Artichoke Biorefinery: From Food to Advanced Technological Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Biomass Preparation
2.2. Chemical Characterisation of Biomass
2.3. Microwave-Assisted Extraction (MAE) of Phenols
2.4. Conventional Extraction (CE) of Phenols
2.5. Microwave-Assisted Extraction of Inulin
2.6. Conventional Extraction of Inulin
2.7. HPLC Analysis of Phenolic Profile
2.8. HPLC Analysis of Inulin
2.9. FT-IR ATR Analysis of Inulin
2.10. Statistical Analysis
3. Results and Discussion
3.1. Waste Biomass Available in the Field
3.2. Chemical Characterisation of Raw Biomass
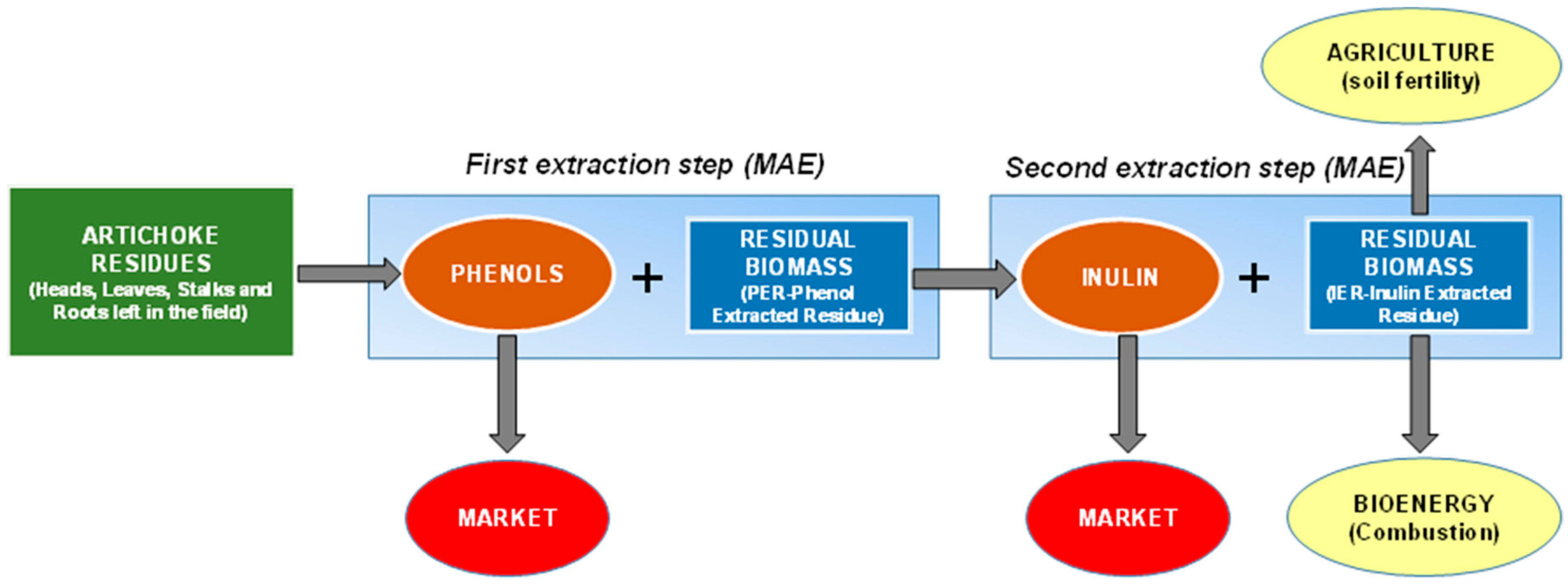
3.3. Sequential Process
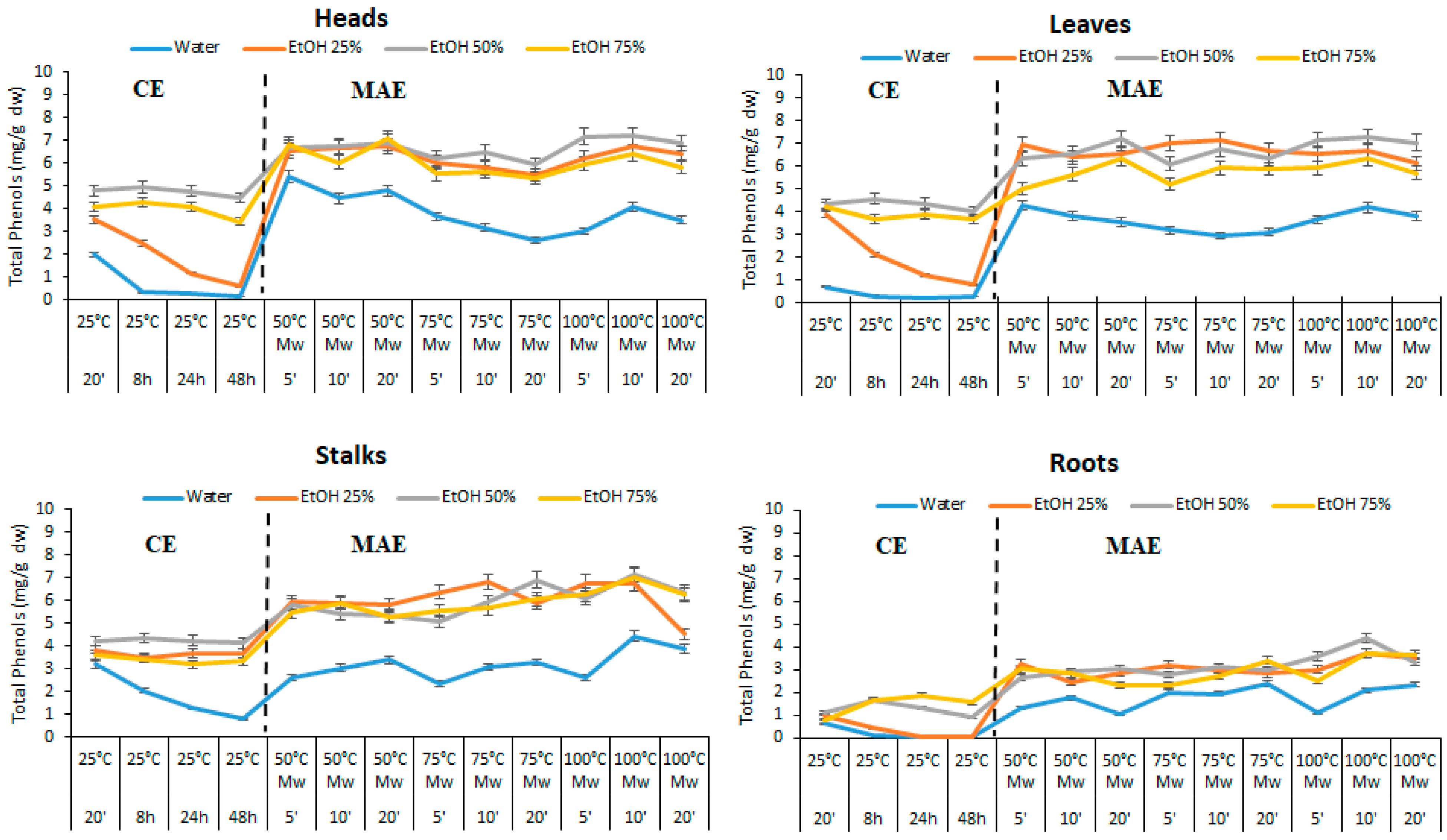
3.3.1. First Step: Microwave-Assisted Extraction (MAE) of Phenols
3.3.2. Second Step: MAE Inulin Extraction
3.3.3. Phenols and Inulin Productivity
3.3.4. Solid Residue Characterization for Potential Application as Bioenergy Feedstock
3.3.5. Solid Residue Characterization for Potential Application as Green Manure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD. Global Material Resources Outlook to 2060; OECD Publishing: Paris, France, 2018. [Google Scholar]
- World Bank. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- European Commission. A New Circular Economy Action Plan. For a Cleaner and More Competitive Europe. COM (2020) 98 Final; EUR-Lex: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. The European Green Deal. COM (2019) 640 Final; EUR-Lex: Brussels, Belgium, 2019. [Google Scholar]
- Tabasso, S.; Ginepro, M.; Tomasso, L.; Montoneri, E.; Nisticò, R.; Francavilla, M. Integrated biochemical and chemical processing of municipal bio-waste to obtain bio based products for multiple uses. The case of soil remediation. J. Clean. Prod. 2020, 245, 119191. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, Innovation, and Green Chemistry in the Production and Valorisation of Phenolic Extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- De Cortato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefinery from agro-bioenergy co/by-porducts and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energ. Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Castellino, M.; Renna, M.; Leoni, B.; Calasso, M.; Difonzo, G.; Santamaria, P.; Gambacorta, G.; Caponio, F.; De Angelis, M.; Paradiso, V.M. Conventional and unconventional recovery of inulin rich extracts for food use from the roots of globe artichoke. Food Hydrocoll. 2020, 107, 105975. [Google Scholar] [CrossRef]
- Grabowska, A.; Caruso, G.; Mehrafarin, A.; Kalisz, A.; Gruszecki, R.; Kunicki, E.; Sękara, A. Application of modern agronomic and biotechnological strategies to valorise worldwide globe artichoke (Cynara cardunculus L.) potential—An analytical overview. Ital. J. Agron. 2018, 13, 279–289. [Google Scholar] [CrossRef]
- Virdis, A.; Motzo, R.; Giunta, F. The phenology of seed-propagated globe artichoke. Ann. Appl. Biol. 2014, 164, 128–137. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. 2018. Available online: http://faostat.fao.org (accessed on 16 October 2020).
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Reuse potential of artichoke (Cynara scolimus L.) waste for the recovery of phenolic compounds and bioenergy. J. Clean. Prod. 2016, 111, 279–284. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–134. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Machado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar] [CrossRef]
- Sihem, D.; Samia, D.; Gaetano, P.; Sara, L.; Giovanni, M.; Hassiba, C.; Laura, G.; Ahmed Noureddine, H. In vitro antioxidant activities and phenolic content in crop residues of Tunisian globe artichoke. Sci. Hortic. 2015, 190, 128–136. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Ierna, A.; Mauromicale, G. Variation of polyphenols in a germplasm collection of globe artichoke. Food Res. Int. 2012, 46, 544–551. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; Palma, M.; De Pepe, R. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds in fruits and beverages consumed as part of the Mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Morand, C.; Sies, H. Polyphenols and health (Special Issue). Arch. Biochem. Biophys. 2016, 559, 1–2. [Google Scholar] [CrossRef]
- Causey, J.L.; Feirtag, J.M.; Gallaher, D.D.; Tungland, B.C.; Slavin, J.L. Effects of dietary inulin on serum lipids, blood glucose and the gastrointestinal environment in hypercholesterolemic men. Nutr. Res. 2000, 20, 191–201. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Larroche, C. Biotechnological applications of inulin-rich feedstocks. Bioresour. Technol. 2019, 273, 641–653. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Endoinulinase production by a new endoinulinase producer Aspergillus tritici BGPUP6 using a low cost substrate. Int. J. Biol. Macromol. 2016, 92, 1113–1122. [Google Scholar] [CrossRef]
- Bach, V.; Jensen, S.; Kidmose, U.; Sørensen, J.N.; Edelenbos, M. The effect of culinary preparation on carbohydrate composition, texture and sensory quality of Jerusalem artichoke tubers (Helianthus tuberosus L.). LWT Food Sci. Technol. 2013, 54, 165–170. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorisation of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by microwave assisted extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A. Response surface methodology analysis of polyphenol recovery from artichoke waste. Am. J. Appl. Sci. 2014, 11, 1463–1471. [Google Scholar] [CrossRef]
- Fabbri, A.; Serranti, S.; Bonifazi, G. Biochemical methane potential (BMP) of artichoke waste: The inoculum effect. Waste Manag. Res. 2014, 32, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.; De Paola, A.; Linsalata, V.; Cardinali, A.; Vanadia, S. The use of artichoke peroxidase to remove phenols from olive mill waste water. Fresenius Environ. Bull. 2010, 19, 3028–3036. [Google Scholar]
- Lopez-Molina, D.; Navarro-Martínez, M.D.; Rojas Melgarejo, F.; Hiner, A.N.P.; Chazarra, S.; Rodríguez-Lopez, J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis; National Renewable Energy Laboratory, Technical Report, NREL/TP 510-42620; Battelle: Columbus, OH, USA, August 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory, Technical Report, NREL/TP 510-42618; Battelle: Columbus, OH, USA, April 2008. [Google Scholar]
- Zuleta, A.; Sambucetti, M.E. Inulin Determination for Food Labeling. J. Agric. Food Chem. 2001, 49, 4570–4572. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knodler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Pesce, G.R.; Fernandes, M.C.; Mauromicale, G. Globe artichoke crop residues and their potential for bioethanol production by dilute acid hydrolysis. Biomass Bioenergy 2020, 134, 105471. [Google Scholar] [CrossRef]
- Unal, H.; Alibas, K. Agricultural residues as biomass energy. Energy Sources Part B Econ. Plan. Policy 2007, 2, 123–140. [Google Scholar] [CrossRef]
- Foti, S.; Mauromicale, G.; Raccuia, S.A.; Fallico, B.; Fanella, F. Possible alternative utilisation of Cynara spp.: Biomass, grain yield and chemical composition of grain. Ind. Crop. Prod. 1999, 10, 219–228. [Google Scholar] [CrossRef]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimisation. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Santiago, B.; Calvo, A.A.; Gullòn, B.; Feijoo, G.; Moreira, M.T.; Gonzàlez-Garcìa, S. Production of flavonol quercetin and fructooligosaccharides from onion (Allium cepa L.) waste: An environmental life cycle approach. Chem. Eng. J. 2020, 392, 123772. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Liu, C.Z. Microwave-assisted extraction of solanesol from tobacco leaves. J. Chromatogr. A 2006, 1129, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crop. Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Cova, C.M.; Bo, L.; Pistocchi, M.; Giorgini, S.; Luque, R.; Cravotto, G. Technology and Process Design for Phenols Recovery from Industrial Chicory (Chicorium intybus) Leftovers. Molecules 2019, 24, 2681. [Google Scholar] [CrossRef]
- Binello, A.; Cravotto, G.; Bo, L.; Stevanato, L.; Bellumori, M.; Innocenti, M.; Mulinacci, N. Efficient and selective green extraction of polyphenols from lemon balm. Comptes Rendus Chim. 2017, 20, 921–926. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Mulinacci, N.; Prucher, D.; Peruzzi, M.; Romani, A.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Commercial and laboratory extracts from artichoke leaves: Estimation of caffeoyl esters and flavonoidic compounds content. J. Pharm. Biomed. Anal. 2004, 34, 349–357. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodriguez, B.; Ramos, L.; Sanz, M.L. Extraction of bioactive carbohydrates from artichoke (Cynara scolymus L.) external bracts using microwave assisted extraction and pressurised liquid extraction. Food Chem. 2016, 196, 1156–1162. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-Transform Mid Infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Cerna, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Copikova, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Vázquez-Vuelvas, O.F.; Chávez-Camacho, F.A.; Meza-Velázquez, J.A.; Mendez-Merino, E.; Ríos-Licea, M.M.; Contreras-Esquivel, J.C. A comparative FTIR study for supplemented agavin as functional food. Food Hydrocoll. 2020, 103, 105642. [Google Scholar] [CrossRef]
- Kardos, N.; Luche, J.L. Sonochemistry of carbohydrate compounds. Carbohydr. Res. 2001, 332, 115–131. [Google Scholar] [CrossRef]
- Grand View Research. Global Polyphenols Market Size & Share, Industry Report, 2019–2025. 2019. Available online: https://www.grandviewresearch.com/industry-analysis/polyphenols-market-analysis (accessed on 21 September 2020).
- Fact.MR. Inulin Market Forecast, Trend Analysis & Competition Tracking—Global Market Insights 2018 to 2028. 2018. Available online: https://www.factmr.com/report/1607/inulin-market (accessed on 6 September 2020).
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Liu, X.; Huang, G.; Xiao, W.; Han, L. The composition characteristics of different crop straw types and their multivariate analysis and comparison. Waste Manag. 2020, 110, 87–97. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671. [Google Scholar] [CrossRef]
- FAO. Save and Grow. A Policymaker’s Guide to the Sustainable Intensification of Smallholder Crop Production; FAO: Rome, Italy, 2011. [Google Scholar]
- Ferrarini, A.; Bini, C.; Amaducci, A. Soil and ecosystem services: Current knowledge and evidences from Italian case studies. Appl. Soil Ecol. 2018, 123, 693–698. [Google Scholar]
- Lal, R. Sequestering carbon in soils of agro-ecosystems. Food Policy 2011, 36, S33–S39. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Ren, W.; Coyne, M.; Jacinthe, P.A.; Tao, B.; Hui, D.; Yang, J.; Matocha, C. Responses of soil carbon sequestration to climate-smart agriculture practices: A meta-analysis. Glob. Chang. Biol. 2019, 25, 2591–2606. [Google Scholar] [CrossRef]
- Lal, R. Beyond COP 21: Potential and challenges of the “4 per Thousand” initiative. J. Soil Water Conserv. 2016, 71, 20A–25A. [Google Scholar] [CrossRef]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Lenzi, A.; Antichi, D.; Bigongiali, F.; Mazzoncini, M.; Migliorini, P.; Tesi, R. Effect of different cover crops on organic tomato production. Renew. Agric. Food Syst. 2009, 24, 92–101. [Google Scholar] [CrossRef]

| Plant Weight (Mean Value) | 22.28 ± 4.32 (kg ww) | ||||
|---|---|---|---|---|---|
| Plant Components | Heads | Leaves | Stalks | Roots | Total Biomass |
| (% dw) | 1.21 ± 0.09 | 32.17 ± 2.41 | 32.57 ± 2.44 | 34.04 ± 2.67 | |
| t (ww) Ha−1 | 2.32 ± 0.14 | 80.27 ± 6.23 | 66.67 ± 5.62 | 32.65 ± 2.71 | 181.91 ± 13.64 |
| t (dw) Ha−1 | 0.4 ± 0.03 | 10.55 ± 0.71 | 10.68 ± 0.83 | 11.16 ± 0.87 | 32.79 ± 2.63 |
| Parameter | |||||
| Moisture (%) | 82.81 ± 1.24 | 86.86 ± 1.52 | 83.98 ± 1.11 | 65.82 ± 0.87 | |
| ASH (% dw) | 7.63 ± 0.57 | 14.8 ± 0.62 | 6.4 ± 0.31 | 17.58 ± 0.76 | |
| Volatile Solids (% dw) | 75.39 ± 1.03 | 72.22 ± 0.93 | 74.99 ± 1.03 | 65.05 ± 0.87 | |
| Fixed Carbon (% dw) | 16.98 ± 0.86 | 12.98 ± 0.71 | 18.61 ± 0.92 | 17.37 ± 0.81 | |
| C (% dw) | 39.63 ± 0.26 | 40.23 ± 0.33 | 39.88 ± 0.29 | 36.25 ± 0.22 | |
| H (% dw) | 6.82 ± 0.13 | 6.31 ± 0.16 | 6.57 ± 0.12 | 6.18 ± 0.14 | |
| N (% dw) | 2.49 ± 0.16 | 2.77 ± 0.13 | 0.87 ± 0.09 | 0.74 ± 06 | |
| S (% dw) | 0.30 ± 0.08 | 0.43 ± 0.09 | 0.22 ± 0.03 | 0.18 ± 0.03 | |
| O (% dw) | 43.13 ± 0.27 | 35.46 ± 0.29 | 46.06 ± 0.32 | 39.07 ± 0.25 | |
| Cl (% dw) | 0.24 ± 0.01 | 0.25 ± 0.02 | 0.26 ± 0.02 | 0.31 ± 0.03 | |
| HHV (MJ/kg dw) | 17.43 ± 0.14 | 17.67 ± 0.12 | 16.98 ± 0.13 | 15.72 ± 0.15 | |
| Protein (% dw) | 15.58 ± 0.39 | 17.32 ± 0.43 | 5.44 ± 0.14 | 4.61 ± 0.18 | |
| Total Carbohydrates (% dw) | 56.42 ± 1.41 | 65.00 ± 1.66 | 60.86 ± 1.58 | 57.79 ± 1.32 | |
| Cellulose (% dw) | 18 ± 0.45 | 15.76 ± 0.39 | 24.15 ± 0.60 | 6.13 ± 0.15 | |
| Hemicellulose (% dw) | 8.27 ± 0.21 | 7.89 ± 0.26 | 10.86 ± 0.31 | 4.6 ± 0.15 | |
| Lignin (% dw) | 14.06 ± 0.39 | 10.78 ± 0.72 | 15.62 ± 0.91 | 13.52 ± 0.48 | |
| Compound | Heads | Leaves | Stalks | Roots |
|---|---|---|---|---|
| (mg/g dw) | (mg/g dw) | (mg/g dw) | (mg/g dw) | |
| Chlorogenic Acid | 2.281 ± 0.114 | 3.891 ± 0.195 | 2.796 ± 0.140 | 1.403 ± 0.070 |
| Cynarin | 0.142 ± 0.007 | 0.036 ± 0.002 | 0.025 ± 0.001 | 0.156 ± 0.008 |
| Caffeic Acid | 0.178 ± 0.009 | 0.110 ± 0.006 | 0.108 ± 0.008 | 0.060 ± 0.003 |
| Luteolin 7-O-glucoside | ND | 0.608 ± 0.030 | ND | ND |
| Luteolin 7-O-glucoronide | ND | 0.545 ± 0.027 | ND | ND |
| Trans Ferulic Acid | 0.040 ± 0.002 | 0.177 ± 0.009 | 0.015 ± 0.004 | 0.504 ± 0.025 |
| 1,5-di-O-Dicaffeoylquinic Acid | 2.783 ± 0.139 | 1.090 ± 0.055 | 2.450 ± 0.123 | 0.890 ± 0.045 |
| 3,4-O Dicaffeoylquinic Acid | 0.805 ± 0.031 | 0.201 ± 0.026 | 0.516 ± 0.029 | 0.270 ± 0.025 |
| Luteolin 7-O-rutinoside | ND | 0.264 ± 0.019 | ND | ND |
| Apigenin 7-O-glucoside | 0.249 ± 0.037 | 0.016 ± 0.002 | 0.017 ± 0,004 | ND |
| Quercitin | 0.002 ± 0.001 | 0.006 ± 0.001 | 0.002 ± 0.001 | 0.002 ± 0.001 |
| Apigenin 7-O-glucoronide | 0.035 ± 0.003 | 0.002 ± 0.001 | ND | ND |
| Apigenin 7-O-rutinoside | 0.008 ± 0.001 | ND | 0.006 ± 0.001 | ND |
| Total Phenols | 6.523 ± 0.326 | 6.943 ± 0.347 | 5.933 ± 0.297 | 3.283 ± 0.164 |
| Phenols | Inulin | Solid Residue (IER) | |
|---|---|---|---|
| Plant components | kg Ha−1 | kg Ha−1 | t Ha−1 |
| Heads | 2.60 ± 0.09 | 26.52 ± 0.93 | 0.21 ± 0.01 |
| Leaves | 73.20 ± 2.56 | 158.22 ± 5.54 | 5.64 ± 0.19 |
| Stalks | 63.33 ± 2.22 | 96.12 ± 3.36 | 6.09 ± 0.21 |
| Roots | 36.61 ± 1.28 | 4602.72 ± 83.09 | 4.17 ± 0.15 |
| Total Yield | 175.74 ± 6.15 | 4883.58 ± 91.13 | 16.10 ± 0.56 |
| Heads | Leaves | Stalks | Roots | Artichoke Residues a | Wheat Straw b,c (Range) | Corn Stover b,c (Range) | |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 4.2 | 5.11 | 4.81 | 5.54 | nr | 2.55–7.36 | 2.46–8.07 |
| ASH (% dw) | 2.35 | 7.31 | 2.95 | 25.33 | 1.6 | 3.37–15.55 | 1.77–16.65 |
| Volatile Solids (% dw) | 88.29 | 87.8 | 93.63 | 70.56 | nr | 60.32–78.40 | 65.28–77.54 |
| Fixed Carbon (% dw) | 9.36 | 4.89 | 3.42 | 4.11 | nr | 9.14–23.84 | 10.80–22.97 |
| C (% dw) | 45.37 | 44.32 | 44.17 | 35.08 | 44.1 | 38.07–47.09 | 39.68–47.70 |
| H (% dw) | 6.52 | 6.52 | 6.33 | 5.13 | 6.3 | 4.05–6.58 | 4.31–8.68 |
| N (% dw) | 3.26 | 3.43 | 1.23 | 1.85 | 1.4 | 0.23–1.04 | 0.15–1.68 |
| S (% dw) | 0.12 | 0.22 | 0.05 | 0.06 | <0.1 | 0.19–0.90 | 0.15–1.04 |
| O (% dw) | 42.38 | 38.19 | 45.27 | 32.55 | 37.52–47.35 | 36.13–49.17 | |
| Cl (% dw) | <0.01 | <0.01 | <0.01 | <0.01 | nr | 0.15–1.50 | 0.15–1.20 |
| Lignin (% dw) | 28.62 | 27.99 | 21.85 | 40.46 | nr | 15.13–27.90 | 14.66–30.02 |
| HHV (MJ kg−1 dw) | 19.23 | 19.19 | 18.32 | 14.54 | 19.6 | 14.59–18.14 | 15.24–18.30 |
| Ash Fusibility | |||||||
| ST (Shrinkage, °C) | 903 | 993 | 897 | 1003 | nr | nr | nr |
| DT (Deformation, °C) | 1360 | 1320 | 1365 | 1273 | nr | 950 | 1150 |
| HT (Hemisphere, °C) | 1390 | 1350 | 1390 | 1333 | nr | nr | nr |
| FT (Flow, °C) | 1480 | 1427 | 1463 | 1487 | nr | 1270 | 1280 |
| Element (mg/kg dw) | Raw Biomass | Residual Biomass | ||||||
|---|---|---|---|---|---|---|---|---|
| Heads | Leaves | Stalks | Roots | Heads | Leaves | Stalks | Roots | |
| Al | 33.31 | 200.37 | 52.26 | 618.28 | 216.37 | 797.67 | 139.02 | 1897.55 |
| As | ND | ND | ND | 0.46 | ND | ND | ND | ND |
| B | 14.46 | 22.65 | 14.98 | 8.71 | 13.91 | 16.07 | 11.26 | 10.80 |
| Ba | ND | 20.15 | 7.61 | 48.25 | 0.00 | 31.36 | 13.33 | 106.58 |
| Be | ND | ND | ND | ND | ND | ND | ND | ND |
| Ca | 2043.87 | 20,661.21 | 5700.07 | 3775.96 | 3231.43 | 23,930.29 | 5656.15 | 6518.26 |
| Cd | ND | ND | ND | ND | ND | ND | ND | ND |
| Co | ND | ND | ND | ND | ND | ND | ND | ND |
| Cr | 0.04 | 0.52 | 0.03 | 3.17 | 0.14 | 6.00 | 3.35 | 8.65 |
| Cu | 4.11 | 2.71 | 1.70 | 9.73 | 2.73 | 0.46 | ND | 16.50 |
| Fe | 72.34 | 425.84 | 393.59 | 2446.44 | 74.81 | 603.48 | 246.45 | 3294.08 |
| Hg | ND | ND | ND | ND | ND | ND | ND | ND |
| K | 13,717.60 | 30,739.82 | 17,866.30 | 5541.83 | 9903.07 | 9402.07 | 7794.11 | 3526.07 |
| Mg | 1765.90 | 1547.33 | 1055.82 | 278.36 | 1630.21 | 704.38 | 534.77 | 426.21 |
| Mn | 17.79 | 45.53 | 10.46 | 96.87 | 22.52 | 47.71 | 9.66 | 160.30 |
| Na | 13.52 | 4402.88 | 1209.16 | 811.20 | 115.68 | 1074.95 | 447.05 | 690.74 |
| Ni | 0.48 | 0.05 | ND | 1.34 | ND | 2.05 | ND | 2.74 |
| P | 3046.05 | 2017.00 | 1755.06 | 1811.49 | 1459.67 | 1544.73 | 830.79 | 725.40 |
| Pb | ND | ND | ND | ND | ND | ND | ND | ND |
| S | 1963.65 | 4843.28 | 1094.01 | 770.70 | 1218.21 | 2242.07 | 485.56 | 647.14 |
| Se | ND | ND | ND | ND | 0.41 | 0.51 | 0.48 | 0.24 |
| Si | 5386.15 | 5021.47 | 3181.10 | 10,076.69 | 4963.29 | 12,607.87 | 4734.35 | 21,903.69 |
| Sr | 1.71 | 63.56 | 24.58 | 32.92 | 3.67 | 76.61 | 30.80 | 68.91 |
| Ti | 2.87 | 31.35 | 4.79 | 228.91 | 2.39 | 60.42 | 7.05 | 389.03 |
| V | ND | 0.45 | ND | 8.86 | ND | 0.38 | ND | 13.39 |
| Zn | 5.47 | ND | ND | ND | ND | ND | ND | ND |
| Organic Matter | C | N | P | K | |
|---|---|---|---|---|---|
| t Ha−1 | t Ha−1 | kg Ha−1 | kg Ha−1 | kg Ha−1 | |
| Heads | 0.19 ± 0.01 | 0.10 ±0.01 | 6.84 ± 0.24 | 0.31 ± 0.02 | 2.08 ± 0.07 |
| Leaves | 4.95 ± 0.17 | 2.50 ± 0.09 | 193.29 ± 6.77 | 8.71 ± 0.30 | 52.98 ± 1.85 |
| Stalks | 5.70 ± 0.20 | 2.69 ± 0.09 | 74.91 ± 2.62 | 5.06 ± 0.18 | 47.46 ± 1.66 |
| Roots | 2.94 ± 0.10 | 1.46 ± 0.05 | 77.07 ± 2.70 | 3.02 ± 0.11 | 14.69 ± 0.51 |
| Total | 13.77 ± 0.48 | 6.75 ± 0.24 | 352.11 ± 11.32 | 17.09 ± 0.60 | 117.22 ± 4.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods 2021, 10, 112. https://doi.org/10.3390/foods10010112
Francavilla M, Marone M, Marasco P, Contillo F, Monteleone M. Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods. 2021; 10(1):112. https://doi.org/10.3390/foods10010112
Chicago/Turabian StyleFrancavilla, Matteo, Mauro Marone, Paolo Marasco, Francesco Contillo, and Massimo Monteleone. 2021. "Artichoke Biorefinery: From Food to Advanced Technological Applications" Foods 10, no. 1: 112. https://doi.org/10.3390/foods10010112
APA StyleFrancavilla, M., Marone, M., Marasco, P., Contillo, F., & Monteleone, M. (2021). Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods, 10(1), 112. https://doi.org/10.3390/foods10010112







