Abstract
Systematic reviews (SRs) reflect the best available evidence for informing vaccination recommendations. This study presents a bibliometric analysis of vaccination-related SRs aiming to uncover research trends. Vaccination-related SRs published from 2011 to 2023 in MEDLINE, Embase, the Cochrane Library and the Living Overview of Evidence database were considered. Trends over time, disease/pathogen, topic, population, geographical location, accessibility, methodological quality, and overlap were descriptively analyzed using R. A total of 2275 SRs were identified, most of which were freely accessible (n = 2083, 91.7%). The annual number of published SRs increased more than twelvefold from 2011 to 2023. COVID-19 (n = 861, 37.8%), influenza (n = 328, 14.4%), Human papillomavirus (n = 248, 10.9%), and pneumococcal disease (n = 152, 6.7%) were the most frequently addressed diseases/pathogens. Efficacy/effectiveness (n = 1066, 46.9%) and safety of vaccines (n = 812, 35.7%) were the most common topics. The methodological quality of SRs on intervention topics (n = 1376) was mostly critically low (n = 1155, 84.0%). Several SRs were identified that covered similar diseases/pathogens, topics and populations, indicating duplication and overlap, particularly for COVID-19. Our analysis showed a large increase in the number of published vaccination-related SRs. The results provide a basis for understanding the current state and priorities in vaccination research and decrease the overlap potential in newly developed SRs.
1. Introduction
Vaccinations are amongst the most crucial and effective means of preventing and controlling infectious diseases (Immunization Agenda 2030 Partners, 2024). To ensure that immunization policies are based on best available evidence, the World Health Organization (WHO) recommends that countries establish National Immunization Technical Advisory Groups (NITAGs) (Immunization Agenda 2030 Partners, 2024). As reemphasized in the Immunization Agenda 2030 (IA2030), NITAGs provide expert advice to governments on vaccine-related decisions, such as introducing new vaccines, setting immunization schedules, and ensuring vaccine safety (Duclos, 2010; Immunization Agenda 2030 Partners, 2024). When developing recommendations, NITAGs are encouraged to use systematic reviews (SRs) reflecting the best available evidence. By applying rigorous and transparent methods, SRs synthesize results of multiple studies. In recent years, the body of evidence has expanded rapidly, challenging NITAGs in accessing technical resources to effectively synthesize and assess the relevant literature (Fernandes et al., 2018; Steffen et al., 2021).
This paper presents a bibliometric analysis of the vaccination-related SRs. Bibliometric analysis is a quantitative method used to evaluate the inter-relationships and impacts of publications (Donthu et al., 2021). Unlike SRs, bibliometric analyses summarize publication details such as author information, journals, and keywords (Ellegaard & Wallin, 2015), and highlight shifts in research focus and emerging areas of interest. However, to our knowledge, only one bibliometric analysis has been published to date, analyzing trends of vaccination-related SRs published from 2008 to 2016 (Fernandes et al., 2018).
The objectives of this paper were (i) to provide an overview of the vaccination-related SR literature by describing trends in time, journal, accessibility, topic, disease, population, geographic region, and methodological quality published between 1 January 2011 and 31 December 2023 and (ii) to identify the extent of duplication and overlap in vaccination-related SRs.
2. Methods
2.1. Data Source: Systematic Reviews from SYSVAC Registry
SRs from the freely accessible SYSVAC registry were considered in this study (WHO, n.d.-a). The following section describes the standard operation used to populate the SYSVAC registry with SRs. In this registry, both SRs and systematic umbrella reviews are included (hereafter referred as SRs). Systematic searches for SRs were carried out in MEDLINE (via Ovid), Embase, the Cochrane Library of Systematic Reviews, and the COVID-19 Living Overview of Evidence (L-OVE) repository (Epistemonikos, n.d.). The last literature search was conducted in June 2024, using search terms related to “vaccination” and “immunization” (see Supplementary Materials for complete search strategy for each database). Vaccination-related SRs published from 01 January 2011 to 31 December 2023 were included. Further eligibility required articles (1) to state if they are a SR or follow minimum criteria defined by Robinson et al. (2016), (2) focus on preventive vaccines for humans, (3) not be a pre-print, (4) not be withdrawn, and (5) be written in English language. The inclusion and exclusion criteria can be found in Table S1, Supplementary Materials.
EndNote (Version 21.3) was used for record management. After removing duplicates and irrelevant records, the remaining records were screened for eligibility by one author based on the title and abstract of the published SRs. The eligibility of full texts of the remaining records was assessed by two reviewers independently. Disagreement between reviewers was resolved by discussion or by consulting with a third reviewer.
Eligible SRs were indexed using keywords according to a keyword coding protocol (see Supplementary Materials, Table S2 for keyword categorization). Keywords enabled SRs to be assigned to categories in seven areas: (1) accessibility of full texts; (2) vaccine-preventable disease or pathogen, e.g., COVID-19 (if applicable); (3) vaccine topic, e.g., safety of vaccines; (4) target population, e.g., adults, health care workers; (5) focus on low and middle-income countries (if applicable); (6) geographical location according to WHO regions (WHO, n.d.-b) (if applicable); and (7) methodological quality using A Measurement Tool to Assess systematic Reviews 2 (AMSTAR 2) (Shea et al., 2017), e.g., high. Keywords were not mutually exclusive. SRs could be classified with multiple keywords or none, except for disease/pathogen or geographical region. Any uncertainties during coding process were resolved by consensus between two reviewers or by consulting a third reviewer.
2.2. Data Analysis
An analysis protocol was developed in advance and is available upon request. For this analysis, the registry content (as of June 2024) was exported to Excel 2019. Data categorizations were checked in Excel, providing complete information of each publication, including the literature title, author list, journal name, publication year, and keywords. The dataset was descriptively analyzed by year of publication, journal, and keywords. Time series analysis on the disease/pathogen and methodological quality keyword category were used to explore trends over time. Subgroup analysis was performed to describe trends for umbrella reviews. To identify SRs covering identical or overlapping topics, the keywords disease/pathogen, topic, and population were examined in a bubble plot. In this plot, the bubble size corresponded to the number of SRs published on each unique keyword combination. Larger bubbles indicated higher productivity of SRs and allowed assessment of several intersections, while smaller bubbles indicated less frequent productivity, reflecting potential gaps in the literature. Both nominal and metric data were described by calculating frequencies with corresponding percentages. Bibliometric analysis and data visualization were conducted using R (version 4.4.1).
3. Results
3.1. Journals and Accessibility of Full Texts
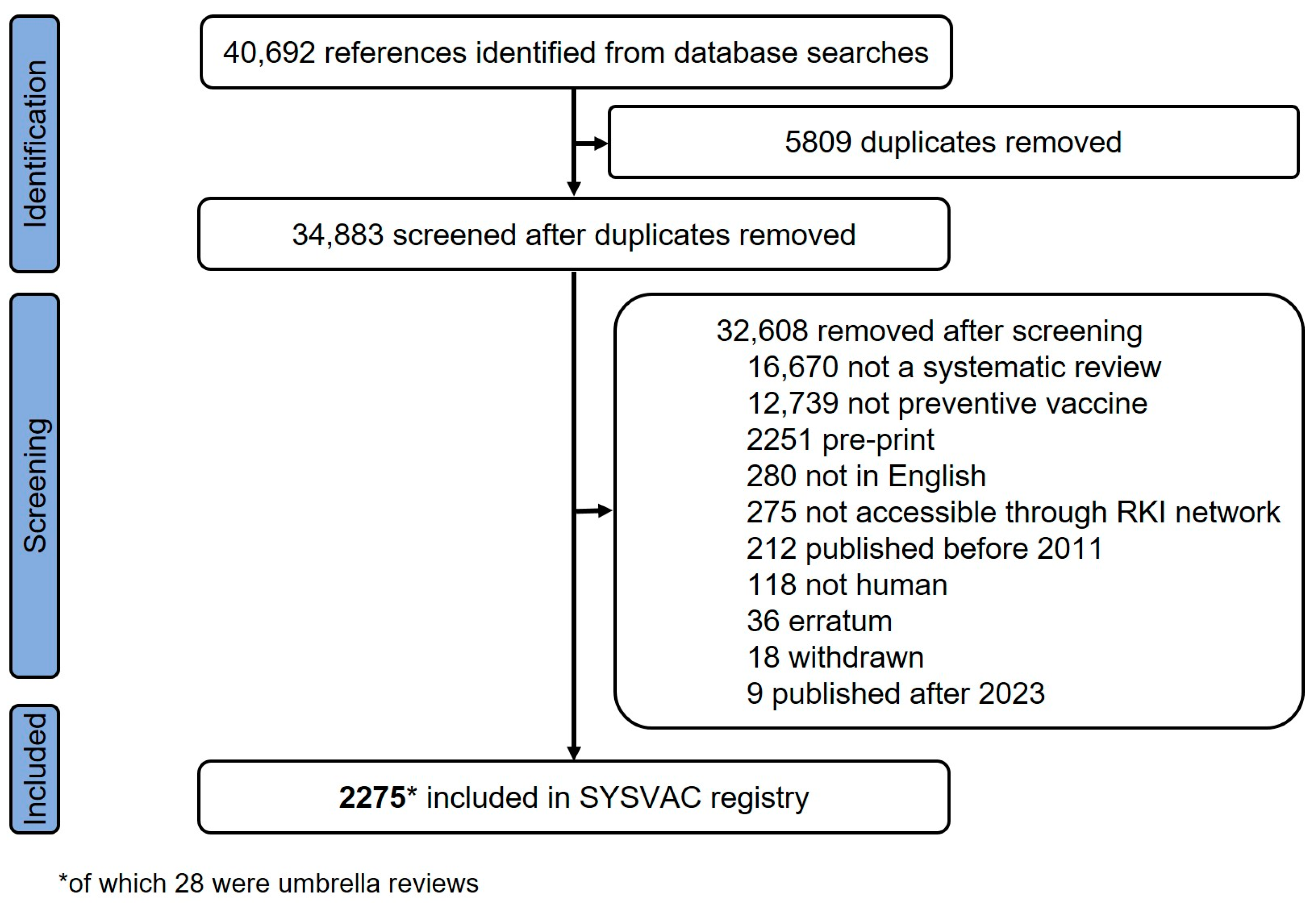
A total of 2275 SRs were included in the bibliometric analysis, 28 of which were umbrella reviews (see Figure 1 for PRISMA flowchart). The 2275 unique SRs were published in 653 different academic journals. The most productive journals were Vaccine (n = 213, 9.4%), Vaccines (n = 197, 8.7%), and Human vaccines & Immunotherapeutics (n = 136, 6.0%) (see Supplementary Materials, Table S3 for top ten of productive journals). Most SRs were published open access (n = 2083, 91.6%), while for the remaining SRs subscription was needed to access full text of publications (n = 192, 8.4%).
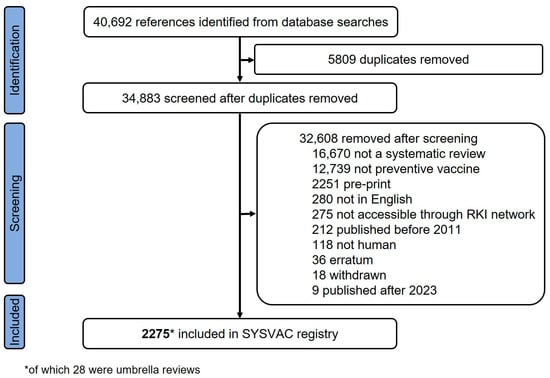
Figure 1.
Flow diagram illustrating the screening process from multiple systematic searches and inclusion of systematic reviews in SYSVAC registry, published between 2011 and 2023. References identified include de-duplicated references identified by London School of Hygiene and Tropical Medicine.
Regarding umbrella reviews (n = 28), PLOS One and Vaccine (n = 3 each, 10.7%) were the most productive journals (see Supplementary Materials, Table S4 for journals). The majority of umbrella reviews were freely accessible in full text (n = 26, 92.9%).
3.2. Trend over Time (2011–2023)
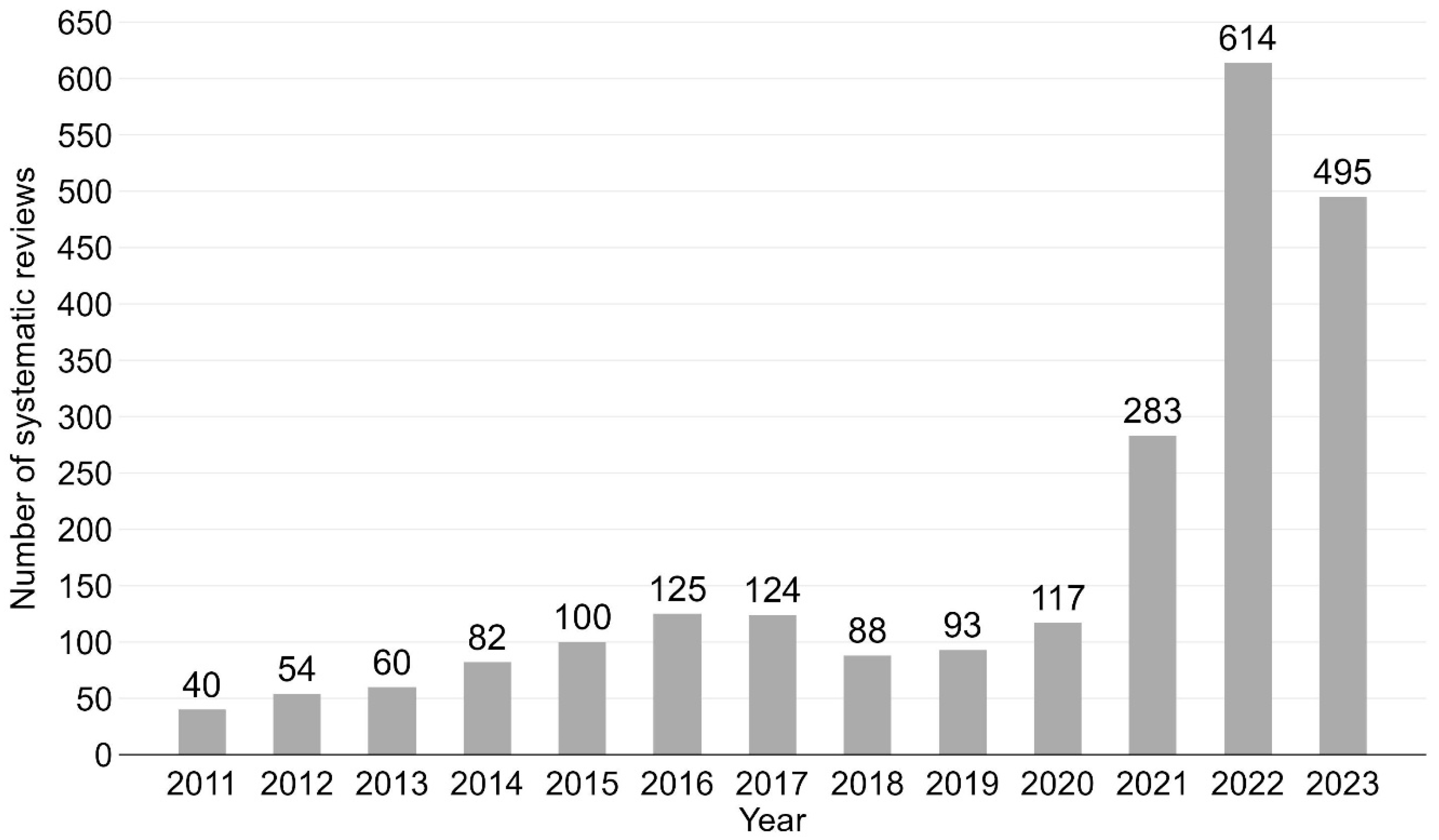
The number of vaccination-related SRs published per year increased continually from 40 in 2011 to 495 in 2023, a more than twelvefold increase (mean number of annual SRs = 175; see Figure 2 for bar chart). The highest number of SRs (n = 614) was published in 2022. Notably few SRs were published from 2011 to 2013 (n = 154, 6.8%), while more than half of the SRs (n = 1392, 61.2%) were published during the last three years (2021–2023). A similar time trend was shown for umbrella reviews (see Supplementary Materials, Figure S1 for bar chart by year).
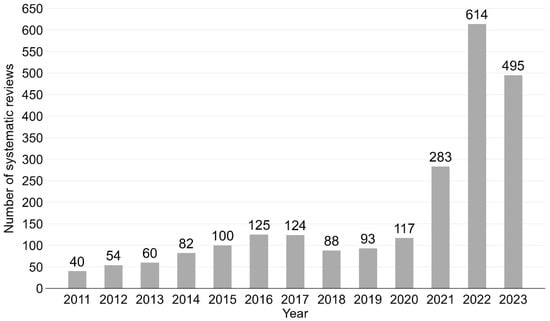
Figure 2.
Vaccination-related systematic reviews by year. Bar chart from 2011 to 2023. N = 2275.
3.3. Trend for Disease/Pathogen
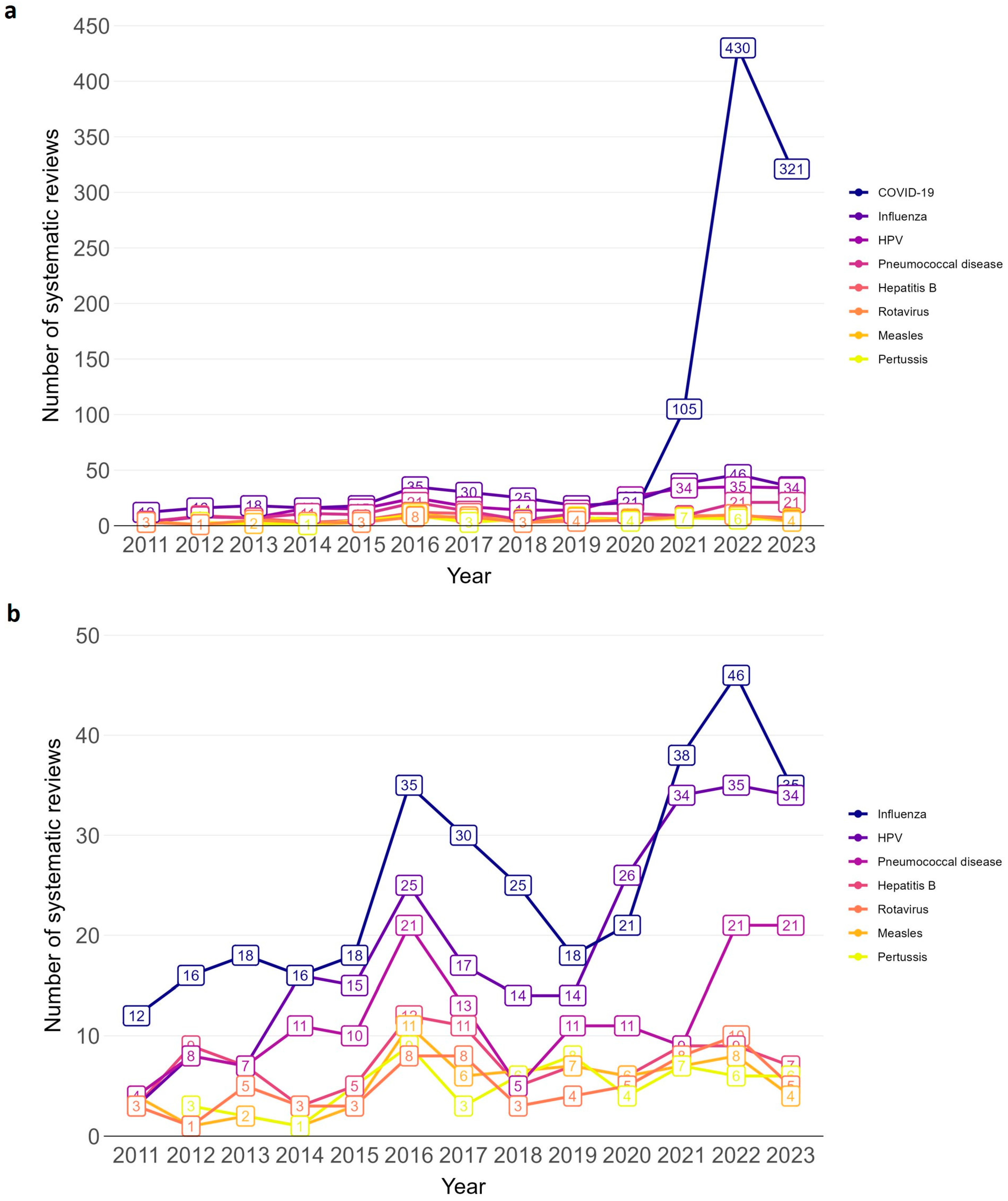
In total, 43 disease/pathogen areas were covered in the SRs (see Supplementary Materials, Table S2 for alphabetical order of disease/pathogen areas). Among 2275 SRs, 1918 (84.3%) focused on specific disease/pathogen areas, of which 1779 (78.2%) focused on one disease/pathogen and 139 (6.1%) on two or more. For eight disease/pathogen areas more than 50 SRs were identified: COVID-19, influenza, Human papillomavirus (HPV), pneumococcal disease, hepatitis B, rotavirus, measles, and pertussis (see Table 1 for number of SRs for each disease/pathogen). These eight diseases/pathogens fluctuated in the annual number of SRs over time (see line chart Figure 3 and Supplementary Materials, Table S5 for absolute numbers). The sharpest increase in the number of SRs was for COVID-19, followed by influenza, HPV and pneumococcal disease. For COVID-19 and influenza, there is a peak in 2022, shortly after the early 2020 SARS-CoV-2 pandemic. The number of SRs for hepatitis B, rotavirus, measles, and pertussis seems to have plateaued in the past three years.

Table 1.
Vaccination-related systematic reviews by disease/pathogen, published from 2011–2023.
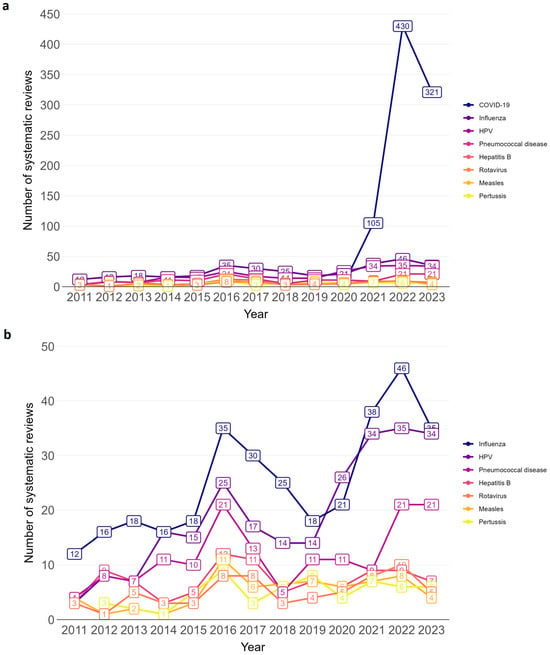
Figure 3.
Annual trend of vaccination-related systematic reviews by disease/pathogen for which ≥50 systematic reviews were identified. (a): including COVID-19; (b): excluding COVID-19. Bar chart from 2011 to 2023.
In contrast, umbrella reviews covered only five disease/pathogen areas (see Supplementary Materials, Table S6 for number of umbrella reviews for each disease/pathogen). The highest number of umbrella reviews focused on influenza with nine umbrella reviews.
3.4. Trends for Vaccination Topic
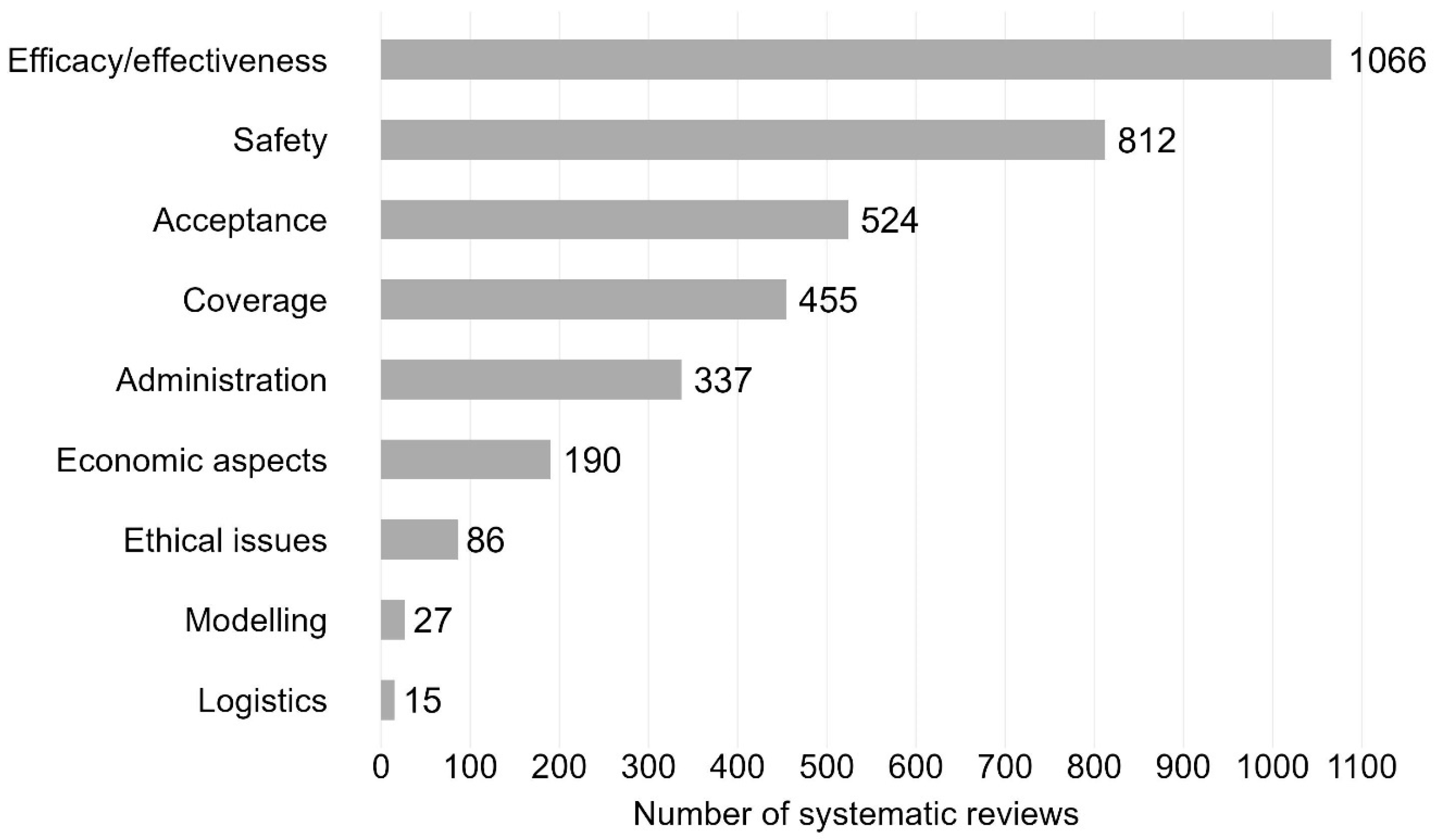
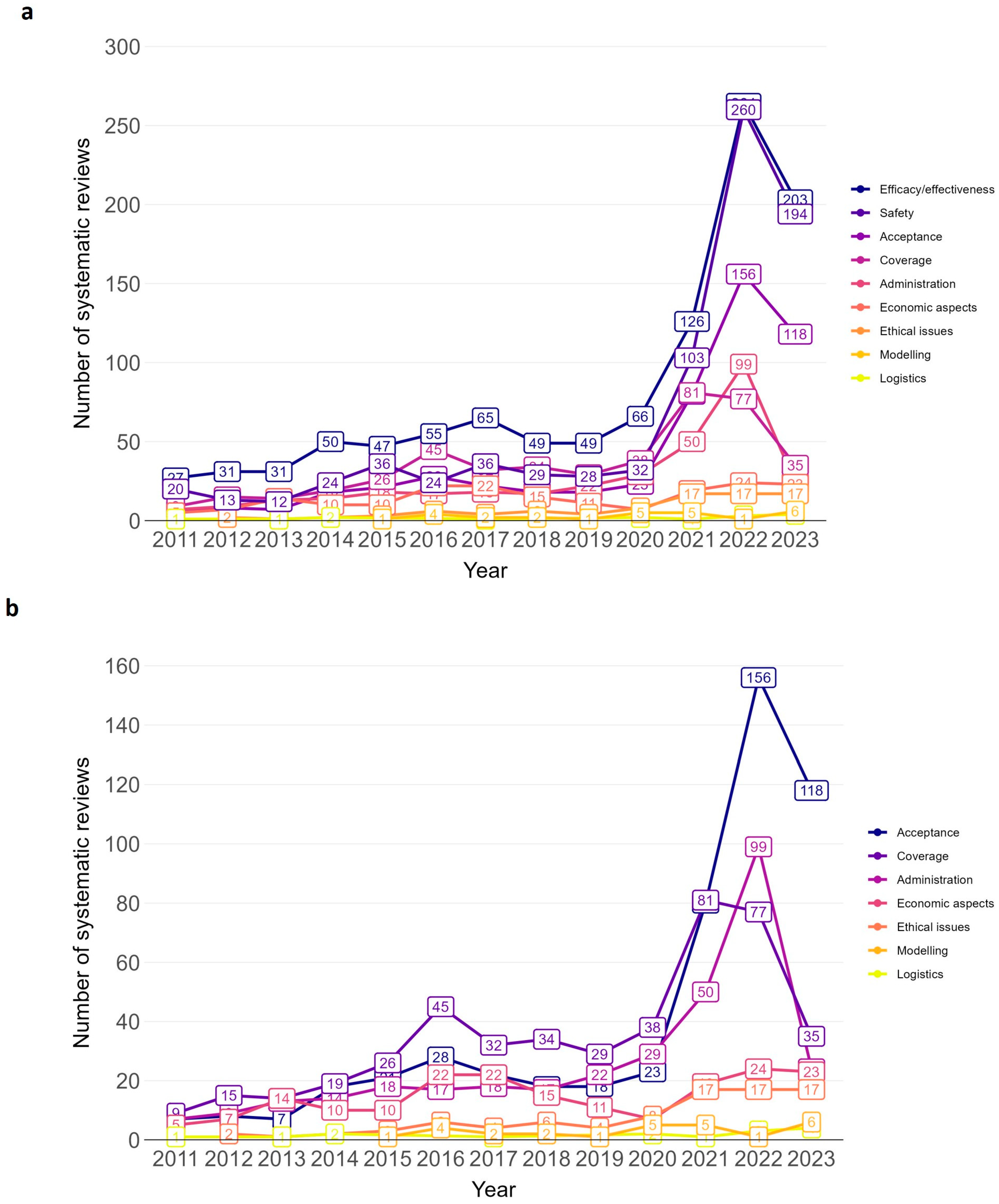
The majority of SRs addressed the efficacy/effectiveness (n = 1066, 46.9%) and safety of vaccines (n = 812, 35.7%) (see Figure 4 for trend by vaccination topic). Further vaccination topics were acceptance (n = 524, 23.0%), coverage (n = 455, 20.0%) and administration (n = 337, 14.8%). Less frequently addressed topics were ethical issues (n = 86, 3.8%), modelling (n = 27, 1.2%), and logistics (n = 15, 0.7%). All nine topic areas exhibited a general trend upwards in the annual number of SRs over time (see line chart Figure 5 and Supplementary Materials, Table S7 for absolute numbers). The sharpest increase in the number of SRs was for efficacy/effectiveness, safety, acceptance, coverage, and administration.
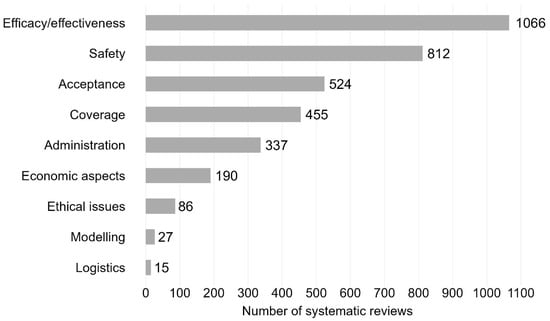
Figure 4.
Vaccination-related systematic reviews by topic. Bar chart including systematic reviews published between 2011 and 2023. N = 2275 systematic reviews. Efficacy/effectiveness includes systematic reviews on immunogenicity (vaccine effects against immune markers or other biological endpoints such as antibodies, immune cells). 1271 systematic reviews with one topic area; 789 with two topic areas; 215 with ≥3 topic areas.
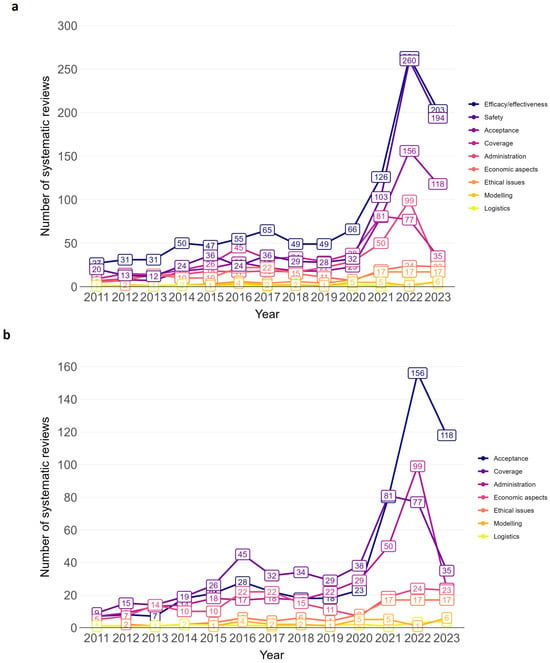
Figure 5.
Annual trend of vaccination-related systematic reviews by topic, (a): all topics; (b): excluding topic efficacy/effectiveness and safety. Bar chart from 2011 to 2023. Efficacy/effectiveness includes systematic reviews on immunogenicity (vaccine effects against immune markers or other biological endpoints such as antibodies, immune cells).
Similar trends were observed for umbrella reviews, with efficacy/effectiveness, safety, and acceptance being the leading topic areas (see Supplementary Materials, Figure S2 for bar chart).
3.5. Geographic Location
Most SRs did not focus on a specific country or WHO region (n = 1951, 85.8%). Among the 324 SRs with a geographical focus, 307 (95%) focused on one WHO region and 17 (5%) on more than one WHO region. The most often addressed WHO region was the Americas with 94 SRs (4.1%), followed by Africa, Europe, and the Western Pacific with 87 (3.8%), 64 (2.8%) and 53 (2.3%) SRs, respectively (see Supplementary Materials, Figure S3 for details on distribution by WHO region). A smaller number of SRs focused on South-East Asia and the Eastern Mediterranean region (n = 26, 1.1% and n = 19, <1.0%), respectively. Only a small portion (n = 96, 4.2%) of the SRs targeted low and middle-income countries.
The majority of SRs were not restricted to a specific country (n = 2084, 91.6%), while 187 (8.2%) SRs had a single-country focus and 4 (0.2%) SRs had a multi-country focus. The United States of America (n = 54, 2.4%), followed by China (n = 29, 1.3%) and Ethiopia (n = 24, 1.1%) were the countries with the most focused SRs, respectively (see Supplementary Materials, Table S8 for other country foci). Of the 190 SRs focusing on a specific country, in 173 (91.1%) SRs the country focus was identical to the country affiliation of the corresponding author.
3.6. Target Population
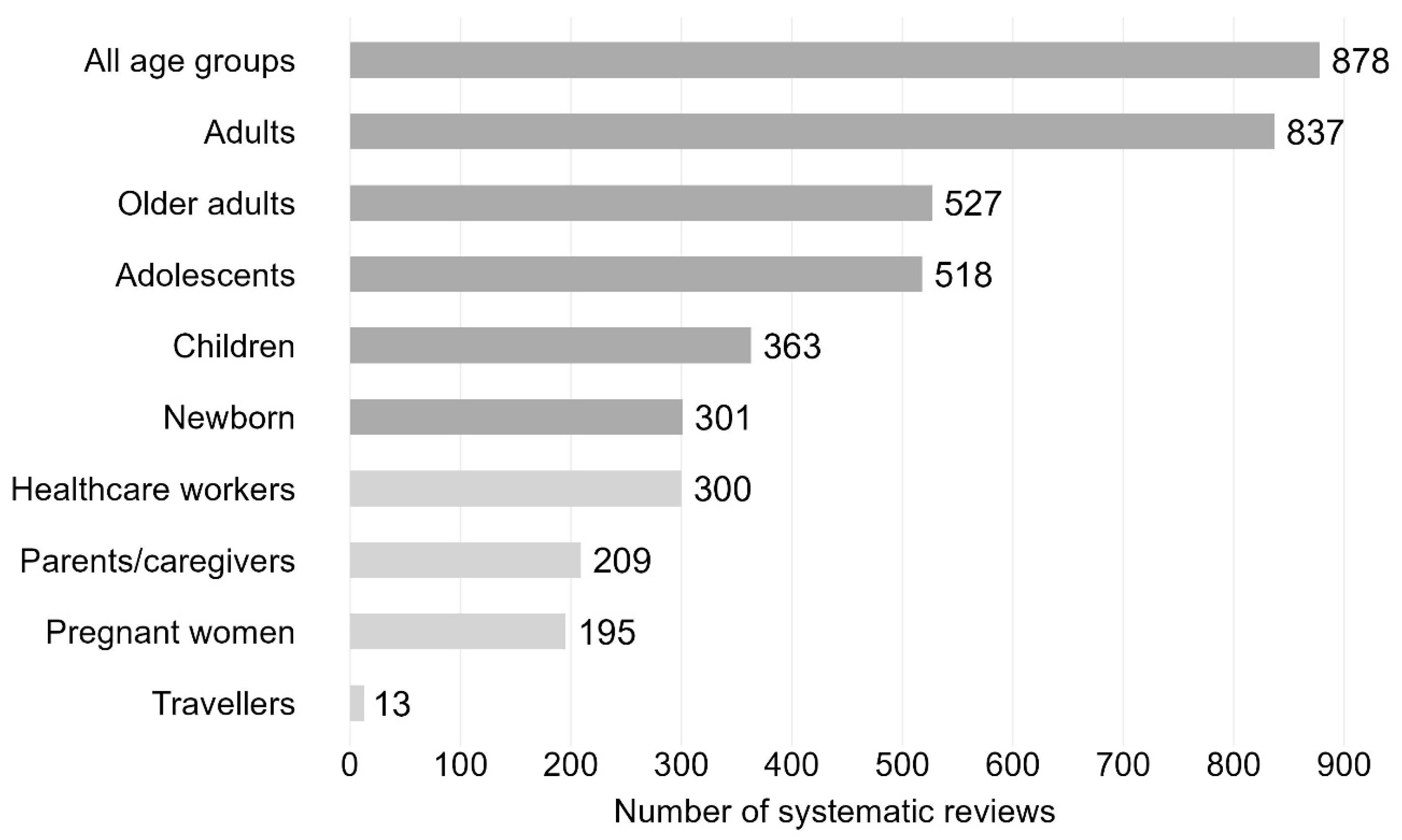
A total of 1019 (44.8%) SRs targeted one particular age or population group, 794 (34.9%) focused on two groups, and 462 (20.3%) on more than two age or population groups. More than a third of the SRs included all age groups (n = 878 SRs; 38.6%), followed by adults aged ≥18–64 years with 837 SRs (36.8%) (see Figure 6 for distribution of further age or population groups). Less often addressed age groups were older adults aged ≥65 years (n = 527, 23.2%), adolescents aged 10–17 years (n = 518, 22.8%), children aged 1–9 years (n = 363, 15.6%) and newborns aged ≤1 year (n = 301, 13.2%). Other population groups were healthcare workers (n = 300, 13.2%), parents/caregivers (n = 209, 9.2%), pregnant women (n = 195, 8.6%), and travellers (n = 13, 0.6%).
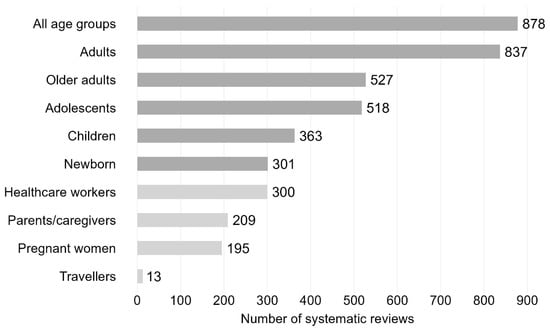
Figure 6.
Vaccination-related systematic reviews by age (dark grey) or population groups (light grey). Bar chart including systematic reviews published between 2011 and 2023. Newborn: ≤1 year; children: 2–9 years; adolescents: 10–17 years; adults: ≥18–64 years; older adults: ≥65 years. N = 2275 systematic reviews.
A similar distribution was observed for umbrella reviews, with all age groups being the most often targeted population group (see Supplementary Materials, Figure S4 for bar chart).
3.7. Methodological Quality
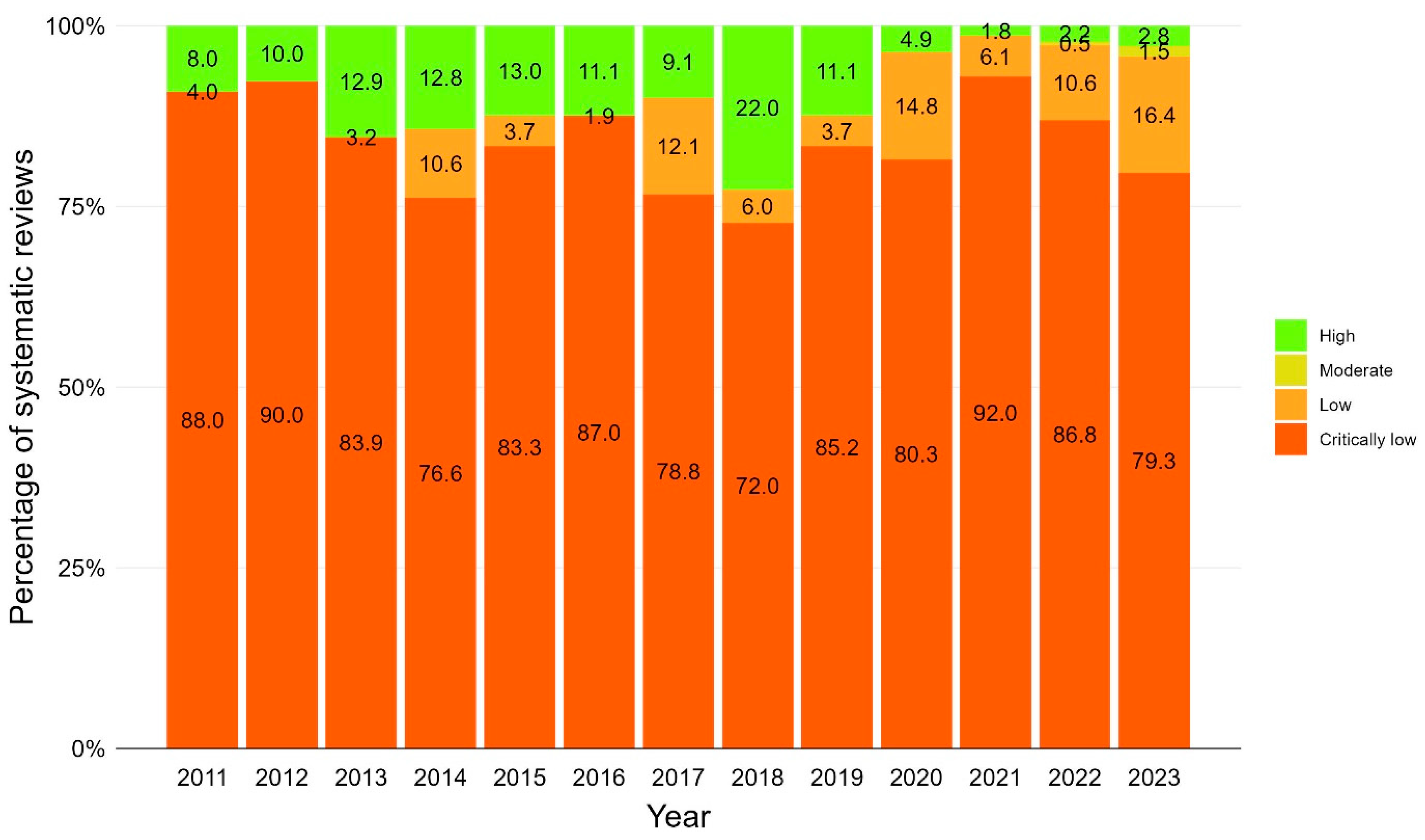
In the majority of the 1376 SRs where AMSTAR 2 was applicable, methodological quality was assessed as critically low, indicating flaws in more than one of seven critical items of the 16-item AMSTAR 2 instrument (n = 1155, 84.0%). The remaining SRs were assessed as low (n = 139, 10.0%; flaw in one critical item), high (n = 75, 5.5%; flaw in no or one non-critical item) or moderate (n = 7, 0.5%; flaws in more than one non-critical item). The methodological quality of SRs remained stable over the time, with the exception of 2018 when the percentage of high-rating SRs increased to 22% (see Figure 7 for stacked bar chart of methodological quality by year and Supplementary Materials, Table S9 for relative frequencies). For information on journals of high-quality SRs, refer to Supplementary Materials, Table S10.
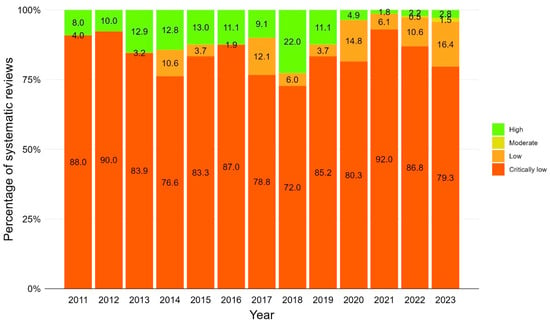
Figure 7.
Methodological quality of vaccination-related systematic reviews over time using AMSTAR 2 (A Measurement Tool to Assess systematic Reviews 2). Stacked bar chart including systematic reviews published between 2011 and 2023. N = 1376 systematic reviews where AMSTAR 2 was applicable.
3.8. Repetition and Overlap of Systematic Reviews
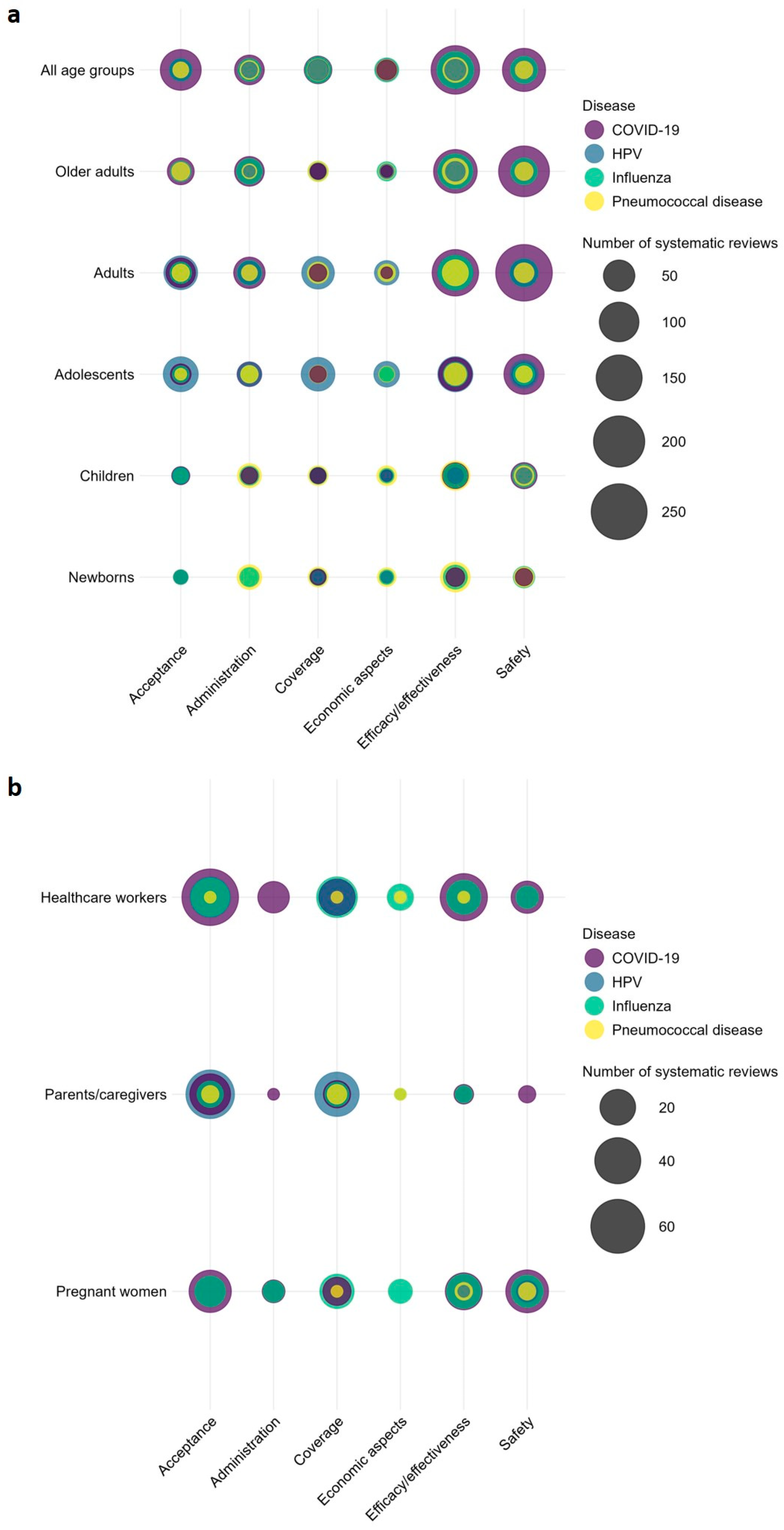
There were several overlapping topics among the SRs (see Figure 8 for bubble plot). Of the 861 SRs on COVID-19, 389 focused on COVID-19 vaccination for adults, with 258 addressing safety outcomes, 155 the efficacy/effectiveness, 50 addressing administration, and 41 focusing on vaccine acceptance. In 2022, seven SRs (Elberry et al., 2022; Kim et al., 2022; Kolahchi et al., 2022; Matar et al., 2022; Palaiodimou et al., 2022; Saluja et al., 2022; Zheng et al., 2022) were published on thrombosis after COVID-19 vaccination. A further eight SRs published in 2022 focused on COVID-19 vaccine hesitancy and acceptance among pregnant women (Azami et al., 2022; Bhattacharya et al., 2022; Bianchi et al., 2022; Galanis et al., 2022; Halemani et al., 2022; Nikpour et al., 2022; Nindrea et al., 2022; Sarantaki et al., 2022). Of 328 SRs on influenza, 97 targeted older adults aged ≥65 years, with 70 on efficacy/effectiveness and 30 on safety outcomes. Among the 248 SRs addressing HPV, 51 targeted caregivers/parents of which 46 focused on barriers and factors associated with HPV vaccination and 36 SRs on determinants of uptake. Among 152 SRs on pneumococcal disease, 56 SRs focused on pneumococcal vaccination of newborns, 42 SRs addressed efficacy/effectiveness, 22 focused on administration and 9 each on coverage and safety. For further overlap of SRs in other topic areas, refer to Figure 8.
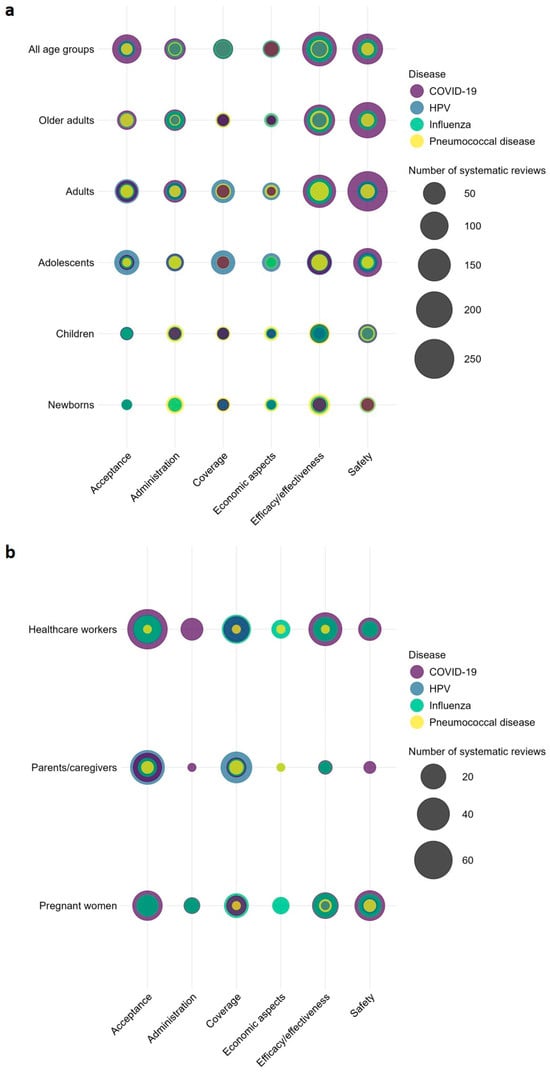
Figure 8.
Comparison of systematic reviews on disease/pathogen and topic by population. (a): age groups and (b): population groups with ≥100 systematic reviews. Bubble plot including systematic reviews published between 2011–2023.
4. Discussion
This bibliometric analysis provides a comprehensive overview of the landscape of SRs on vaccination topics published in the past 13 years. A total of 2275 SRs were identified. The results indicate a considerable increase in vaccination-related SRs during this time period. Several factors might have contributed to this increase, including the introduction of new vaccines or advances in research funding with support from international organizations or initiatives such as WHO (Immunization Agenda 2030 Partners, 2024) or GAVI (GAVI, n.d.). Moreover, from early 2020 onwards, the COVID-19 pandemic had a strong impact on research orientation and hot topic areas. Several vaccines, such as mRNA vaccines, were developed at an unprecedented pace, leading to an increase in SRs to assess their efficacy/effectiveness, safety and technological innovation (Pardi et al., 2018). Nonetheless, the trend in the number of SRs in vaccination appears to be in line with the growth of general scientific output. Until 2023, more than 30 million articles had been indexed in MEDLINE (National Library of Medicine, n.d.). The expansion of primary research may have favoured the emergence and proliferation of systematic literature reviews.
In recent years, there have been some bibliometric analyses of articles on vaccine-preventable diseases such as COVID-19, HPV, pertussis or Anthrax, revealing publishing activity in this area (Ahmad et al., 2022; Alsulaiman et al., 2025; Bruel et al., 2021; Tan et al., 2024). Similarly to our results, Vaccine was the most productive journal. However, to our knowledge only one previous bibliometric analysis focused on vaccination-related SRs in terms of journals, trends over time, disease/pathogen, topic, population and geographical focus. In 2018, Fernandes et al. analyzed SRs published on vaccination from 2008 to 2016 and found the most addressed disease/pathogen areas being influenza, HPV and pneumococcal disease. These trends have remained the same during the past seven years, in addition to COVID-19. As this study showed, the efficacy/effectiveness and safety remained the predominant vaccination topics.
During the past decades, interest in vaccine research has grown due to the development of new vaccines to prevent serious illness (Steffen et al., 2021). However, the mass proliferation of SRs has raised concerns about their quality and rigorousness (Ioannidis, 2016). To our knowledge, this is the first study analyzing the trends of the methodological quality of vaccination-related SRs. Our analysis shows that most SRs were critically low in methodological quality using AMSTAR 2, questioning the internal validity of these SRs (see chapter 3.7). AMSTAR 2 is one of the most widely used instruments for methodological quality assessment of SRs, yet the assessment of some items is inherently subjective. According to the developers, there are seven items that can critically affect the validity of a SR and its conclusions (item 2: protocol registration, item 4: adequacy of literature search strategy, item 7: justification for excluding individual studies, item 9: risk of bias assessment, item 11: appropriateness of meta-analytical methods, item 13: risk of bias interpretation, and item 15: publication bias). In our analysis, there was more than one flaw in the seven critical items present, underscoring the need for stronger adherence to reporting and methodological standards such as PRISMA and PROSPERO (Schiavo, 2019; Tugwell & Tovey, 2021). Nonetheless, there may be items not always regarded as critical and considered less important (Shea et al., 2017). Depending on this, SRs with critically low quality might still yield useful information and may be considered by NITAGs. Interestingly, results showed that the percentages of high-quality SRs increased in 2018. This may have been favoured by the release of the AMSTAR 2 instrument and the Cochrane Collaboration Handbook for review authors in 2017 (Higgins et al., 2017; Shea et al., 2017). As both releases aimed at setting methodological standards for the conduct of SRs, they might have contributed to an improvement of the methodological quality of SRs published in the following year. Going into detail on the individual AMSTAR 2 items is beyond the scope of this paper. Furthermore, a comparison with other assessment tools, such as ROBIS, which assesses the risk of bias of SRs, may be necessary to confirm the validity of the findings. To date, however, only AMSTAR 2 has been routinely applied within the SYSVAC registry. Future analyses are planned, focusing on the methodological quality of the SRs included in the SYSVAC registry.
The potential for duplication and overlap rises with the increase of SRs. Our results showed numerous SRs targeting similar diseases/pathogens, vaccination topics and population groups. This overlap underscores the redundancy of research outputs with identical settings. It emphasizes the importance of improving resource deployment. A more practical approach for authors may be to register their SR protocols in advance and foster collaborations to combine efforts and minimize waste of limited resources. Policymakers and journal editors can play a crucial role in promoting these practices, ensuring a more efficient and effective approach to SR production. We therefore recommend that researchers should be encouraged, e.g., by journal editors, to check the landscape of existing SRs before planning to conduct a new SR. Moreover, in cases when SRs on a particular topic already exist, journal editors should ask authors of new submissions to clearly explain the rational for performing a new SR. Lastly, policymakers are encouraged to use existing SRs, instead of asking for new ones to support their policy-making. On the other hand, the findings from this bibliometric analysis can be leveraged by NITAGs and authors of new SRs to identify gaps in the vaccination literature and identify areas where further SRs are needed. By examining the overlap of existing SRs, NITAGs and authors can determine which topics have been thoroughly explored and underexplored. The SYSVAC registry can be a resource to identify these emerging gaps. NITAGs and authors can conduct new SRs that avoid redundancy and contribute novel insights to the vaccination field. This targeted approach ensures new SRs to address relevant and impactful research, offering high value to NITAGs and policymakers, who rely on robust and non-redundant evidence to inform their decisions.
The SYSVAC registry and this bibliometric analysis have two limitations. First, there is a potential for language bias due to the inclusion criteria of the SYSVAC registry which is restricted to SRs written in English language (n = 280 non-English articles, see flowchart, Figure 1). This limitation may affect the representation of evidence from low and middle-income countries or non-English-speaking regions, where research may be conducted and disseminated in local languages. Additionally, the exclusion of SRs due to lack of institutional access can lead to an overrepresentation of SRs from well-funded institutions with better access to resources. Nonetheless, systematic searches with no language restrictions are conducted to embed SRs into the registry, ensuring a broad and thorough collection of evidence from multiple databases (see Supplementary Materials for comprehensive search strategies). Additionally, the number of SRs not written in English or not accessible is relatively small, minimizing their likely impact on the SR pool (see Figure 1 for flowchart). A list of these excluded SRs is available upon request. However, some SRs on vaccination topics may not be included in the registry because they are deemed irrelevant to the work of NITAGs, such as the review of the evolution of studies concerning HPV vaccination (Bruel et al., 2021). We also acknowledge that research trends may have shifted since 2024. However, this analysis is limited to SRs published up to 2023 to ensure data quality and consistency, as the incorporation of SRs published in 2024 into the SYSVAC registry has not yet been completed. As of April 2025, 282 SRs published in 2024 were included in the SYSVAC registry WHO (n.d.-a). Second, the presented descriptive analysis on identified overlaps of SR topics is based on the developed and assigned keywords, but not on the specific research question of the SR (see Supplementary Materials, Table S2 for keyword categorization). It is currently unclear to what extent the keywords match the question of interest of the SR such as the intervention, comparator or outcomes. A further assessment by the user is essential. Future analyses combining bibliometrics with qualitative content analysis could bridge the gap and provide a further understanding of the alignment between SR scope and content.
5. Conclusions
The number of vaccination-related SRs increased tremendously from 2011 to 2023. However, the potential for overlap and duplication remains high, emphasizing the importance of improving resource deployment. This bibliometric analysis can provide a basis for understanding the current state and priorities in the vaccination research. The SYSVAC registry can facilitate identify existing SRs and decrease the potential of overlap in newly developed SRs (www.nitag-resource.org/sysvac-systematic-reviews) (accessed on 22 April 2025).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/publications13020025/s1. Table S1: Eligibility criteria; Table S2: Keywords; Table S3: Top ten of productive journals with systematic reviews; Table S4: Productive journals with umbrella reviews; Figure S1: Vaccination-related umbrella reviews by year; Table S5: Annual number of systematic reviews by disease/pathogen; Table S6: Vaccination-related umbrella reviews by disease/pathogen; Table S7: Annual number of systematic reviews by topic; Figure S2: Vaccination-related umbrella reviews by topic; Figure S3: Vaccination related-systematic reviews by WHO region and low- and middle-income country area; Table S8: Vaccination-related systematic reviews by country; Figure S4: Vaccination-related umbrella reviews by age or population groups; Table S9: Methodological quality of vaccination-related systematic reviews; Table S10: Productive journals with high methodological quality systematic reviews.
Author Contributions
A.P.: conceptualization, methodology, formal analysis and investigation, writing—original draft preparation, writing—review and editing, supervision, funding acquisition, project administration; L.H.: conceptualization, writing—review and editing; C.A.S.: conceptualization, writing—review and editing; H.H.L., A.I.D. and M.B.: conceptualization, writing—review and editing; O.W.: conceptualization, writing—review and editing, supervision, funding acquisition; V.P.: conceptualization, methodology, writing—review and editing, supervision; T.H.: conceptualization, methodology, writing—review and editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the German Federal Ministry of Health through the Global Health Protection Program (Grant number: ZM I 5 2519GHP713).
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its Supplementary Materials.
Acknowledgments
Thanks to Matthäus Lottes for methodological input and Elizabeth Lynch for linguistic suggestions on the manuscript (both Robert Koch Institute).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| AMSTAR 2 | A Measurement Tool to Assess systematic Reviews 2 |
| GNN | Global NITAG Network |
| HPV | Human papillomavirus |
| IA2030 | Immunization Agenda 2030 |
| NITAG | National Immunization Technical Advisory Group |
| RSV | Respiratory Syncytial Virus |
| SR | Systematic review |
| WHO | World Health Organization |
References
- Ahmad, T., Baig, M., Othman, S. S., Malibary, H., Ahmad, S., Rasheed, S. M., Al Bataineh, M. T., & Al-Omari, B. (2022). Bibliometric analysis and visualization mapping of anthrax vaccine publications from 1991 through 2021. Vaccines, 10(7), 1007. [Google Scholar] [CrossRef] [PubMed]
- Alsulaiman, J. W., Alzoubi, A., Alrawashdeh, A., Al-Dekah, A. M., Abubaker, S., Amayreh, W., Sweileh, W. M., Alzoubi, H. M., & Kheirallah, K. A. (2025). Mapping trends and hotspots of research on COVID-19 vaccine effectiveness: A comprehensive bibliometric analysis of global research. Journal of Infection and Public Health, 18(1), 102597. [Google Scholar] [CrossRef] [PubMed]
- Azami, M., Nasirkandy, M. P., Gouvarchin Ghaleh, H. E., & Ranjbar, R. (2022). COVID-19 vaccine acceptance among pregnant women worldwide: A systematic review and meta-analysis. PLoS ONE, 17(9), e0272273. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, O., Siddiquea, B. N., Shetty, A., Afroz, A., & Billah, B. (2022). COVID-19 vaccine hesitancy among pregnant women: A systematic review and meta-analysis. BMJ Open, 12(8), e061477. [Google Scholar] [CrossRef]
- Bianchi, F. P., Stefanizzi, P., Di Gioia, M. C., Brescia, N., Lattanzio, S., & Tafuri, S. (2022). COVID-19 vaccination hesitancy in pregnant and breastfeeding women and strategies to increase vaccination compliance: A systematic review and meta-analysis. Expert Review of Vaccines, 21(10), 1443–1454. [Google Scholar] [CrossRef]
- Bruel, S., Dutzer, D., Pierre, M., Botelho-Nevers, E., Pozzetto, B., Gagneux-Brunon, A., Chauvin, F., & Frappé, P. (2021). Vaccination for Human Papillomavirus: An historic and bibliometric study. Human Vaccines and Immunotherapeutics, 17(4), 934–942. [Google Scholar] [CrossRef]
- Donthu, N., Kumar, S., Mukherjee, D., Pandey, N., & Lim, W. M. (2021). How to conduct a bibliometric analysis: An overview and guidelines. Journal of Business Research, 133, 285–296. [Google Scholar] [CrossRef]
- Duclos, P. (2010). National Immunization Technical Advisory Groups (NITAGs): Guidance for their establishment and strengthening. Vaccine, 28, A18–A25. [Google Scholar] [CrossRef]
- Elberry, M. H., Abdelgawad, H. A. H., Hamdallah, A., Abdella, W. S., Ahmed, A. S., Ghaith, H. S., & Negida, A. (2022). A systematic review of vaccine-induced thrombotic thrombocytopenia in individuals who received COVID-19 adenoviral-vector-based vaccines. Journal of Thrombosis and Thrombolysis, 53(4), 798–823. [Google Scholar] [CrossRef]
- Ellegaard, O., & Wallin, J. A. (2015). The bibliometric analysis of scholarly production: How great is the impact? Scientometrics, 105(3), 1809–1831. [Google Scholar] [CrossRef]
- Epistemonikos. (n.d.). Living overview of evidence repository. Available online: https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d (accessed on 1 June 2024).
- Fernandes, S., Jit, M., Bozzani, F., Griffiths, U. K., Scott, J. A. G., & Burchett, H. E. D. (2018). A bibliometric analysis of systematic reviews on vaccines and immunisation. Vaccine, 36(17), 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P., Vraka, I., Siskou, O., Konstantakopoulou, O., Katsiroumpa, A., & Kaitelidou, D. (2022). Uptake of COVID-19 vaccines among pregnant women: A systematic review and meta-analysis. Vaccines, 10(5), 766. [Google Scholar] [CrossRef] [PubMed]
- GAVI. (n.d.). GAVI—The vaccine alliance. GAVI’s strategy. Available online: https://www.gavi.org/our-alliance/strategy (accessed on 6 November 2024).
- Halemani, K., Dhiraaj, S., Latha, T., Mishra, P., & Issac, A. (2022). The prevalence of COVID vaccine acceptance among pregnant women: A systematic review and meta-analysis. Clinical Epidemiology and Global Health, 17, 101144. [Google Scholar] [CrossRef]
- Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., & Welch, V. A. (2017). Cochrane handbook for systematic reviews of interventions version 5.2.0 [updated June 2017]. The Cochrane Collaboration. Available online: www.handbook.cochrane.org (accessed on 22 April 2025).
- Immunization Agenda 2030 Partners. (2024). Immunization agenda 2030: A global strategy to leave no one behind. Vaccine, 42, S5–S14. [Google Scholar] [CrossRef]
- Ioannidis, J. P. (2016). The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q, 94(3), 485–514. [Google Scholar] [CrossRef]
- Kim, A. Y., Woo, W., Yon, D. K., Lee, S. W., Yang, J. W., Kim, J. H., Park, S., Koyanagi, A., Kim, M. S., Lee, S., Shin, J. I., & Smith, L. (2022). Thrombosis patterns and clinical outcome of COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A Systematic Review and Meta-Analysis. International Journal of Infectious Diseases, 123, 166. [Google Scholar] [CrossRef]
- Kolahchi, Z., Khanmirzaei, M., & Mowla, A. (2022). Acute ischemic stroke and vaccine-induced immune thrombotic thrombocytopenia post COVID-19 vaccination; a systematic review. Journal of the Neurological Sciences, 439, 120327. [Google Scholar] [CrossRef]
- Matar, R. H., Than, C. A., Nakanishi, H., Daniel, R. S., Smayra, K., Sim, B. L., Beran, A., & Danoun, O. A. (2022). Outcomes of patients with thromboembolic events following coronavirus disease 2019 AstraZeneca vaccination: A systematic review and meta-analysis. Blood Coagul Fibrinolysis, 33(2), 90–112. [Google Scholar] [CrossRef]
- National Library of Medicine. (n.d.). MEDLINE PubMed production statistics. Available online: https://www.nlm.nih.gov/bsd/medline_pubmed_production_stats.html (accessed on 4 December 2024).
- Nikpour, M., Sepidarkish, M., Omidvar, S., & Firouzbakht, M. (2022). Global prevalence of acceptance of COVID-19 vaccines and associated factors in pregnant women: A systematic review and meta-analysis. Expert Review of Vaccines, 21(6), 843–851. [Google Scholar] [CrossRef]
- Nindrea, R. D., Djanas, D., Warsiti, Darma, I. Y., Hendriyani, H., & Sari, N. P. (2022). The risk factors and pregnant women’s willingness toward the SARS-CoV-2 vaccination in various countries: A systematic review and meta-analysis. Clinical Epidemiology and Global Health, 14, 100982. [Google Scholar] [CrossRef]
- Palaiodimou, L., Stefanou, M., de Sousa, D. A., Coutinho, J. M., Papadopoulou, M., Papaevangelou, V., Vassilakopoulos, T. I., Tsiodras, S., Filippou, D. K., & Tsivgoulis, G. (2022). Cerebral venous sinus thrombosis in the setting of COVID-19 vaccination: A systematic review and meta-analysis. Journal of Neurology, 269(7), 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines—A new era in vaccinology. Nature Review Drug Discovery, 17, 261–279. [Google Scholar] [CrossRef]
- Robinson, K. A., Chou, R., Berkman, N. D., Newberry, S. J., Fu, R., Hartling, L., Dryden, D., Butler, M., Foisy, M., Anderson, J., Motu’apuaka, M., Relevo, R., Guise, J.-M., & Chang, S. (2016). Twelve recommendations for integrating existing systematic reviews into new reviews: EPC guidance. Journal of Clinical Epidemiology, 70, 38–44. [Google Scholar] [CrossRef]
- Saluja, P., Gautam, N., Yadala, S., & Venkata, A. N. (2022). Thrombotic thrombocytopenic purpura (TTP) after COVID-19 vaccination: A systematic review of reported cases. Thrombosis Research, 214, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sarantaki, A., Kalogeropoulou, V. E., Taskou, C., Nanou, C., & Lykeridou, A. (2022). COVID-19 vaccination and related determinants of hesitancy among pregnant women: A systematic review and meta-analysis. Vaccines, 10(12), 2055. [Google Scholar] [CrossRef]
- Schiavo, J. H. (2019). PROSPERO: An international register of systematic review protocols. Medical Reference Services Quarterly, 38(2), 171–180. [Google Scholar] [CrossRef] [PubMed]
- Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., Moher, D., Tugwell, P., Welch, V., Kristjansson, E., & Henry, D. A. (2017). AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ, 358, j4008. [Google Scholar] [CrossRef]
- Steffen, C. A., Henaff, L., Durupt, A., El Omeiri, N., Ndiaye, S., Batmunkh, N., Liyanage, J. B. L., Hasan, Q., Mosina, L., Jones, I., O’Brien, K., & Hombach, J. (2021). Evidence-informed vaccination decision-making in countries: Progress, challenges and opportunities. Vaccine, 39(15), 2146–2152. [Google Scholar] [CrossRef]
- Tan, C., Xiao, Y., Chen, S., Liu, T., Zhou, J., Zhang, S., Hu, Y., Zhou, J., She, Z., Tian, B., Wu, A., & Li, C. (2024). Bibliometrics analysis and knowledge mapping of pertussis vaccine research: Trends from 1994 to 2023. Infection. [Google Scholar] [CrossRef]
- Tugwell, P., & Tovey, D. (2021). PRISMA 2020. Journal of Clinical Epidemiology, 134, A5–A6. [Google Scholar] [CrossRef]
- WHO. (n.d.-a). SYSVAC. Available online: https://www.nitag-resource.org/sysvac-systematic-reviews (accessed on 22 April 2025).
- WHO. (n.d.-b). WHO member states regions. Available online: http://www.who.int/about/regions/en/ (accessed on 24 October 2024).
- Zheng, X., Gao, F., Wang, L., Meng, Y., Ageno, W., & Qi, X. (2022). Incidence and outcomes of splanchnic vein thrombosis after diagnosis of COVID-19 or COVID-19 vaccination: A systematic review and meta-analysis. Journal of Thrombosis and Thrombolysis, 55(1), 18–31. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).