chronic Obstructive Pulmonary Disease Model. Foods 2021, 10, 2929. https://doi.org/10.3390/foods10122929
Jung SY, Kim G-D, Choi DW, Shin D-U, Eom J-E, Kim SY, Chai OH, Kim H-J, Lee S-Y, Shin HS. Epilobiumpyrricholophum Extract Suppresses Porcine Pancreatic Elastase and Cigarette Smoke Extract-Induced Inflammatory response in a Chronic Obstructive Pulmonary Disease Model. Foods. 2021; 10(12):2929. https://doi.org/10.3390/foods10122929
Chicago/Turabian StyleJung, Sun Young, Gun-Dong Kim, Dae Woon Choi, Dong-Uk Shin, Ji-Eun Eom, Seung Yong Kim, Ok Hee Chai, Hyun-Jin Kim, So-Young Lee, and Hee Soon Shin. 2021. "Epilobiumpyrricholophum Extract Suppresses Porcine Pancreatic Elastase and Cigarette Smoke Extract-Induced Inflammatory response in a Chronic Obstructive Pulmonary Disease Model" Foods 10, no. 12: 2929. https://doi.org/10.3390/foods10122929
APA StyleJung, S. Y., Kim, G.-D., Choi, D. W., Shin, D.-U., Eom, J.-E., Kim, S. Y., Chai, O. H., Kim, H.-J., Lee, S.-Y., & Shin, H. S. (2021). Epilobiumpyrricholophum Extract Suppresses Porcine Pancreatic Elastase and Cigarette Smoke Extract-Induced Inflammatory response in a Chronic Obstructive Pulmonary Disease Model. Foods, 10(12), 2929. https://doi.org/10.3390/foods10122929






