Quantitative and Comparative Investigation of Plasmalogen Species in Daily Foodstuffs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection
2.3. Lipid Extraction
2.4. Chromatographic Separation
2.5. Quantification by Triple Quadrupole MS and MS/MS
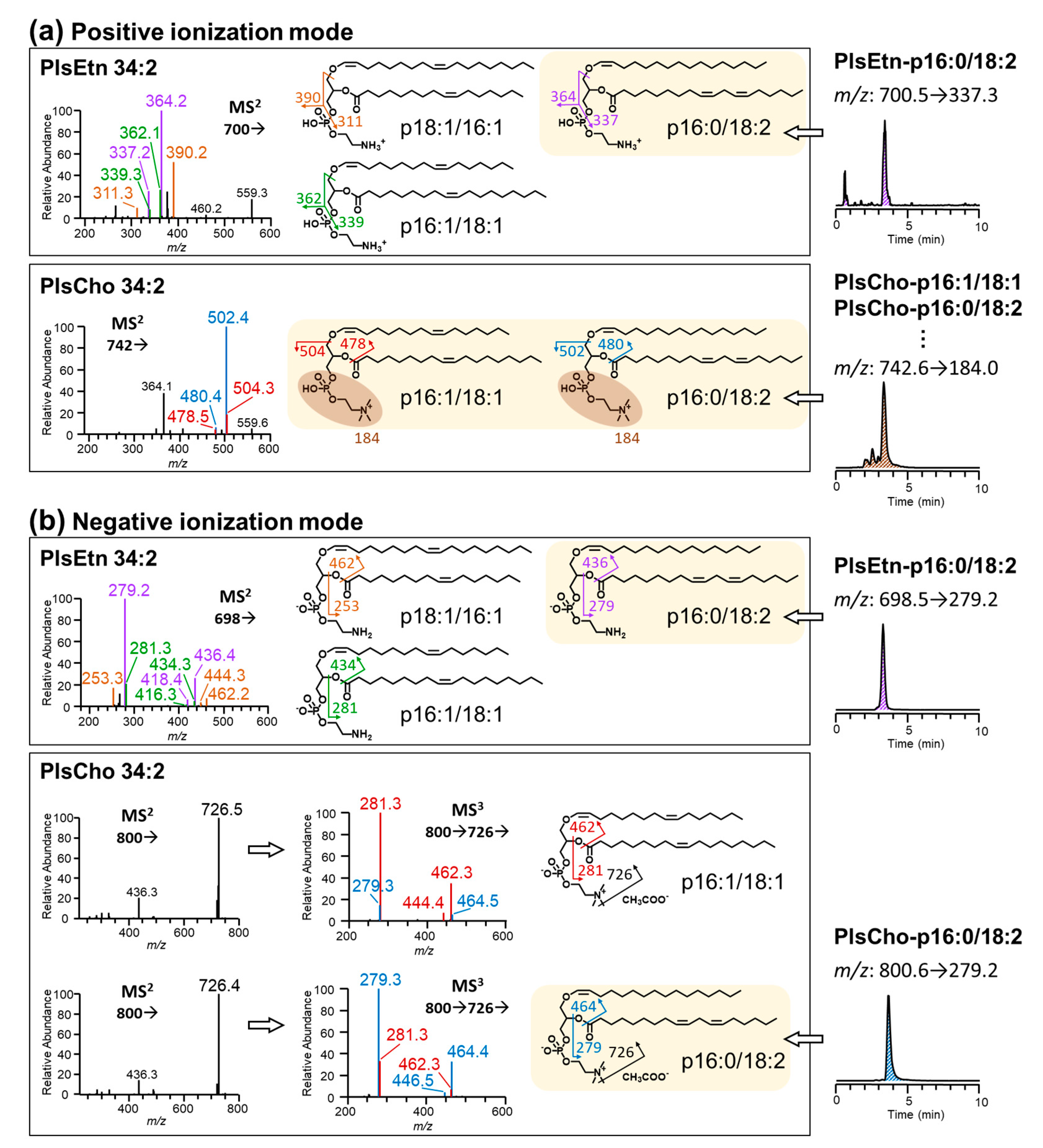
2.6. Identification by Orbitrap Tandem MS
2.7. Data Process and Statistics
3. Results and Discussion
3.1. Comparison of LC-MS/MS Methods under Positive and Negative Ionization Modes
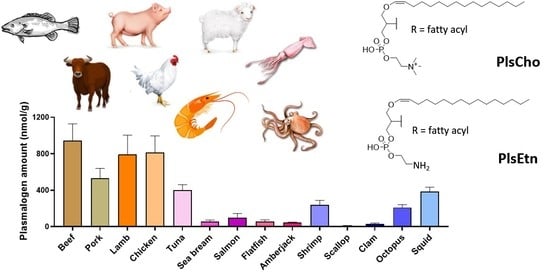
3.2. Total Plasmalogen Amount
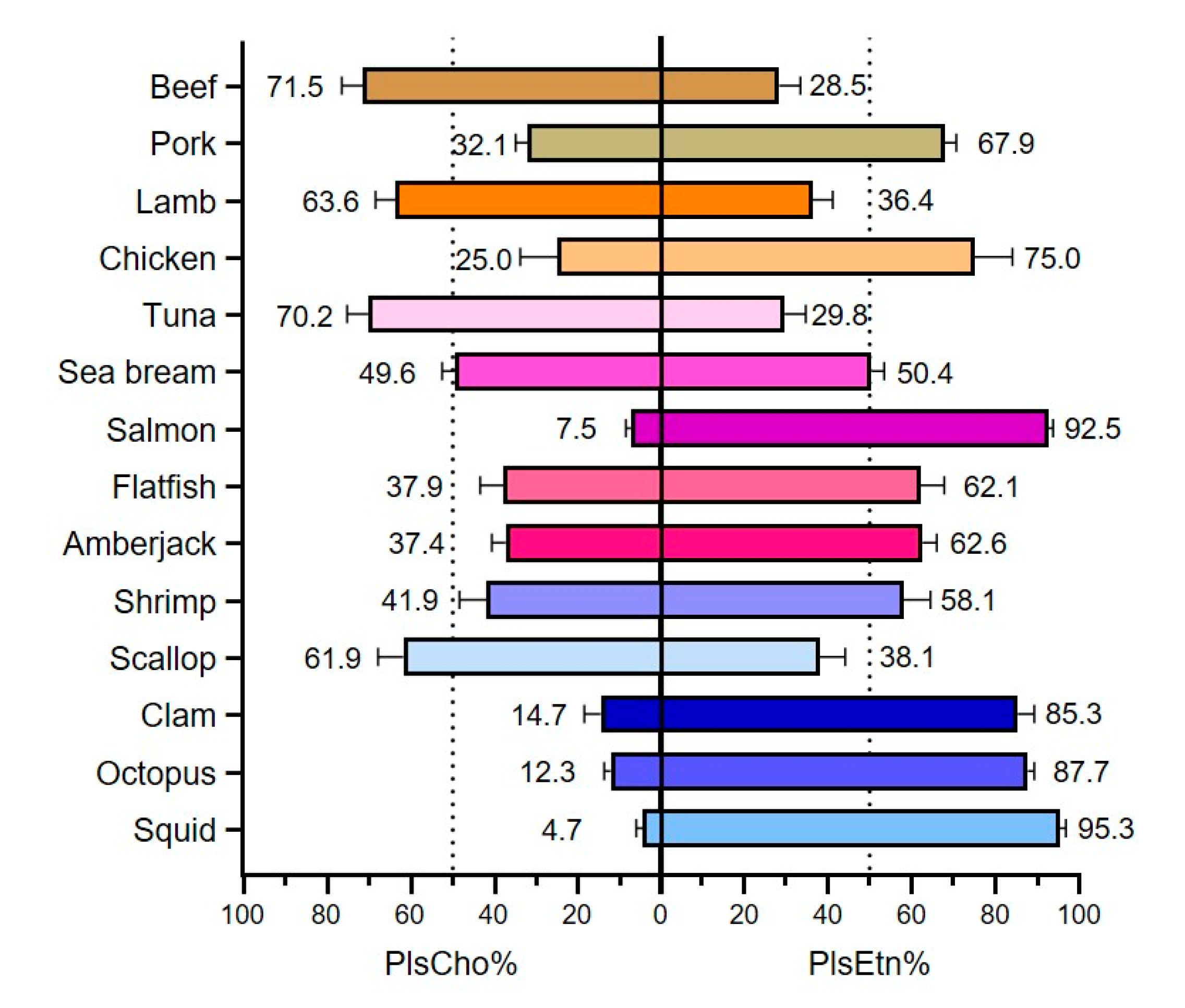
3.3. Types of Plasmalogen (Phosphate Headgroup)
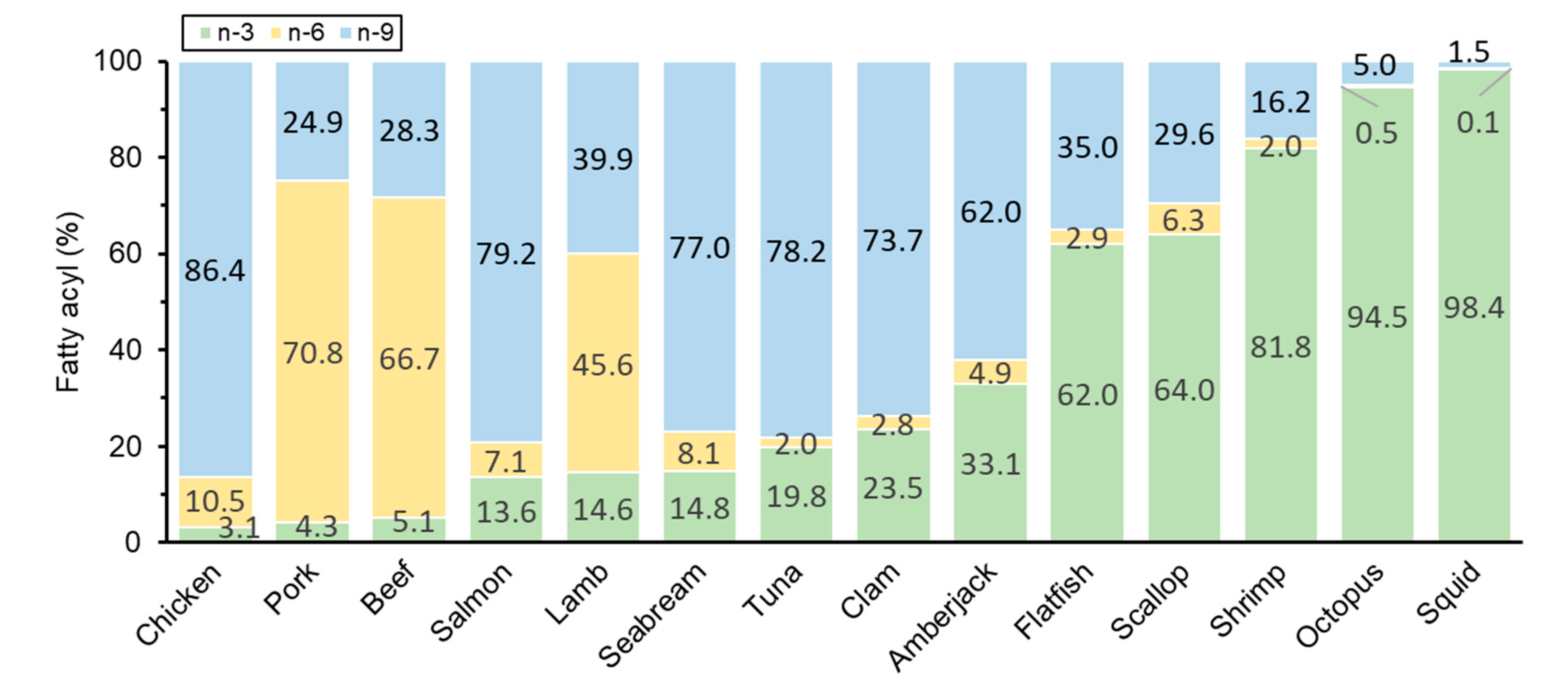
3.4. Fatty Acyl Composition of Plasmalogen
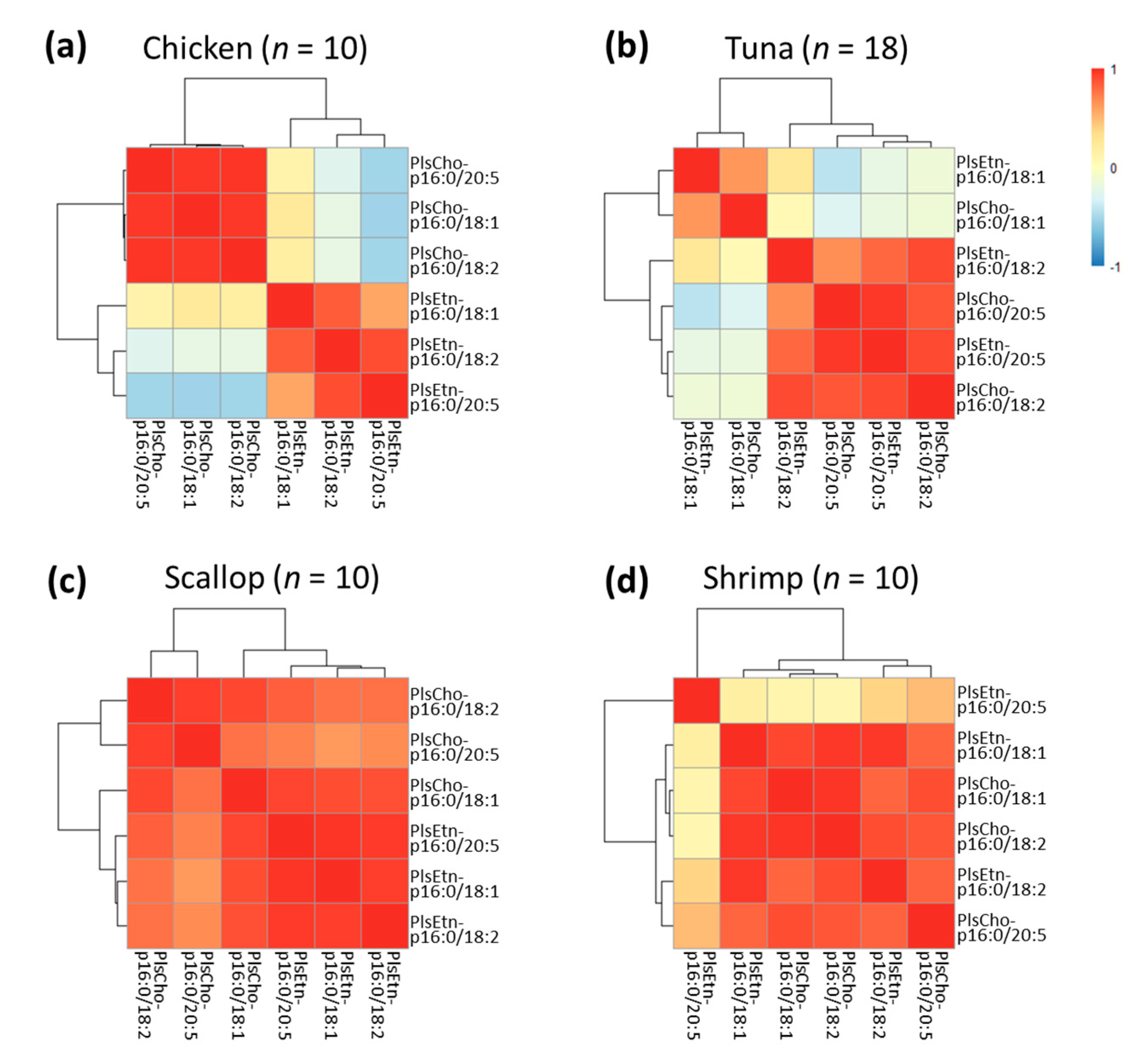
3.5. Correlations among Different Plasmalogen Species in These Foodstuffs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta-Mol. Basis. Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Goodenowe, D.B.; Cook, L.L.; Liu, J.; Lu, Y.; Jayasinghe, D.A.; Ahiahonu, P.W.K.; Heath, D.; Yamazaki, Y.; Flax, J.; Krenitsky, K.F.; et al. Peripheral ethanolamine plasmalogen deficiency: A logical causative factor in Alzheimer’s disease and dementia. J. Lipid Res. 2007, 48, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Oma, S.; Mawatari, S.; Saito, K.; Wakana, C.; Tsuboi, Y.; Yamada, T.; Fujino, T. Changes in Phospholipid Composition of Erythrocyte Membrane in Alzheimer’s Disease. Dement. Geriatr. Cogn. Dis. Extra 2012, 2, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Wang, Y.; Cairns, N.J.; Lantos, P.L.; Dallner, G.; Sindelar, P.J. Decrease and Structural Modifications of Phosphatidylethanolamine Plasmalogen in the Brain with Alzheimer Disease. J. Neuropathol. Exp. Neurol. 1999, 58, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Dragonas, C.; Bertsch, T.; Sieber, C.C.; Brosche, T. Plasmalogens as a marker of elevated systemic oxidative stress in Parkinson’s disease. Clin. Chem. Lab. Med. 2009, 47, 894–897. [Google Scholar] [CrossRef]
- Luoma, A.M.; Kuo, F.; Cakici, O.; Crowther, M.N.; Denninger, A.R.; Avila, R.L.; Brites, P.; Kirschner, D.A. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic. Biol. Med. 2015, 84, 296–310. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Darwish, W.S.; Terada, K.; Chiba, H.; Hui, S.-P. Choline and ethanolamine plasmalogens prevent lead-induced cytotoxicity and lipid oxidation in HepG2 cells. J. Agric. Food Chem. 2019, 67, 7716–7725. [Google Scholar] [CrossRef]
- Sejimo, S.; Hossain, M.S.; Akashi, K. Scallop-derived plasmalogens attenuate the activation of PKCδ associated with the brain inflammation. Biochem. Biophys. Res. Commun. 2018, 503, 837–842. [Google Scholar] [CrossRef]
- Yamashita, S.; Kanno, S.; Honjo, A.; Otoki, Y.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Analysis of Plasmalogen Species in Foodstuffs. Lipids 2016, 51, 199–210. [Google Scholar] [CrossRef]
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by31P NMR: Towards specific interest in human health. Food Chem. 2012, 135, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lancaster, G.I.; Meikle, P.J. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog. Lipid Res. 2019, 74, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Yamada, T.; Asada, T.; Tsuboi, Y.; Wakana, C.; Mawatari, S.; Kono, S. Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. EBioMedicine 2017, 17, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral ingestion of plasmalogens can attenuate the LPS-induced memory loss and microglial activation. Biochem. Biophys. Res. Commun. 2018, 496, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, S.; Katafuchi, T.; Miake, K.; Fujino, T. Dietary plasmalogen increases erythrocyte membrane plasmalogen in rats. Lipids Health Dis. 2012, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef]
- Boselli, E.; Pacetti, D.; Lucci, P.; Frega, N.G. Characterization of Phospholipid Molecular Species in the Edible Parts of Bony Fish and Shellfish. J. Agric. Food Chem. 2012, 60, 3234–3245. [Google Scholar] [CrossRef]
- Czyżowska, A.; Wilkowska, A.; Staszczak (Mianowska), A.; Nowak, A. Characterization of Phytochemicals in Berry Fruit Wines Analyzed by Liquid Chromatography Coupled to Photodiode-Array Detection and Electrospray Ionization/Ion Trap Mass Spectrometry (LC-DAD-ESI-MSn) and Their Antioxidant and Antimicrobial Activity. Foods 2020, 9, 1783. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Deng, Z.; Li, H.; Zhang, B. The Composition and Antioxidant Activity of Bound Phenolics in Three Legumes, and Their Metabolism and Bioaccessibility of Gastrointestinal Tract. Foods 2020, 9, 1816. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L. Analytical Strategies for Fingerprinting of Antioxidants, Nutritional Substances, and Bioactive Compounds in Foodstuffs Based on High Performance Liquid Chromatography–Mass Spectrometry: An Overview. Foods 2020, 9, 1734. [Google Scholar] [CrossRef]
- Yamashita, S.; Abe, A.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Separation and detection of plasmalogen in marine invertebrates by high-performance liquid chromatography with evaporative light-scattering detection. Lipids 2014, 49, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, J.; Wang, H.; Zhu, X.; Li, L.; Lu, W.; Song, G.; Shen, Q. Quantitative and comparative study of plasmalogen molecular species in six edible shellfishes by hydrophilic interaction chromatography mass spectrometry. Food Chem. 2021, 334, 127558. [Google Scholar] [CrossRef]
- Hui, S.-P.; Chiba, H.; Kurosawa, T. Liquid chromatography–mass spectrometric determination of plasmalogens in human plasma. Anal. Bioanal. Chem. 2011, 400, 1923–1931. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 13 October 2019).
- Berry, K.A.Z.; Murphy, R.C. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J. Am. Soc. Mass. Spectrom. 2004, 15, 1499–1508. [Google Scholar] [CrossRef]
- Otoki, Y.; Nakagawa, K.; Kato, S.; Miyazawa, T. MS/MS and LC-MS/MS analysis of choline/ethanolamine plasmalogens via promotion of alkali metal adduct formation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1004, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Otoki, Y.; Kato, S.; Kimura, F.; Furukawa, K.; Yamashita, S.; Arai, H.; Miyazawa, T.; Nakagawa, K. Accurate quantitation of choline and ethanolamine plasmalogen molecular species in human plasma by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017, 134, 77–85. [Google Scholar] [CrossRef]
- Donato, P.; Cacciola, F.; Tranchida, P.Q.; Dugo, P.; Mondello, L. Mass spectrometry detection in comprehensive liquid chromatography: Basic concepts, instrumental aspects, applications and trends. Mass. Spectrom. Rev. 2012, 31, 523–559. [Google Scholar] [CrossRef]
- Murray, J.; Burt, J.R. The Composition of Fish. Available online: http://www.fao.org/3/x5916e00.htm (accessed on 23 June 2020).
- United States Department of Agriculture. Water in Meat and Poultry. Available online: https://www.fsis.usda.gov/wps/wcm/connect/42a903e2-451d-40ea-897a-22dc74ef6e1c/Water_in_Meats.pdf?MOD=AJPERES (accessed on 13 October 2019).
- Innis, S.M.; Kuhnlein, H.V. The Fatty Acid Composition of Northern-Canadian Marine and Terrestrial Mammals. Acta Med. Scand. 1987, 222, 105–109. [Google Scholar] [CrossRef]
- World Health Organization. Population Nutrient Intake Goals for Preventing Diet-Related Chronic Diseases; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Alles, M.S.; Eussen, S.R.B.M.; van der Beek, E.M. Nutritional Challenges and Opportunities during the Weaning Period and in Young Childhood. Ann. Nutr. Metab. 2014, 64, 284–293. [Google Scholar] [CrossRef]
- Fogerty, A.C.; Whitfield, F.B.; Svoronos, D.; Ford, G.L. The composition of the fatty acids and aldehydes of the ethanolamine and choline phospholipids of various meats. Int. J. Food Sci. Technol. 1991, 26, 363–371. [Google Scholar] [CrossRef]
- Hu, X.P.; An, Q.D.; Zhou, D.Y.; Lu, T.; Yin, F.W.; Song, L.; Zhao, Q.; Zhang, J.H.; Qin, L.; Zhu, B.W.; et al. Lipid profiles in different parts of two species of scallops (Chlamys farreri and Patinopecten yessoensis). Food Chem. 2018, 243, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Sieiro, M.P.; Aubourg, S.P.; Rocha, F. Seasonal study of the lipid composition in different tissues of the common octopus (Octopus vulgaris). Eur. J. Lipid Sci. Technol. 2006, 108, 479–487. [Google Scholar] [CrossRef]
- Sanina, N.M.; Goncharova, S.N.; Kostetsky, E.Y. Seasonal changes of fatty acid composition and thermotropic behavior of polar lipids from marine macrophytes. Phytochemistry 2008, 69, 1517–1527. [Google Scholar] [CrossRef]
- Vasilev, D.; Dimovska, N.; Hajrulai-Musliu, Z.; Teodorović, V.; Nikolić, A.; Karabasil, N.; Dimitrijević, M.; Mirilović, M. Fatty acid profile as a discriminatory tool for the origin of lamb muscle and adipose tissue from different pastoral grazing areas in North Macedonia—A short communication. Meat Sci. 2020, 162, 108020. [Google Scholar] [CrossRef]
- Lee, T. Biosynthesis and possible biological functions of plasmalogens. Biochim. Biophys. Acta Lipids Lipid Metab. 1998, 1394, 129–145. [Google Scholar] [CrossRef]


| Type | Foodstuff | Part | Sample Numbers (n) | Production Area |
|---|---|---|---|---|
| Livestock & Poultry | Beef | Leg | 12 | Kamikawa, Hokkaido (43°50’ N 142°21’ E) |
| Pork | Leg | 10 | Tokachi, Hokkaido (42°54’ N 143°22’ E) | |
| Lamb | Leg | 6 | Tokachi, Hokkaido (43°05’ N 142°51’ E) | |
| Chicken | Breast | 10 | Iburi, Hokkaido (42°33’ N 141°22’ E) (4); Okhotsk, Hokkaido (44°11’ N 143°05’ E) (6) | |
| Fish | Tuna | Lean meat | 18 | Oshima, Hokkaido (41°43’ N 141°01’ E) (14); Nagasaki, Kyushu (33°27’ N 129°45’ E) (4) |
| Sea bream | Lean meat | 10 | Nemuro, Hokkaido (43°20’ N 145°35’ E) (6); Kisarazu, Chiba (35°22’ N 139°53’ E) (4) | |
| Salmon | Lean meat | 10 | Kushiro, Hokkaido (43°03’ N 144°51’ E) (6); Minamisannriku, Miyagi (38°43’ N 141°34’ E) (4) | |
| Flatfish | Lean meat | 4 | Kushiro, Hokkaido (43°03’ N 144°51’ E) | |
| Amberjack | Lean meat | 6 | Shiribeshi, Hokkaido (43°18′ N 140°36′ E) | |
| Mollusk | Shrimp | Muscle | 10 | Nemuro, Hokkaido (43°20’ N 145°35’ E) |
| Scallop | Muscle | 10 | Nemuro, Hokkaido (43°20’ N 145°35’ E) | |
| Clam | Muscle | 16 | Kushiro, Hokkaido (43°03’ N 144°51’ E) | |
| Octopus | Arms | 4 | Oshima, Hokkaido (41°43’ N 141°01’ E) | |
| Squid | Mantle | 4 | Oshima, Hokkaido (41°43’ N 141°01’ E) |
| Ionization Mode | Molecular Species | Precursor Ion | Fragmentation | MRM Transition | Collision Energy | Tube Lens |
|---|---|---|---|---|---|---|
| Positive | PlsEtn-p16:0/18:1 | [M + H]+ | 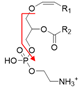 | 702.5 → 339.3 | 20 | 115 |
| PlsEtn-p16:0/18:2 | [M + H]+ | 700.5 → 337.3 | 20 | 110 | ||
| PlsEtn-p16:0/20:5 | [M + H]+ | 722.5 → 359.3 | 17 | 115 | ||
| PlsEtn-p16:0/17:0 | [M + H]+ | 690.5 → 327.3 | 20 | 120 | ||
| PlsCho-p16:0/18:1 | [M + H]+ | 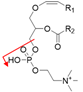 | 744.6 → 184.0 | 25 | 126 | |
| PlsCho-p16:0/18:2 | [M + H]+ | 742.6 → 184.0 | 25 | 110 | ||
| PlsCho-p16:0/20:5 | [M + H]+ | 764.6 → 184.0 | 25 | 120 | ||
| PlsCho-p16:0/17:0 | [M + H]+ | 732.6 → 184.0 | 25 | 110 | ||
| Negative | PlsEtn-p16:0/18:1 | [M − H]− | 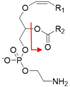 | 700.5 → 281.2 | 33 | 134 |
| PlsEtn-p16:0/18:2 | [M − H]− | 698.5 → 279.2 | 33 | 149 | ||
| PlsEtn-p16:0/20:5 | [M − H]− | 720.5 → 301.2 | 28 | 134 | ||
| PlsEtn-p16:0/17:0 | [M − H]− | 688.5 → 269.2 | 33 | 143 | ||
| PlsCho-p16:0/18:1 | [M + CH3COO]− | 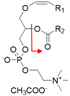 | 802.6 → 281.2 | 40 | 149 | |
| PlsCho-p16:0/18:2 | [M + CH3COO]− | 800.6 → 279.2 | 41 | 115 | ||
| PlsCho-p16:0/20:5 | [M + CH3COO]− | 822.6 → 301.2 | 31 | 111 | ||
| PlsCho-p16:0/17:0 | [M + CH3COO]− | 790.6 → 269.2 | 37 | 125 |
| Foodstuff | PlsEtn-p16:0/18:1 | PlsEtn-p16:0/18:2 | PlsEtn-p16:0/20:5 | PlsCho-p16:0/18:1 | PlsCho-p16:0/18:2 | PlsCho-p16:0/20:5 |
|---|---|---|---|---|---|---|
| Beef | 66.11 ± 11.79 | 179.21 ± 65.08 | 30.78 ± 22.12 | 201.15 ± 22.13 | 450.74 ± 70.24 | 16.95 ± 10.70 |
| Pork | 94.42 ± 12.34 | 242.70 ± 60.50 | 20.98 ± 6.94 | 37.77 ± 6.51 | 133.27 ± 47.15 | 1.70 ± 0.24 |
| Lamb | 86.92 ± 23.75 | 121.58 ± 48.15 | 81.23 ± 31.59 | 229.07 ± 52.29 | 239.46 ± 67.45 | 34.36 ± 11.27 |
| Chicken | 533.23 ± 125.27 | 54.41 ± 12.21 | 19.83 ± 6.86 | 171.04 ± 78.37 | 31.22 ± 14.23 | 5.04 ± 2.69 |
| Tuna | 87.95 ± 31.05 | 2.37 ± 1.13 | 30.11 ± 18.47 | 224.52 ± 23.61 | 5.76 ± 2.87 | 49.03 ± 33.97 |
| Sea bream | 20.20 ± 8.08 | 2.38 ± 1.14 | 5.39 ± 2.10 | 21.86 ± 6.07 | 2.05 ± 0.74 | 2.71 ± 0.96 |
| Salmon | 72.69 ± 39.28 | 6.45 ± 3.04 | 9.72 ± 4.40 | 3.01 ± 1.07 | 0.37 ± 0.10 | 3.29 ± 1.31 |
| Flatfish | 16.35 ± 6.84 | 1.13 ± 0.22 | 18.67 ± 6.67 | 3.65 ± 1.16 | 0.53 ± 0.15 | 16.73 ± 2.40 |
| Amberjack | 16.91 ± 3.64 | 1.41 ± 0.32 | 10.61 ± 1.40 | 11.67 ± 1.05 | 0.85 ± 0.18 | 4.63 ± 0.83 |
| Shrimp | 14.17 ± 2.63 | 1.66 ± 0.30 | 121.93 ± 22.72 | 24.71 ± 6.98 | 3.17 ± 1.04 | 74.13 ± 24.98 |
| Scallop | 1.14 ± 0.52 | 0.10 ± 0.04 | 2.65 ± 1.03 | 1.82 ± 0.59 | 0.54 ± 0.16 | 3.75 ± 1.05 |
| Clam | 19.94 ± 9.50 | 0.58 ± 0.18 | 4.01 ± 1.39 | 1.00 ± 0.30 | 0.22 ± 0.09 | 2.68 ± 0.55 |
| Octopus | 1.62 ± 0.11 | 0.21 ± 0.03 | 181.48 ± 29.25 | 8.84 ± 1.52 | 0.90 ± 0.19 | 15.83 ± 2.51 |
| Squid | 0.79 ± 0.09 | 0.11 ± 0.02 | 366.16 ± 50.43 | 5.03 ± 0.26 | 0.30 ± 0.02 | 12.37 ± 3.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Chen, Z.; Jia, J.; Chiba, H.; Hui, S.-P. Quantitative and Comparative Investigation of Plasmalogen Species in Daily Foodstuffs. Foods 2021, 10, 124. https://doi.org/10.3390/foods10010124
Wu Y, Chen Z, Jia J, Chiba H, Hui S-P. Quantitative and Comparative Investigation of Plasmalogen Species in Daily Foodstuffs. Foods. 2021; 10(1):124. https://doi.org/10.3390/foods10010124
Chicago/Turabian StyleWu, Yue, Zhen Chen, Jiaping Jia, Hitoshi Chiba, and Shu-Ping Hui. 2021. "Quantitative and Comparative Investigation of Plasmalogen Species in Daily Foodstuffs" Foods 10, no. 1: 124. https://doi.org/10.3390/foods10010124
APA StyleWu, Y., Chen, Z., Jia, J., Chiba, H., & Hui, S.-P. (2021). Quantitative and Comparative Investigation of Plasmalogen Species in Daily Foodstuffs. Foods, 10(1), 124. https://doi.org/10.3390/foods10010124