Lipid Compositions and Geographical Discrimination of 94 Geographically Authentic Wheat Samples Based on UPLC-MS with Non-Targeted Lipidomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Extraction Methods
2.3. UPLC Q/TOF-MS Conditions
2.4. Data Processing
3. Results and Discussion
3.1. Optimization of Analytical Methods
3.2. Identification of Chemical Compositions in Wheat
3.2.1. Identification of Fatty Acids
3.2.2. Identification of Glycerophospholipids
3.2.3. Identification of Galactolipids
3.2.4. Identification of Other Minor Polar Lipids
3.2.5. Identification of Glycerides
3.3. Multivariate Modeling for Chemical Characteristics Discrimination of Geographical Authentic Wheat Samples
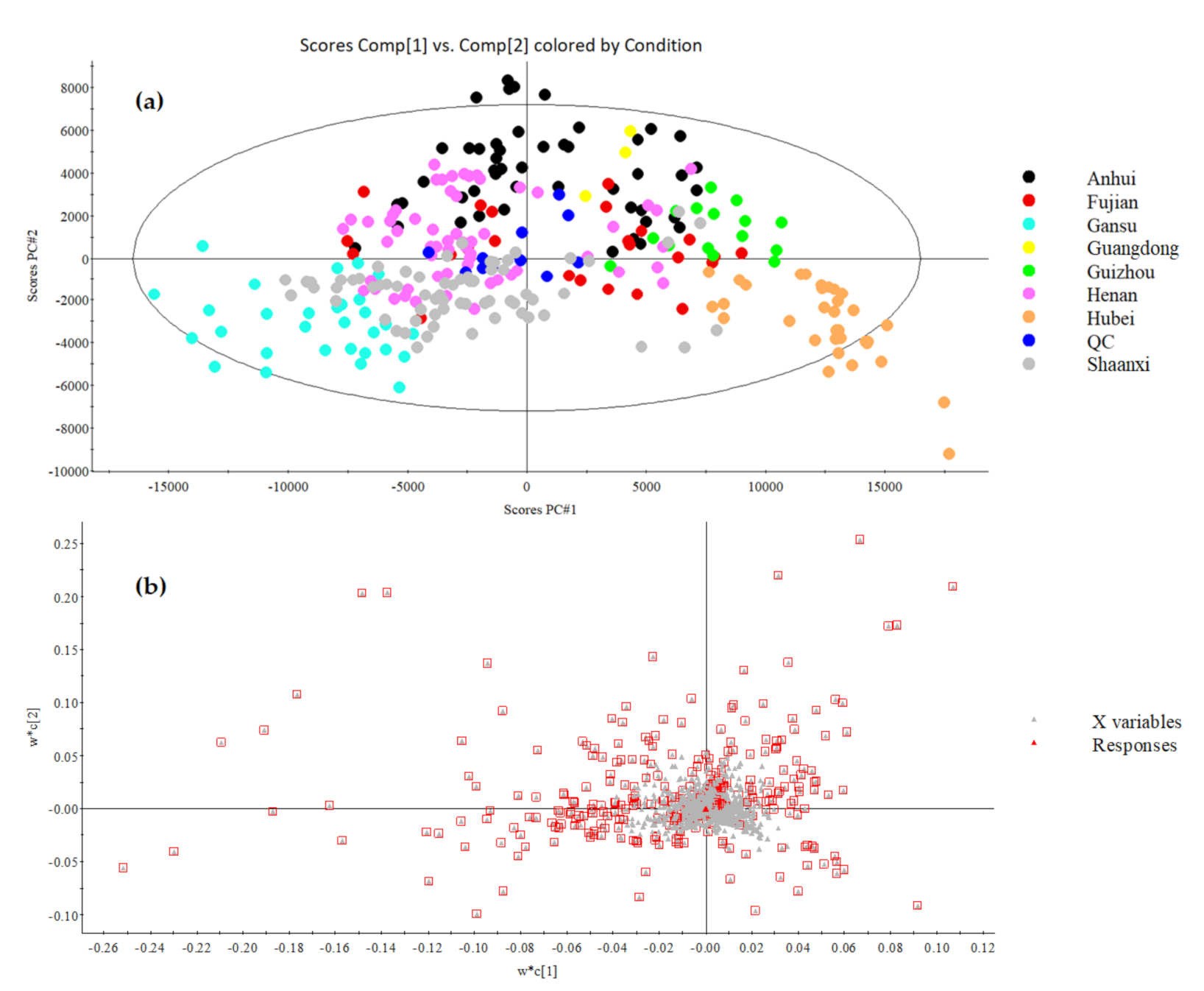
3.3.1. PCA Analysis
3.3.2. PLS-DA Analysis
3.4. Identified Markers for Wheat Geographical Discrimination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borg, J.; Kiaer, L.; LeCarpentier, C.; Goldringer, I.; Gauffreteau, A.; Saint-Jean, S.; Barot, S.; Enjalbert, J. Unfolding the potential of wheat cultivar mixtures: A meta-analysis perspective and identification of knowledge gaps. Field Crop. Res. 2018, 221, 298–313. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook-2020. 12-10 Output of Major Farm Products. Available online: http://www.stats.gov.cn/tjsj/ndsj/2020/indexch.htm (accessed on 20 November 2020).
- Xu, Z.; Yu, Z.; Zhao, J. Theory and application for the promotion of wheat production in China: Past, present and future. J. Sci. Food Agric. 2013, 93, 2339–2350. [Google Scholar] [CrossRef]
- Cheong, B.E.; Ho, W.W.H.; Biddulph, B.; Wallace, X.; Rathjen, T.; Rupasinghe, T.W.T.; Roessner, U.; Dolferus, R. Phenotyping reproductive stage chilling and frost tolerance in wheat using targeted metabolome and lipidome profiling. Metabolomics 2019, 15, 144. [Google Scholar] [CrossRef] [Green Version]
- Wadood, S.A.; Guo, B.; Zhang, X.; Wei, Y. Geographical origin discrimination of wheat kernel and white flour using near-infrared reflectance spectroscopy fingerprinting coupled with chemometrics. Int. J. Food Sci. Technol. 2019, 54, 2045–2054. [Google Scholar] [CrossRef]
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control. 2014, 39, 172–184. [Google Scholar] [CrossRef]
- Cavanna, D.; Loffi, C.; Dall’Asta, C.; Suman, M. A non-targeted high-resolution mass spectrometry approach for the assessment of the geographical origin of durum wheat. Food Chem. 2020, 317, 126366. [Google Scholar] [CrossRef]
- González-Thuillier, I.; Salt, L.; Chope, G.; Penson, S.; Skeggs, P.; Tosi, P.; Powers, S.J.; Ward, J.L.; Wilde, P.J.; Shewry, P.R.; et al. Distribution of Lipids in the Grain of Wheat (cv. Hereward) Determined by Lipidomic Analysis of Milling and Pearling Fractions. J. Agric. Food Chem. 2015, 63, 10705–10716. [Google Scholar] [CrossRef] [Green Version]
- Geng, P.; Harnly, J.M.; Chen, P. Differentiation of Whole Grain from Refined Wheat (T. aestivum) Flour Using Lipid Profile of Wheat Bran, Germ, and Endosperm with UHPLC-HRAM Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6189–6211. [Google Scholar] [CrossRef]
- Jouhet, J. Importance of the hexagonal lipid phase in biological membrane organization. Front. Plant Sci. 2013, 4, 494. [Google Scholar] [CrossRef] [Green Version]
- Janssen, F.; Wouters, A.G.; Linclau, L.; Waelkens, E.; Derua, R.; Dehairs, J.; Moldenaers, P.; Vermant, J.; Delcour, J.A. The role of lipids in determining the air-water interfacial properties of wheat, rye, and oat dough liquor constituents. Food Chem. 2020, 319, 126565. [Google Scholar] [CrossRef]
- Bekes, F. Relationship between lipid content and composition and loaf volume of twenty-six common spring wheats. Cereal Chem. 1986, 63, 327–331. [Google Scholar]
- Tsuzuki, W.; Suzuki, Y.; Yamada, S.; Kano, S.; Ohnishi, H.; Fujimoto, T.; Horigane, A. Effect of oxygen absorber on accumulation of free fatty acids in brown rice and whole grain wheat during storage. LWT 2014, 58, 222–229. [Google Scholar] [CrossRef]
- Ribeiro, M.; Nunes-Miranda, J.D.; Branlard, G.; Carrillo, J.M.; Rodriguez-Quijano, M.; Igrejas, G. One Hundred Years of Grain Omics: Identifying the Glutens That Feed the World. J. Proteome Res. 2013, 12, 4702–4716. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications-a review. Int. J. Food Sci. Technol. 2016, 52, 38–58. [Google Scholar] [CrossRef]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics Provides Valuable Insight for the Study of Durum Wheat: A Review. J. Agric. Food Chem. 2019, 67, 3069–3085. [Google Scholar] [CrossRef]
- Righetti, L.; Rubert, J.; Galaverna, G.; Hurkova, K.; Dall’Asta, C.; Hajslova, J.; Stranska-Zachariasova, M. A novel approach based on untargeted lipidomics reveals differences in the lipid pattern among durum and common wheat. Food Chem. 2018, 240, 775–783. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; A Georgiou, C. Geographical origin and botanical type honey authentication through elemental metabolomics via chemometrics. Food Chem. 2020, 338, 127936. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Liu, Z.; Lu, N.; Hai, G.; Shao, S.; Zheng, Q.; Zhang, X.; Fu, H.; Bai, C.; et al. Differentiating Westlake Longjing tea from the first- and second-grade producing regions using ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry-based untargeted metabolomics in combination with chemometrics. J. Sep. Sci. 2020, 43, 2794–2803. [Google Scholar] [CrossRef]
- Lyu, C.-G.; Kang, C.-Z.; Kang, L.-P.; Yang, J.; Wang, S.; He, Y.-L.; Deng, A.-P.; Wang, H.-Y.; Huang, L.; Guo, L. Structural characterization and discrimination of Ophiopogon japonicas (Liliaceae) from different geographical origins based on metabolite profiling analysis. J. Pharm. Biomed. Anal. 2020, 185, 113212. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2004, 51, 57–67. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodríguez, L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. TrAC Trends Anal. Chem. 2009, 28, 263–278. [Google Scholar] [CrossRef]
- Sun, T.; Wang, X.; Cong, P.; Xu, J.; Xue, C. Mass spectrometry-based lipidomics in food science and nutritional health: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2530–2558. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, F.D.; Broeckling, C.D.; Cockrell, D.M.; Haley, S.D.; Peairs, F.B.; Jahn, C.E.; Heuberger, A.L. GC-MS Metabolomics to Evaluate the Composition of Plant Cuticular Waxes for Four Triticum aestivum Cultivars. Int. J. Mol. Sci. 2018, 19, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, M.D.; Moreau, R.; DerMarderosian, A.; McKeown, N.; Jacques, P.F. Accelerated Solvent Extraction of Alkylresorcinols in Food Products Containing Uncooked and Cooked Wheat. J. Agric. Food Chem. 2012, 60, 4799–4802. [Google Scholar] [CrossRef] [PubMed]
- Wadood, S.A.; Boli, G.; Xiaowen, Z.; Raza, A.; Wei, Y. Geographical discrimination of Chinese winter wheat using volatile compound analysis by HS-SPME/GC-MS coupled with multivariate statistical analysis. J. Mass Spectrom. 2020, 55, e4453. [Google Scholar] [CrossRef]
- Finnie, S.M.; Jeannotte, R.; Faubion, J.M. Quantitative Characterization of Polar Lipids from Wheat Whole Meal, Flour, and Starch. Cereal Chem. J. 2009, 86, 637–645. [Google Scholar] [CrossRef]
- Rodionova, O.; Pomerantsev, A. Chemometric tools for food fraud detection: The role of target class in non-targeted analysis. Food Chem. 2020, 317, 126448. [Google Scholar] [CrossRef]
- Gao, B.; Holroyd, S.E.; Moore, J.C.; Laurvick, K.; Gendel, S.M.; Xie, Z. Opportunities and Challenges Using Non-targeted Methods for Food Fraud Detection. J. Agric. Food Chem. 2019, 67, 8425–8430. [Google Scholar] [CrossRef]
- Righetti, L.; Rubert, J.; Galaverna, G.; Folloni, S.; Ranieri, R.; Stranska-Zachariasova, M.; Hajslova, J.; Dall’Asta, C. Characterization and Discrimination of Ancient Grains: A Metabolomics Approach. Int. J. Mol. Sci. 2016, 17, 1217. [Google Scholar] [CrossRef] [Green Version]
- Stark, T.D.; Weiss, P.; Friedrich, L.; Hofmann, T. The wheat species profiling by non-targeted UPLC–ESI–TOF-MS analysis. Eur. Food Res. Technol. 2020, 246, 1617–1626. [Google Scholar] [CrossRef]
- SciFinder. Available online: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf (accessed on 20 November 2020).
- Pubchem. Available online: http://pubchem.ncbi.nlm.nih.gov/ (accessed on 15 May 2013).
- Home: LIPID MAPS Lipidomics Gateway. Available online: http://www.lipidmaps.org/ (accessed on 28 June 2013).
- De Vos, R.C.H.; Moco, S.; Lommen, A.; Keurentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Claassen, C.; Kuballa, J.; Rohn, S. Polar Lipids in Starch-Rich Commodities to be Analyzed with LC-MS-Based Metabolomics-Optimization of Ionization Parameters and High-Throughput Extraction Protocols. Metabolites 2019, 9, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Razek, A. Elucidation of polynutrients and fatty acid patterns of wheat germ oil extracted by n-hexane and chloroform/methanol. J. Appl. Sci. Res 2011, 7, 1840–1846. [Google Scholar]
- Kara, G.M. Some chemical and physical properties, fatty acid composition and bioactive compounds of wheat germ oils extracted from different wheat cultivars. Tarım Bilim. Derg. 2016, 22, 433–443. [Google Scholar] [CrossRef]
- Geng, P.; Harnly, J.M.; Chen, P. Differentiation of bread made with whole grain and refined wheat (T. aestivum) flour using LC/MS-based chromatographic fingerprinting and chemometric approaches. J. Food Compos. Anal. 2016, 47, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Barcelona, M.J. Structural determination and quantitative analysis of bacterial phospholipids using liquid chromatography/electrospray ionization/mass spectrometry. J. Microbiol. Methods 1998, 33, 23–35. [Google Scholar] [CrossRef]
- Kim, Y.H.; Gil, J.H.; Hong, J.; Yoo, J.S. Tandem mass spectrometric analysis of fatty acyl groups of galactolipid molecular species from wheat flour. Microchem. J. 2001, 68, 143–155. [Google Scholar] [CrossRef]
- Zhu, Y.; Soroka, D.N.; Sang, S. Structure Elucidation and Chemical Profile of Sphingolipids in Wheat Bran and Their Cytotoxic Effects against Human Colon Cancer Cells. J. Agric. Food Chem. 2013, 61, 866–874. [Google Scholar] [CrossRef]
- Vesper, H.; Schmelz, E.-M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H. Sphingolipids in Food and the Emerging Importance of Sphingolipids to Nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Merrill, J.A.H.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Sullards, M.C. Analysis of sphingomyelin, glucosylceramide, ceramide, sphingosine, and sphingosine 1-phosphate by tandem mass spectrometry. Methods Enzymol. 2000, 312, 32–45. [Google Scholar] [PubMed]
- Koistinen, V.M.; Hanhineva, K. Mass spectrometry-based analysis of whole-grain phytochemicals. Crit. Rev. Food Sci. Nutr. 2017, 57, 1688–1709. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gao, B.; Zhang, X.; Xu, Y.; Shi, H.; Yu, L. Chemical profiling of triacylglycerols and diacylglycerols in cow milk fat by ultra-performance convergence chromatography combined with a quadrupole time-of-flight mass spectrometry. Food Chem. 2014, 143, 199–204. [Google Scholar] [CrossRef]
- Marzilli, L.A.; Fay, L.B.; Dionisi, F.; Vouros, P. Structural characterization of triacylglycerols using electrospray ionization-MSn ion-trap MS. J. Am. Oil Chem. Soc. 2003, 80, 195–202. [Google Scholar] [CrossRef]
- Jakab, A.; Héberger, K.; Forgács, E. Comparative analysis of different plant oils by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2002, 976, 255–263. [Google Scholar] [CrossRef]
- Kou, Z.-X.; Yao, Y.-H.; Hu, Y.; Zhang, B.-P. Discussion on position of China’s north-south transitional zone by comparative analysis of mountain altitudinal belts. J. Mt. Sci. 2020, 17, 1901–1915. [Google Scholar] [CrossRef]
- Wang, P.; Wu, D.; Yang, J.; Ma, Y.; Feng, R.; Huo, Z. Summer maize growth under different precipitation years in the Huang-Huai-Hai Plain of China. Agric. For. Meteorol. 2020, 285, 107927. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Fan, J.; Saito, M.; Tatsumi, E. Radical-Scavenging Activity and Isoflavone Content of Sufu (Fermented Tofu) Extracts from Various Regions in China. Food Sci. Technol. Res. 2004, 10, 324–327. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Nie, F.; Zhang, C.; Xiang, S. A unified framework for semi-supervised dimensionality reduction. Pattern Recognit. 2008, 41, 2789–2799. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B.; Sun, S.; Zhang, L.; Yan, J. Determining the Geographic Origin of Wheat Using Multielement Analysis and Multivariate Statistics. J. Agric. Food Chem. 2011, 59, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Huang, G.; Feng, F.; Liu, X.; Guo, R.; Gu, F.; Zhong, X.; Mei, X. Dynamic changes in membrane lipid composition of leaves of winter wheat seedlings in response to PEG-induced water stress. BMC Plant Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hristov, N.; Mladenov, N.; Djuric, V.; Kondić-Špika, A.; Marjanović-Jeromela, A.; Šimić, D. Genotype by environment interactions in wheat quality breeding programs in southeast Europe. Euphytica 2009, 174, 315–324. [Google Scholar] [CrossRef]
- Sroan, B.S.; MacRitchie, F. Mechanism of gas cell stabilization in breadmaking. II. The secondary liquid lamellae. J. Cereal Sci. 2009, 49, 41–46. [Google Scholar] [CrossRef]
- Khalid, K.H.; Ohm, J.-B.; Simsek, S. Whole wheat bread: Effect of bran fractions on dough and end-product quality. J. Cereal Sci. 2017, 78, 48–56. [Google Scholar] [CrossRef]
- Mills, E.; Wilde, P.; Salt, L.; Skeggs, P. Bubble Formation and Stabilization in Bread Dough. Food Bioprod. Process. 2003, 81, 189–193. [Google Scholar] [CrossRef]

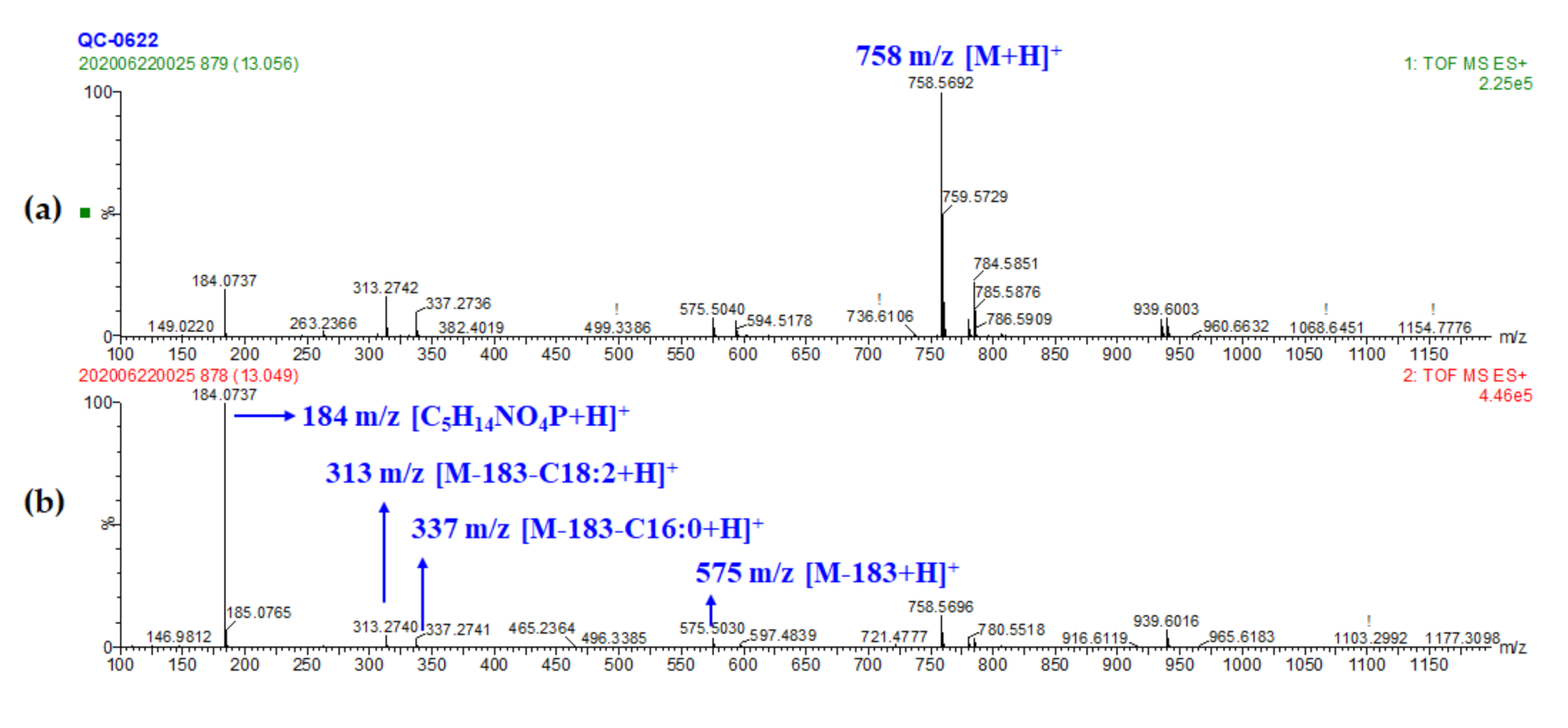
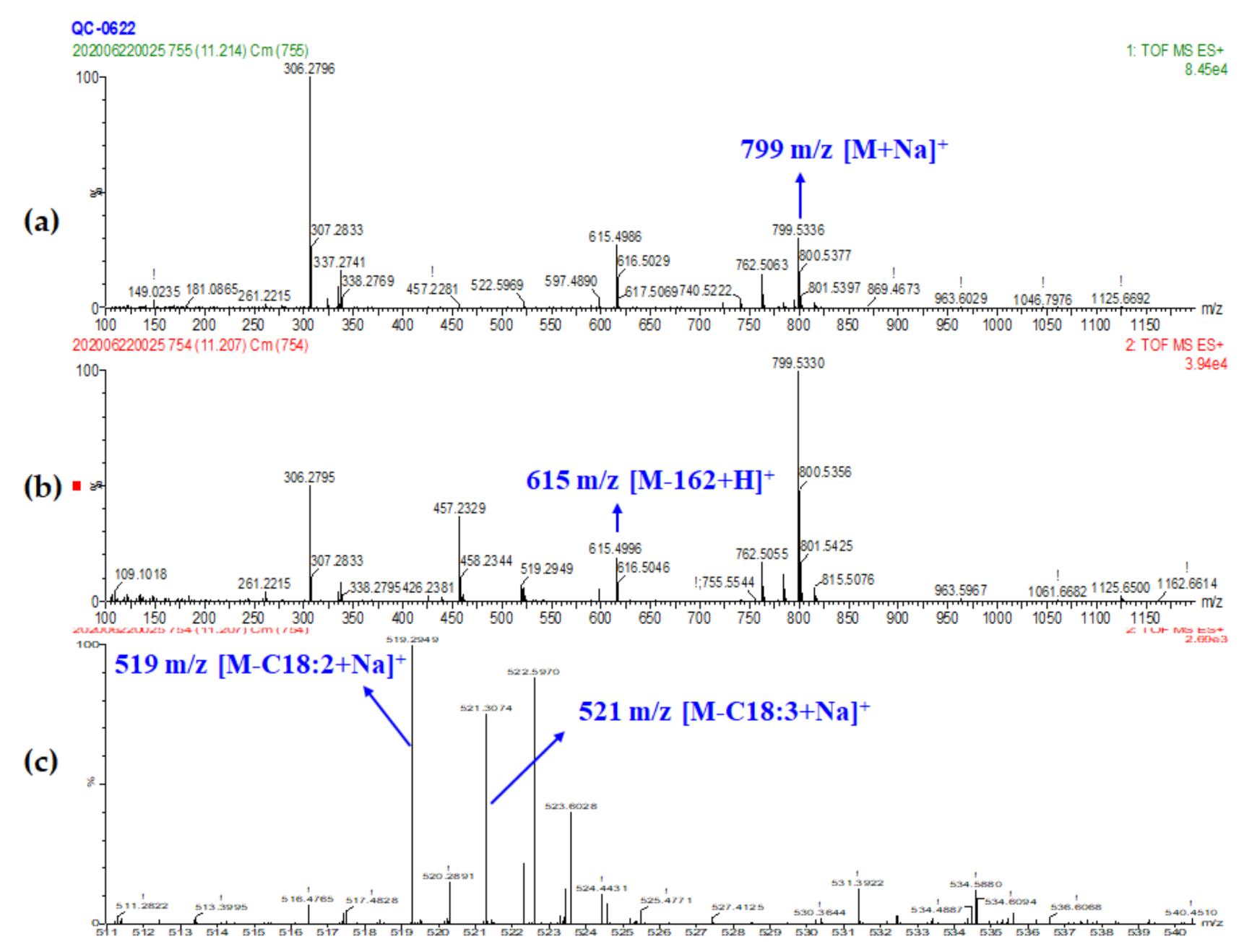
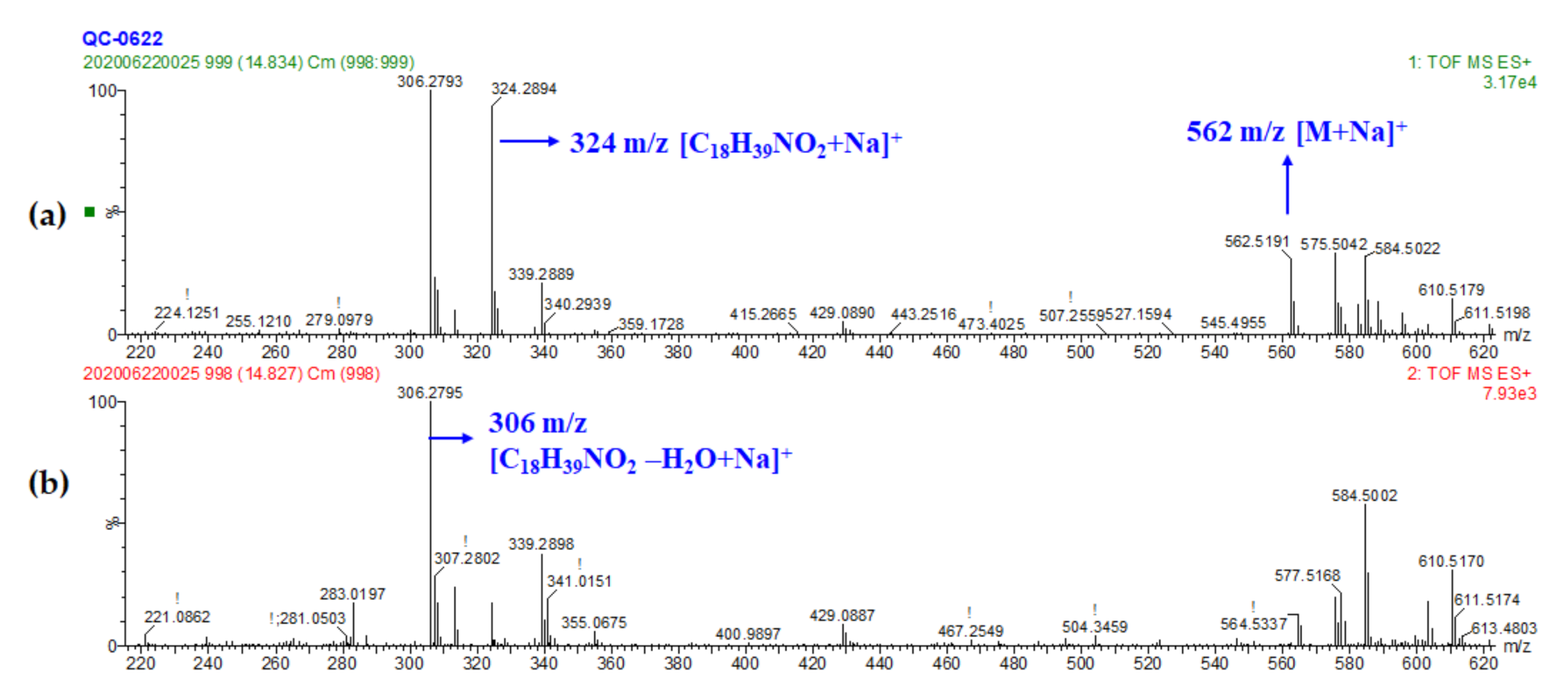
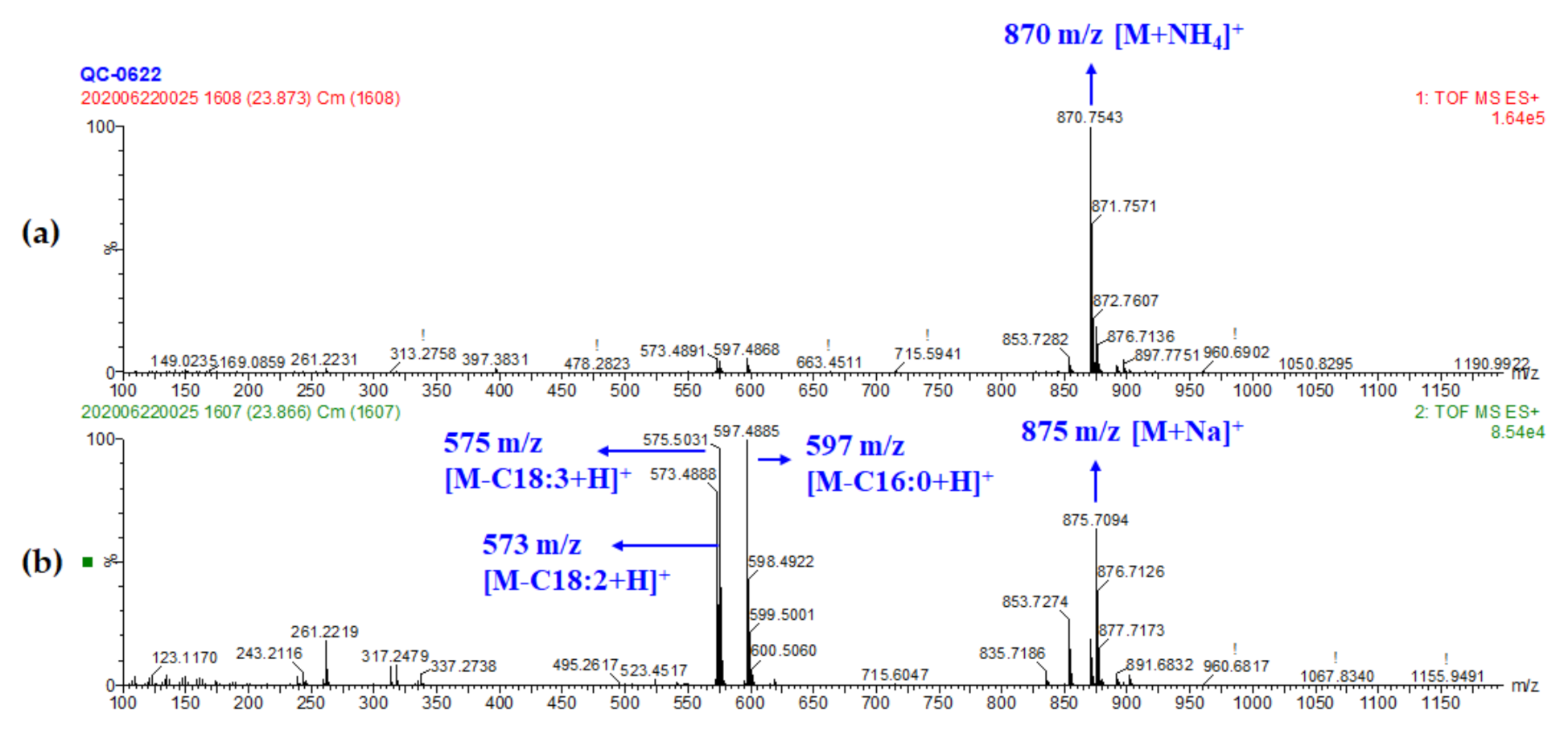

| Peak No. | RT (min) | Exptl. Mass | Calc. Mass | Difference (ppm) | Chemical Formula | Tentative Identification | Adducts # | Fragments |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.79 | 279.2325 | 279.2324 | 0.36 | C18H30O2 | Linolenic acid | M+H | 261.2218, 243.2115, 223.1702 |
| 2 | 5.70 | 518.3240 | 518.3247 | −1.35 | C26H48NO7P | Lyso PC (18:3) | M+H, M+Na | 184.0731, 261.2234 |
| 3 | 6.21 | 520.3404 | 520.3403 | 0.19 | C26H50NO7P | Lyso PC (18:2) | M+H, M+H-H2O, M+Na | 184.0739, 337.2746, 263.2377 |
| 4 | 6.57 | 263.2378 | 263.2375 | 1.14 | C18H32O2 | Linoleic acid | M+H-H2O | 245.2271 |
| 5 | 6.60 | 485.2899 | 485.2879 | 4.12 | C22H45O9P | Lyso PG (16:0) | M+H, M+Na | 313.2745 |
| 6 | 6.73 | 496.3403 | 496.3403 | 0 | C24H50NO7P | Lyso PC (16:0) | M+H, M+Na, M+H-H2O | 184.0740, 518.3212, 478.3301, 313.2742 |
| 7 | 6.85 | 522.3562 | 522.3560 | 0.38 | C26H52NO7P | Lyso PC (18:1) | M+H, M+Na | 184.0737, 544.3376, 339.2884 |
| 8 | 7.24 | 265.2527 | 265.2531 | −1.51 | C18H34O2 | Oleic acid | M+H-H2O | 247.2427 |
| 9 | 7.71 | 524.3718 | 524.3716 | 0.38 | C26H54NO7P | Lyso PC (18:0) | M+H, M+Na | 184.0740, 546.3522, 341.3028 |
| 10 | 9.59 | 338.3422 | 338.3423 | −0.29 | C22H43ON | 13-docosenamide | M+H | 321.3154 |
| 11 | 10.53 | 961.5863 | 961.5864 | −0.10 | C51H86O15 | DGDG (18:3/18:2) | M+Na, M+NH4 | 615.4992, 335.2579, 337.2722 |
| 12 | 10.56 | 780.5539 | 780.5543 | −0.51 | C44H78NO8P | PC (18:2/18:3) | M+H, M+Na | 184.0740, 804.5352, 615.4985, 337,2734, 335.2593 |
| 13 | 10.90 | 397.3834 | 397.3834 | 0 | C29H50O | β-Sitosterol | M+H-H2O | 261.2611, 243.2099 |
| 14 15 | 11.21 11.50 | 799.5336 958.6453 | 799.5336 958.6467 | 0 −1.46 | C45H76O10 C51H88O15 | MGDG (18:3/18:2) DGDG (18:1/18:3) | M+Na, M+NH4 M+NH4. M+Na | 615.4988, 519.2949, 521.3074 617.5142, 963.6005, 339.2884, 335.2583, 261.2213 |
| 16 | 11.54 | 782.5692 | 782.5700 | −1.02 | C44H80NO8P | PC (18:2/18:2) | M+H, M+Na | 184.0739, 804.5507, 617.5141, 337.2737, 263.2372 |
| 17 18 | 11.72 11.72 | 756.5547 937.5855 | 756.5543 937.5864 | 0.53 −0.96 | C42H78NO8P C49H86O15 | PC (16:0/18:3) DGDG (16:0/18:3) | M+H, M+Na M+Na, M+NH4 | 184.0737, 778.5366, 573.4870, 313.2751, 335.2583 591.4999, 313.2751, 335.2583 |
| 19 | 11.90 | 405.3738 | 405.3733 | 1.23 | C27H48O2 | AR (21:0) | M+H | 169.0864 |
| 20 | 11.99 | 740.5226 | 740.5230 | −0.54 | C41H74NO8P | PE (18:2/18:2) | M+H | 599.5045, 164.0087, 337.2759, 263.2316 |
| 21 | 12.36 | 801.5483 | 801.5493 | −1.19 | C45H78O10 | MGDG (18:2/18:2) | M+Na, M+NH4 | 617.5139, 337.2738, 263.2366 |
| 22 | 12.88 | 965.6168 | 965.6177 | −0.93 | C51H90O15 | DGDG (18:2/18:1) | M+Na, M+NH4 | 619.5295, 337.2737, 339.2901 |
| 23 | 12.94 | 784.5849 | 784.5856 | −0.87 | C44H82NO8P | PC (18:2/18:1) | M+H, M+Na | 184.0738, 601.5189. 337.2745, 339.2901 |
| 24 | 13.00 | 939.6007 | 939.6021 | −1.49 | C49H88O15 | DGDG (16:0/18:2) | M+Na, M+NH4 | 593.5148, 313.2745, 337.2736, 263.2358 |
| 25 | 13.04 | 758.5695 | 758.5700 | −0.67 | C42H80NO8P | PC (16:0/18:2) | M+H, M+Na | 184.0737, 575.5029, 313.2738, 337.2738, 263.2369, 239.2382 |
| 26 | 13.68 | 716.5225 | 716.5230 | −0.70 | C39H74NO8P | PE (16:0/18:3) | M+H, M+Na | 575.5034, 164.0086, 313.2756 |
| 27 | 14.02 | 803.5647 | 803.5649 | −0.25 | C45H80O15 | MGDG (18:2/18:1) | M+Na, M+NH4 | 601.5180, 337.2742, 339.2883 |
| 28 | 14.09 | 639.4965 | 639.4964 | 0.16 | C39H68O5 | DG (18:2/18:2) | M+Na, M+H | 337.2743, 263.2377 |
| 29 | 14.14 | 777.5486 | 777.5493 | −0.90 | C43H78O10 | MGDG (18:2/16:0) | M+Na, M+NH4 | 575.5027, 337.2739, 313.2733 |
| 30 | 14.82 | 562.5194 | 562.5175 | 3.37 | C34H69NO3 | Cer (d18:0/16:0) | M+Na | 324.2898, 306.2793 |
| 31 | 14.86 | 786.6000 | 786.6013 | −1.59 | C44H84NO8P | PC (18:1/18:1) | M+H, M+Na | 184.0738, 603.5320, 339.2904 |
| 32 | 14.88 | 941.6179 | 941.6177 | 0.21 | C49H90O15 | DGDG (18:1/16:0) | M+Na, M+NH4 | 577.5204, 595.5320, 339.2892, 313.2736 |
| 33 | 14.98 | 760.5846 | 760.5856 | −1.34 | C42H82NO8P | PC (16:0/18:1) | M+H, M+Na | 184.0738, 313.2736, 339.2881 |
| 34 | 15.08 | 734.5682 | 734.5700 | −2.45 | C40H80NO8P | PC (16:0/16:0) | M+H, M+Na | 184.0738, 313.2724 |
| 35 | 15.13 | 967.6302 | 967.6334 | 3.31 | C51H92O15 | DGDG (18:0/18:2) | M+Na, M+NH4 | 621.5435, 341.3051, 337.2723 |
| 36 | 15.84 | 601.5196 | 601.5196 | 0 | C39H70O5 | DG (18:1/18:2) | M+H-H2O, M+Na | 641.5116, 337.2740, 339.2924 |
| 37 | 15.91 | 575.5035 | 575.5039 | −0.67 | C37H68O5 | DG (18:2/16:0) | M+H-H2O, M+Na | 615.4962, 313.2730, 239.2368, 263.2388, 337.2751 |
| 38 | 16.21 | 641.5121 | 641.5121 | 0.07 | C39H70O5 | DG (18:2/18:1) | M+Na, M+H-H2O | 339.2895, 337.2743 |
| 39 | 17.57 | 603.5346 | 603.5352 | −0.99 | C39H72O5 | DG (18:1/18:1) | M+H-H2O, M+Na | 339.2897 |
| 40 | 17.64 | 577.5201 | 577.5196 | 0.87 | C37H70O5 | DG (16:0/18:1) | M+H-H2O, M+Na | 339.2900, 313.2752 |
| 41 | 18.90 | 877.7283 | 877.7285 | −0.23 | C57H96O6 | TG (18:3/18:2/18:2) | M+H | 597.4886, 599.5010, 261.2220, 337.2750 |
| 42 | 20.17 | 853.7276 | 853.7285 | −1.08 | C55H9606 | TG (18:2/18:2/16:1) | M+H | 599.5036, 573,4883 |
| 43 | 20.59 | 892.7370 | 892.7394 | −2.74 | C57H94O6 | TG (18:2/18:3/18:3) | M+NH4, M+Na | 597.4885, 595.4723, 261.2222, 263.2357 |
| 44 | 21.43 | 855.7402 | 855.7442 | −4.67 | C55H98O6 | TG (16:1/18:1/18:2) | M+H | 575.5041, 601.5191, 591.4982, 263.2359 |
| 45 | 21.87 | 894.7540 | 894.7551 | −1.24 | C57H96O6 | TG (18:3/18:2/18:2) | M+NH4, M+Na, M+H | 597.4882, 599.5024, 261.2225, 263.2365 |
| 46 | 22.27 | 868.7374 | 868.7394 | −2.31 | C55H94O6 | TG (16:0/18:3/18:3) | M+NH4 | 573.4875, 595.4715, 261.2215, 239.2369 |
| 47 | 23.42 | 896.7692 | 896.7707 | −1.66 | C57H98O6 | TG (18:3/18:1/18:2) | M+NH4, M+Na, M+H | 599.5039,597.4883, 263.2372, 261.2220, 265.2502 |
| 48 | 23.87 | 870.7543 | 870.7551 | −0.92 | C55H96O6 | TG (18:3/18:2/16:0) | M+NH4, M+H, M+Na | 597.4885, 575.5031, 573.4888, 313.2735, 261.2219, 263.2371 |
| 49 | 25.12 | 898.7851 | 898.7864 | −1.50 | C57H100O6 | TG (18:1/18:2/18:2) | M+NH4, M+Na, M+H | 601.5190, 599.5042, 263.2372, 265.2530 |
| 50 | 25.37 | 872.7695 | 872.7707 | −1.41 | C55H98O6 | TG (16:0/18:2/18:2) | M+NH4, M+Na, M+H | 575.5036, 599.5036, 263.2372, 239.2371 |
| 51 | 26.46 | 926.8163 | 926.8177 | −1.55 | C59H104O6 | TG (20:1/18:2/18:2) | M+NH4, M+Na | 629.5507, 599.5030, 263.2381, 293.2830 |
| 52 | 26.59 | 900.8008 | 900.8020 | −1.33 | C57H102O6 | TG (18:2/18:1/18:1) | M+NH4, M+Na | 601.5192, 603.5335, 265.2534, 263.2368 |
| 53 | 26.81 | 874.7850 | 874.7864 | −1.60 | C55H100O6 | TG (18:2/18:1/16:0) | M+NH4, M+Na | 601.5189, 577.5184, 575.5033, 263.2371, 265.2524, 239.2369 |
| 54 | 27.00 | 848.7700 | 848.7707 | −0.82 | C53H98O6 | TG (18:2/16:0/16:0) | M+NH4, M+Na | 575.5038, 551.5039, 263.2372, 239.2381 |
| 55 | 27.66 | 954.8481 | 954.8490 | −0.90 | C61H108O6 | TG (18:2/22:1/18:2) | M+NH4 | 657.5815, 599.5043, 263.2386 |
| 56 | 27.79 | 928.8327 | 928.8333 | −0.67 | C59H106O6 | TG (18:2/18:1/20:1) | M+NH4 | 601.5201, 631.5652, 629.5511 |
| 57 | 27.97 | 902.8168 | 902.8177 | −0.95 | C57H104O6 | TG (20:1/16:1/18:1) | M+NH4, M+Na | 603.5355, 575.5048, 631.5648 |
| 58 | 28.23 | 876.8003 | 876.8020 | −1.99 | C55H102O6 | TG (16:0/18:1/18:1) | M+NH4, M+Na | 577.5192, 603.5347, 265.2528, 239.2381 |
| 59 | 28.49 | 850.7857 | 850.7864 | −0.83 | C53H100O6 | TG (18:1/16:0/16:0) | M+NH4, M+Na | 577.5188, 551.5038, 239.2375, 265.2530 |
| 60 | 28.84 | 824.7705 | 824.7707 | −0.23 | C51H98O6 | TG (16:0/16:0/16:0) | M+NH4 | 551.5038, 239.2386 |
| 61 | 29.04 | 982.8810 | 982.8803 | 0.71 | C63H112O6 | TG (24:1/18:2/18:2) | M+NH4, M+Na | 685.6135, 599.5028 |
| 62 | 29.38 | 930.8482 | 930.8490 | −0.89 | C59H108O6 | TG (18:1/18:1/20:1) | M+NH4 | 631.5659, 603.5343, 265.2540 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Zheng, W.; Zhang, Y.; Gao, B.; Yu, L. Lipid Compositions and Geographical Discrimination of 94 Geographically Authentic Wheat Samples Based on UPLC-MS with Non-Targeted Lipidomic Approach. Foods 2021, 10, 10. https://doi.org/10.3390/foods10010010
Jin M, Zheng W, Zhang Y, Gao B, Yu L. Lipid Compositions and Geographical Discrimination of 94 Geographically Authentic Wheat Samples Based on UPLC-MS with Non-Targeted Lipidomic Approach. Foods. 2021; 10(1):10. https://doi.org/10.3390/foods10010010
Chicago/Turabian StyleJin, Mengchu, Wenhao Zheng, Yaqiong Zhang, Boyan Gao, and Liangli (Lucy) Yu. 2021. "Lipid Compositions and Geographical Discrimination of 94 Geographically Authentic Wheat Samples Based on UPLC-MS with Non-Targeted Lipidomic Approach" Foods 10, no. 1: 10. https://doi.org/10.3390/foods10010010
APA StyleJin, M., Zheng, W., Zhang, Y., Gao, B., & Yu, L. (2021). Lipid Compositions and Geographical Discrimination of 94 Geographically Authentic Wheat Samples Based on UPLC-MS with Non-Targeted Lipidomic Approach. Foods, 10(1), 10. https://doi.org/10.3390/foods10010010





