The Current and Potential Application of Medicinal Cannabis Products in Dentistry
Abstract
1. Introduction
1.1. History of Herbal Remedies to Treat Oral and Dental Diseases
1.2. History of Cannabis sativa L. in the Treatment of Oral and Dental Diseases
2. Current Uses of Cannabinoids in Modern Dentistry
| Utility | Reference (Patent Number) | |
|---|---|---|
| 1. | Cannabis-based composition comprising cannabis-extract, derivatives, and/or at least one synthetic cannabinoid intended for the treatment of dental pulp infection, pulp inflammation, dental (jaw) bone defects | [48] |
| 2. | Cannabinoid-based oral care composition (tooth paste, a tooth powder, or a mouthwash solution) for the treatment of oral infectious disease, including periimplantitis, periodontitis, oral mucositis, and dental pain. Cannabinoid may be cannabidiol and/or cannabigerol. | [49] |
| 3. | Extract of C. sativa L. (toothpaste, oral cleanser, or oral spray) for the treatment of dental caries. | [50] |
| 4. | Cannabinoid-based chewing gum compositions intended for the alleviation of pain. | [51] |
2.1. Targeting the Endocannabinoid System (ECS)
| Chemical Structures of Major Secondary Metabolites of Cannabis sativa L. | Significant Properties | References | |
|---|---|---|---|
| Major cannabinoids | |||
| 1. | 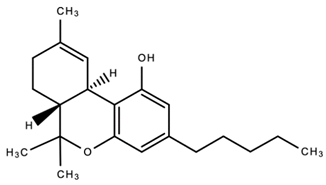 Delta 9-tetrahydrocannabinol (Δ9-THC) | Anti-microbial Anti-inflammatory Analgesic Antioxidant Anti-cancer Anti-tumor | [84,98,99,100,101,102,103,104,105] |
| 2. | 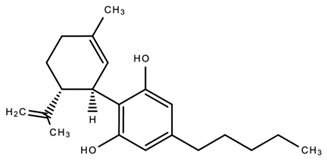 Cannabidiol (CBD) | Anti-microbial Anti-inflammatory Analgesic Anti-cancer Anti-metastatic Antioxidant Analgesic Anti-nociceptive | [35,84,98,99,100,101,102,103,106,107,108,109,110] |
| 3. |  Cannabichromene (CBC) | Anti-microbial Antibacterial and Anti-fungal Analgesic Anti-nociceptive Antioxidant Anti-inflammatory Anti-depressant | [84,111,112,113,114,115,116] |
| 4. | 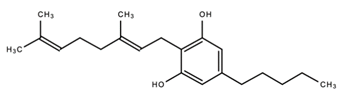 Cannabigerol (CBG) | Anti-microbial Analgesic Antioxidant | [84,117,118,119] |
| 5. | 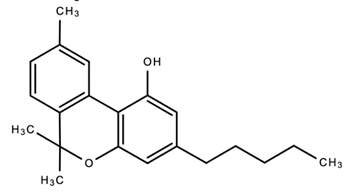 Cannabinol (CBN) | Anti-microbial Analgesic Antioxidant | [84,120,121] |
| Major Terpenes | |||
| 1. | 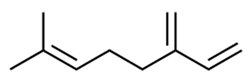 β-myrcene | Antimicrobial Antioxidant Potent analgesic Antioxidant; neuroprotective; anti-inflammatory Anti-cancer. | [85,86,122,123,124,125] |
| 2. | 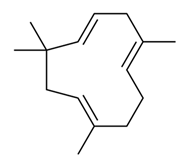 α-humulene | Antimicrobial Antioxidant Anticancer Anti-inflammatory Analgesic Angiogenic | [39,86,122,124,125,126,127,128,129,130] |
| 3. | 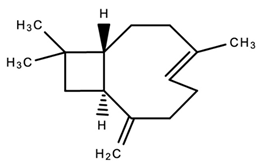 β-caryophyllene | Antimicrobial Antioxidant Anti-Cancer Anti-inflammatory Analgesic Anxiolytic | [86,122,125,130,131,132,133,134] |
| 4. | 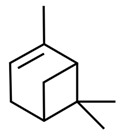 (+)-α-pinene | Antimicrobial Anti-inflammatory Antioxidant Analgesic | [92,122,123,125] |
| 5. | 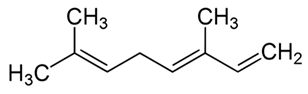 trans-β-ocimene | Antifungal; antibacterial; antioxidant; antiviral; anti-inflammatory | [87,88,89,90,91,122,125] |
| 6. | 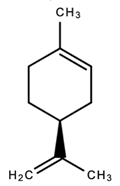 (-)-limonene | Antimicrobial Antioxidant Analgesic Anti-inflammatory; antioxidant; antiviral; antidiabetic; anticancer Antidepressant; Anticonvulsant Anti-cancer Anxiolytic | [35,86,122,125,135,136,137,138,139,140] |
| 7. | 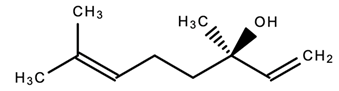 Linalool (Lavender scent) | Anxiolytic; anti-inflammatory; antimicrobial; anticancer; antidepressant Antioxidant | [39,85,122,125] |
| 8. | 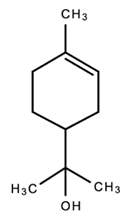 α-terpineol | Antimicrobial; Anti-inflammatory; Analgesic; Nociception inhibition; Antimicrobial | [35,92,122,125,141,142] |
| Major Flavonoids | |||
| 1. | 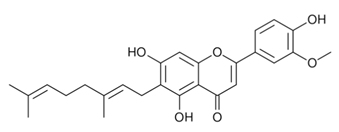 Cannflavin A | Analgesic Anti-inflammatory | [33,143,144,145,146] |
| 2. | 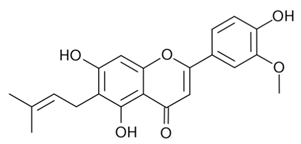 Cannflavin B | Analgesic Anti-inflammatory | [33,143,144,145,146] |
2.2. Implications of Cannabinoid Receptors in the Mouth
2.3. Potential Applications of CBD and Other Secondary Metabolites in Modern Dentistry
2.3.1. Emerging Trends and Potential Value of Medical Cannabis in Dentistry
2.3.2. Toothache
2.3.3. Burning Mouth Syndrome
2.3.4. Dental Caries
2.3.5. Dental Anxiety
2.3.6. Periodontal Disease
2.3.7. Oral Mucositis and Other Forms of Oral Cancers
3. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Righolt, A.J.; Jevdjevic, M.; Marcenes, W.; Listl, S. Global-, Regional-, and Country-Level Economic Impacts of Dental Diseases in 2015. J. Dent. Res. 2018, 97, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S. Jamaica’s Oral Cancer Mortality Rate Is Nearly 100%. Jamaica Observer, 1 April 2017. Available online: https://www.jamaicaobserver.com/news/Jamaica-s-oral-cancer-mortality-rate-is-nearly-100-_94348 (accessed on 2 April 2021).
- Listl, S.; Galloway, J.; Mossey, P.A.; Marcenes, W. Global Economic Impact of Dental Diseases. J. Dent. Res. 2015, 94, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Botelho, J.; Proença, L.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. Economic burden of periodontal disease in Europe and the United states of America—An updated forecast. medRxiv 2021. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 2 April 2021).
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet (Lond. Engl.) 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Petti, S.; Glendor, U.; Andersson, L. World traumatic dental injury prevalence and incidence, a meta-analysis-One billion living people have had traumatic dental injuries. Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol. 2018, 34, 71–86. [Google Scholar] [CrossRef]
- Zheng, L.W.; Hua, H.; Cheung, L.K. Traditional Chinese medicine and oral diseases: Today and tomorrow. Oral Dis. 2011, 17, 7–12. [Google Scholar] [CrossRef]
- Meyer-Hamme, G.; Beckmann, K.; Radtke, J.; Efferth, T.; Greten, H.J.; Rostock, M.; Schröder, S. A survey of chinese medicinal herbal treatment for chemotherapy-induced oral mucositis. Evid.-Based Complement. Altern. Med. 2013, 2013, 284959. [Google Scholar] [CrossRef]
- Agbor, A.M.; Naidoo, S. A review of the role of African traditional medicine in the management of oral diseases. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 133. [Google Scholar] [CrossRef]
- Ayyanar, M.; Ignacimuthu, S. Herbal medicines for wound healing among tribal people in Southern India: Ethnobotanical and Scientific evidences. Int. J. Appl. Res. Nat. Prod. 2009, 2, 29–42. [Google Scholar]
- Shah, N.C. Herbal folk medicines in Northern India. J. Ethnopharmacol. 1982, 6, 293–301. [Google Scholar] [CrossRef]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Megersa, M.; Jima, T.T.; Goro, K.K. The Use of Medicinal Plants for the Treatment of Toothache in Ethiopia. Evid.-Based Complement. Altern. Med. 2019, 2019, 2645174. [Google Scholar] [CrossRef]
- Debbarma, M.; Pala, N.A.; Kumar, M.; Bussmann, R.W. TRADITIONAL KNOWLEDGE OF MEDICINAL PLANTS IN TRIBES OF TRIPURA IN NORTHEAST, INDIA. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 156–168. [Google Scholar] [CrossRef]
- Batiha, G.E.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Beyi, M.W. Ethnobotanical investigation of traditional medicinal plants in Dugda District, Oromia Region. SM J. Med. Plant Stud. 2018, 2, 1–19. [Google Scholar] [CrossRef]
- Kefalew, A.; Asfaw, Z.; Kelbessa, E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia Regional State, Ethiopia. J. Ethnobiol. Ethnomed. 2015, 11, 25. [Google Scholar] [CrossRef]
- Kidane, L.; Gebremedhin, G.; Beyene, T. Ethnobotanical study of medicinal plants in Ganta Afeshum District, Eastern Zone of Tigray, Northern Ethiopia. J. Ethnobiol. Ethnomed. 2018, 14, 64. [Google Scholar] [CrossRef]
- George, D.; Bhat, S.S.; Antony, B. Comparative evaluation of the antimicrobial efficacy of aloe vera tooth gel and two popular commercial toothpastes: An in vitro study. Gen. Dent. 2009, 57, 238–241. [Google Scholar] [PubMed]
- Al-Timimi, E.A.; Al-Casey, M. Effect of thymus vulgaris extract on streptococci and mutans streptococci, in comparison to chlorhexidine gluconate (in vivo study). J. Baghdad Coll. Dent. 2012, 24, 122–127. [Google Scholar]
- Xu, J.S.; Li, Y.; Cao, X.; Cui, Y. The effect of eugenol on the cariogenic properties of Streptococcus mutans and dental caries development in rats. Exp. Ther. Med. 2013, 5, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Jalaluddin, M.; Rout, P.; Mohanty, R.; Dileep, C.L. Emerging trends of herbal care in dentistry. J. Clin. Diagn. Res. JCDR 2013, 7, 1827–1829. [Google Scholar] [CrossRef]
- Elikkottil, J.; Gupta, P.; Gupta, K. The analgesic potential of cannabinoids. J. Opioid Manag. 2009, 5, 341–357. [Google Scholar] [CrossRef]
- Mikuriya, T.H. Marijuana in medicine: Past, present and future. Calif. Med. 1969, 110, 34–40. [Google Scholar]
- Zuardi, A.W. History of cannabis as a medicine: A review. Revista Brasileira de Psiquiatria (Sao Paulo, Brazil: 1999) 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Radwan, M.M.; Ross, S.A.; Slade, D.; Ahmed, S.A.; Zulfiqar, F.; Elsohly, M.A. Isolation and characterization of new Cannabis constituents from a high potency variety. Planta Med. 2008, 74, 267–272. [Google Scholar] [CrossRef]
- Radwan, M.M.; Elsohly, M.A.; Slade, D.; Ahmed, S.A.; Wilson, L.; El-Alfy, A.T.; Khan, I.A.; Ross, S.A. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry 2008, 69, 2627–2633. [Google Scholar] [CrossRef]
- Radwan, M.M.; Elsohly, M.A.; Slade, D.; Ahmed, S.A.; Khan, I.A.; Ross, S.A. Biologically active cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911. [Google Scholar] [CrossRef]
- Barrett, M.L.; Gordon, D.; Evans, F.J. Isolation from Cannabis sativa L. of cannflavin—A novel inhibitor of prostaglandin production. Biochem. Pharmacol. 1985, 34, 2019–2024. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Pertwee, R.G.; McPartland, J.M.; Russo, E.B. Non-Phytocannabinoid constituents of cannabis and Herbal Synergy. In Handbook of Cannabis, 1st ed.; Pertwee, R.G., Ed.; Oxford University Press: Oxford, UK, 2014; Volume 1, pp. 280–295. [Google Scholar]
- Flores-Sanchez, I.J.; Verpoorte, R. PKS activities and biosynthesis of cannabinoids and flavonoids in Cannabis sativa L. plants. Plant Cell Physiol. 2008, 49, 1767–1782. [Google Scholar] [CrossRef]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef]
- Rea, K.A.; Casaretto, J.A.; Al-Abdul-Wahid, M.S.; Sukumaran, A.; Geddes-McAlister, J.; Rothstein, S.J.; Akhtar, T.A. Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry 2019, 164, 162–171. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Allaker, R.P.; Douglas, C.W. Novel anti-microbial therapies for dental plaque-related diseases. Int. J. Antimicrob. Agents 2009, 33, 8–13. [Google Scholar] [CrossRef]
- Cannabidiol360. How Effective Is CBD for Dental Pain? 6 September 2018. Available online: https://cannabidiol360.com/cbd-for-dental-pain/ (accessed on 2 April 2021).
- Ward, A. CBD Toothpaste Sounds Like a Good Idea but Lacks Conclusive Studies Proving It Is, 3 June 2019. Available online: https://www.greenentrepreneur.com/article/334497 (accessed on 2 April 2021).
- Sanger, B. New Cannabis Toothpaste Has People Losing Their Minds. 12 August 2019. Available online: https://herb.co/news/health/cannabis-toothpaste/ (accessed on 3 April 2021).
- Kaufman, H. 5 Benefits of UTILIZING CBD Oral Care. 19 May 2020. Available online: https://www.americanspa.com/cbd/5-benefits-utilizing-cbd-oral-care (accessed on 3 April 2021).
- Bourque, A. Cannabis for Your Mouth: R&d, Patents, and Your Next Visit to the Dentist. 29 June 2019. Available online: https://www.forbes.com/sites/andrebourque/2019/06/29/cannabis-for-your-mouth-rd-patents-and-your-next-visit-to-the-dentist/?sh=6ae0d25a30fb (accessed on 3 April 2021).
- Stahl, V.; Vasudevan, K. Comparison of Efficacy of Cannabinoids versus Commercial Oral Care Products in Reducing Bacterial Content from Dental Plaque: A Preliminary Observation. Cureus 2020, 12, e6809. [Google Scholar] [CrossRef]
- Vasudevan, K.; Stahl, V. Cannabinoids infused mouthwash products are as effective as chlorhexidine on inhibition of total-culturable bacterial content in dental plaque samples. J. Cannabis Res. 2020, 2, 20. [Google Scholar] [CrossRef]
- Stahl, V. Cannabis and Derivatives Thereof for the Treatment of Pain and Inflammation Related with Dental Pulp and Bone Regeneration Related to Dental Jaw Bone Defects. U.S. Patent No. US20200222361A1, 12 November 2020. [Google Scholar]
- Anastassov, G.; Changoer, L. Oral Care Composition Comprising Cannabinoids. U.S. Patent No. US10172786B2, 8 January 2019. [Google Scholar]
- 마진열; 임남희; 정윤희; 이주혜; 이지혜; 김Ꙋ익; 유기종. Composition for Prevention or Treatment of Dental Caries Comprising Extract of Cannabis sativa. South Korea Patent No. KR20120133135A, 10 December 2012. [Google Scholar]
- Van Damme, P.A.; Anastassov, G.E.; Lekhram, C. Chewing Gum Compositions Comprising Cannabinoids. U.S. Patent No. EP2280687B1, 27 February 2019. [Google Scholar]
- Di Marzo, V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009, 60, 77–84. [Google Scholar] [CrossRef]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Lafenêtre, P. Roles of the endocannabinoid system in learning and memory. Curr. Top. Behav. Neurosci. 2009, 1, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Silva, F.J.; Cardinal, P.; Cota, D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J. Psychopharmacol. (Oxf. Engl.) 2012, 26, 114–124. [Google Scholar] [CrossRef]
- Woodhams, S.G.; Sagar, D.R.; Burston, J.J.; Chapman, V. The role of the endocannabinoid system in pain. Handb. Exp. Pharmacol. 2015, 227, 119–143. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef]
- Gamage, T.F.; Lichtman, A.H. The endocannabinoid system: Role in energy regulation. Pediatr. Blood Cancer 2012, 58, 144–148. [Google Scholar] [CrossRef]
- Karasu, T.; Marczylo, T.H.; Maccarrone, M.; Konje, J.C. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum. Reprod. Update 2011, 17, 347–361. [Google Scholar] [CrossRef]
- Walker, O.S.; Holloway, A.C.; Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019, 12, 3. [Google Scholar] [CrossRef]
- Taylor, A.H.; Ang, C.; Bell, S.C.; Konje, J.C. The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum. Reprod. Update 2007, 13, 501–513. [Google Scholar] [CrossRef]
- Pacher, P.; Steffens, S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin. Immunopathol. 2009, 31, 63–77. [Google Scholar] [CrossRef]
- Manzanares, J.; Cabañero, D.; Puente, N.; García-Gutiérrez, M.S.; Grandes, P.; Maldonado, R. Role of the endocannabinoid system in drug addiction. Biochem. Pharmacol. 2018, 157, 108–121. [Google Scholar] [CrossRef]
- Parolaro, D.; Realini, N.; Vigano, D.; Guidali, C.; Rubino, T. The endocannabinoid system and psychiatric disorders. Exp. Neurol. 2010, 224, 3–14. [Google Scholar] [CrossRef]
- Vinod, K.Y.; Hungund, B.L. Role of the endocannabinoid system in depression and suicide. Trends Pharmacol. Sci. 2006, 27, 539–545. [Google Scholar] [CrossRef]
- Fakhoury, M. Role of the Endocannabinoid System in the Pathophysiology of Schizophrenia. Mol. Neurobiol. 2017, 54, 768–778. [Google Scholar] [CrossRef]
- Pazos, M.R.; Núñez, E.; Benito, C.; Tolón, R.M.; Romero, J. Role of the endocannabinoid system in Alzheimer’s disease: New perspectives. Life Sci. 2004, 75, 1907–1915. [Google Scholar] [CrossRef]
- André, A.; Gonthier, M.P. The endocannabinoid system: Its roles in energy balance and potential as a target for obesity treatment. Int. J. Biochem. Cell Biol. 2010, 42, 1788–1801. [Google Scholar] [CrossRef]
- Serra, G.; Fratta, W. A possible role for the endocannabinoid system in the neurobiology of depression. Clin. Pract. Epidemiol. Ment. Health CP EMH 2007, 3, 25. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Zou, M.; Li, D.; Li, L.; Wu, L.; Sun, C. Role of the endocannabinoid system in neurological disorders. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2019, 76, 95–102. [Google Scholar] [CrossRef]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the endocannabinoid system in diabetes and diabetic complications. Br. J. Pharmacol. 2016, 173, 1116–1127. [Google Scholar] [CrossRef]
- Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Bifulco, M. The endocannabinoid signaling system in cancer. Trends Pharmacol. Sci. 2013, 34, 273–282. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Alhouayek, M.; Boldrup, L.; Fowler, C.J. Altered mRNA Expression of Genes Involved in Endocannabinoid Signaling in Squamous Cell Carcinoma of the Oral Tongue. Cancer Investig. 2019, 37, 327–338. [Google Scholar] [CrossRef]
- Mandal, D.A. Phytocannabinoids. 26 February 2019. Available online: https://www.news-medical.net/health/Phytocannabinoids.aspx (accessed on 2 April 2021).
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef]
- Demain, A.L.; Fang, A. The natural functions of secondary metabolites. Adv. Biochem. Eng./Biotechnol. 2000, 69, 1–39. [Google Scholar] [CrossRef]
- Earlenbaugh, E. What Are FLAVONOIDS? 11 March 2021. Available online: https://cannigma.com/plant/what-are-cannabis-flavonoids/ (accessed on 2 April 2021).
- Bennett, P. What Are Cannabis Flavonoids and What Do They Do? 9 February 2018. Available online: https://www.leafly.com/news/cannabis-101/what-are-marijuana-flavonoids (accessed on 3 April 2021).
- Rahn, B. What Are Cannabis Terpenes and What Do They Do? 18 November 2019. Available online: https://www.leafly.com/news/cannabis-101/terpenes-the-flavors-of-cannabis-aromatherapy (accessed on 3 April 2021).
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Rao, V.S.; Menezes, A.M.; Viana, G.S. Effect of myrcene on nociception in mice. J. Pharm. Pharmacol. 1990, 42, 877–878. [Google Scholar] [CrossRef]
- Johnson, J.; Theisen, E. What Are Terpenes? 6 March 2020. Available online: https://www.medicalnewstoday.com/articles/what-are-terpenes (accessed on 28 July 2020).
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. Int. J. Publ. Br. Indus. Biol. Res. Assoc. 2013, 62, 349–354. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.; Gonçalves, M.J.; Hrimpeng, K.; Pinto, J.; Pinto, E. Antifungal activity of the essential oil of Angelica major against Candida, Cryptococcus, Aspergillus and dermatophyte species. J. Nat. Med. 2015, 69, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Golfakhrabadi, F.; Khanavi, M.; Ostad, S.N.; Saeidnia, S.; Vatandoost, H.; Abai, M.R.; Hafizi, M.; Yousefbeyk, F.; Rad, Y.R.; Baghenegadian, A.; et al. Biological activities and composition of ferulago carduchorum essential Oil. J. Arthropod. Borne Dis. 2014, 9, 104–115. [Google Scholar]
- Kim, M.J.; Yang, K.W.; Kim, S.S.; Park, S.M.; Park, K.J.; Kim, K.S.; Choi, Y.H.; Cho, K.K.; Hyun, C.G. Chemical composition and anti-inflammation activity of essential oils from Citrus unshiu flower. Nat. Prod. Comm. 2014, 9, 727–730. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodiver. 2008, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Brock, P.L.; Vaughan, B.M.; Vollmer, D.L. Comparison of antimicrobial activities of natural essential oils and synthetic fragrances against selected environmental pathogens. Biochim. Open 2017, 5, 8–13. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, H.N.; Turner, C.E.; Clark, A.M.; ElSohly, M.A. Synthesis and antimicrobial activities of certain cannabichromene and cannabigerol related compounds. J. Pharm. Sci. 1982, 71, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Klahn, P. Cannabinoids-Promising Antimicrobial Drugs orIntoxicants with Benefits? Antibiotics 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Koltai, H.; Namdar, D. Cannabis Phytomolecule ‘Entourage’: From Domestication to Medical Use. Trends Plant Sci. 2020, 25, 976–984. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016, 23, S23–S32. [Google Scholar] [CrossRef]
- Velasco, G.; Hernández-Tiedra, S.; Dávila, D.; Lorente, M. The use of cannabinoids as anticancer agents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 259–266. [Google Scholar] [CrossRef]
- Guzmán, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Hinz, B. Cannabinoids as Anticancer Drugs. Adv. Pharmacol. (San Diego Calif.) 2017, 80, 397–436. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef]
- Zurier, R.B. Prospects for cannabinoids as anti-inflammatory agents. J. Cell. Biochem. 2003, 88, 462–466. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Zuegg, J.; Beare, N.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanus, L.O. Cannabidiol—Recent advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef]
- Izzo, A.A.; Capasso, R.; Aviello, G.; Borrelli, F.; Romano, B.; Piscitelli, F.; Gallo, L.; Capasso, F.; Orlando, P.; Di Marzo, V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br. J. Pharmacol. 2012, 166, 1444–1460. [Google Scholar] [CrossRef]
- Davis, W.M.; Hatoum, N.S. Neurobehavioral actions of cannabichromene and interactions with delta 9-tetrahydrocannabinol. Gen. Pharmacol. 1983, 14, 247–252. [Google Scholar] [CrossRef]
- Turner, C.E.; Elsohly, M.A. Biological activity of cannabichromene, its homologs and isomers. J. Clin. Pharmacol. 1981, 21, 283S–291S. [Google Scholar] [CrossRef]
- DeLong, G.T.; Wolf, C.E.; Poklis, A.; Lichtman, A.H. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010, 112, 126–133. [Google Scholar] [CrossRef]
- Wirth, P.W.; Watson, E.S.; ElSohly, M.; Turner, C.E.; Murphy, J.C. Anti-inflammatory properties of cannabichromene. Life Sci. 1980, 26, 1991–1995. [Google Scholar] [CrossRef]
- Lygos CBX Adds Cannabichromene to Its Growing Portfolio of High-Quality, Pure and SUSTAINABLE Cannabinoids.19 January 2021. Available online: https://lygos.com/lygos-cbx-adds-cannabichromene-to-its-growing-portfolio-of-high-quality-pure-and-sustainable-cannabinoids/ (accessed on 3 April 2021).
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the Hidden Antibiotic Potential of Cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Ronci, M.; Leone, S.; Brunetti, L.; et al. Antioxidant and Neuroprotective Effects Induced by Cannabidiol and Cannabigerol in Rat CTX-TNA2 Astrocytes and Isolated Cortexes. Int. J. Mol. Sci. 2020, 21, 3575. [Google Scholar] [CrossRef]
- Marsicano, G.; Moosmann, B.; Hermann, H.; Lutz, B.; Behl, C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J. Neurochem. 2002, 80, 448–456. [Google Scholar] [CrossRef]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020, 177, 4330–4352. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Karas, J.A.; Wong, L.; Paulin, O.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical Characterization and Evaluation of the Antibacterial Activity of Essential Oils from Fibre-Type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene alpha-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.S.; Leal, P.C.; Pianowisky, L.; Calixto, J.B. Pharmacokinetics and tissue distribution of the sesquiterpene alpha-humulene in mice. Planta Med. 2008, 74, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Matsuda, T.; Toshimitsu, T.; Mori, A.; Mae, T.; Tsukagawa, M.; Kitahara, M.; Shimizu, M. Regulation of interleukin-8 secretion in human intestinal epithelial Caco-2 cells by alpha-humulene. BioFactors (Oxf. Engl.) 2004, 21, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Bouajaj, S.; Benyamna, A.; Bouamama, H.; Romane, A.; Falconieri, D.; Piras, A.; Marongiu, B. Antibacterial, allelopathic and antioxidant activities of essential oil of Salvia officinalis L. growing wild in the Atlas Mountains of Morocco. Nat. Prod. Res. 2013, 27, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Pieri, F.A.; Souza, M.C.; Vermelho, L.L.; Vermelho, M.L.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.; da Veiga-Junior, V.F.; Moreira, M.A. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet. Res. 2016, 12, 216. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef]
- Zahi, M.R.; El Hattab, M.; Liang, H.; Yuan, Q. Enhancing the antimicrobial activity of d-limonene nanoemulsion with the inclusion of ε-polylysine. Food Chem. 2017, 221, 18–23. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef]
- Espina, L.; Gelaw, T.K.; de Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef]
- Lima, N.G.; De Sousa, D.P.; Pimenta, F.C.; Alves, M.F.; De Souza, F.S.; Macedo, R.O.; Cardoso, R.B.; de Morais, L.C.; Melo Diniz, M.; de Almeida, R.N. Anxiolytic-like activity and GC-MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol. Biochem. Behav. 2013, 103, 450–454. [Google Scholar] [CrossRef]
- de Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Held, S.; Schieberle, P.; Somoza, V. Characterization of α-Terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007, 55, 8040–8046. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Oliveira, M.G.; Santana, M.F.; Santana, M.T.; Guimarães, A.G.; Siqueira, J.S.; Almeida, R.N. α-Terpineol reduces nociceptive behavior in mice. Pharm. Biol. 2011, 49, 583–586. [Google Scholar] [CrossRef]
- Forget Aspirin, These Marijuana-Based Painkillers Are 30 Times More Powerful. 26 July 2019. Available online: https://www.chicagotribune.com/marijuana/sns-tft-marijuana-based-painkillers-20190726-6hcou2ju7nacrcpnqfndyco7fe-story.html (accessed on 4 April 2021).
- Cannabis CannaFlavins 30x Stronger Than Aspirin in Cell Experiment. 27 December 2019. Available online: https://www.rxleaf.com/cannaflavins-antiinflammation-pain-relief-cannabis-medicine/ (accessed on 4 April 2021).
- Barrett, M.L.; Scutt, A.M.; Evans, F.J. Cannflavin A and B, prenylated flavones from Cannabis sativa L. Experientia 1986, 42, 452–453. [Google Scholar] [CrossRef]
- Erridge, S.; Mangal, N.; Salazar, O.; Pacchetti, B.; Sodergren, M.H. Cannflavins—From plant to patient: A scoping review. Fitoterapia 2020, 146, 104712. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Grimaldi, C.; Santoro, A.; Gazzerro, P.; Laezza, C.; Bifulco, M. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 117–131. [Google Scholar] [CrossRef]
- Conti, S.; Costa, B.; Colleoni, M.; Parolaro, D.; Giagnoni, G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002, 135, 181–187. [Google Scholar] [CrossRef]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef]
- Sumariwalla, P.F.; Gallily, R.; Tchilibon, S.; Fride, E.; Mechoulam, R.; Feldmann, M. A novel synthetic, nonpsychoactive cannabinoid acid (HU-320) with antiinflammatory properties in murine collagen-induced arthritis. Arthritis Rheum. 2004, 50, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, C.L.; Chen, M.; Manivannan, A.; Cabay, L.; Pertwee, R.G.; Coutts, A.; Forrester, J.V. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 2007, 82, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Yuill, M.B.; Hale, D.E.; Guindon, J.; Morgan, D.J. Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Mol. Pain 2017, 13, 1744806917728227. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Radtke, A.; Decker, J.; Koch, M.; Belge, G. The Synthetic Cannabinoid WIN 55,212-2 Elicits Death in Human Cancer Cell Lines. Anticancer Res. 2017, 37, 6341–6345. [Google Scholar] [CrossRef]
- Roberto, D.; Klotz, L.H.; Venkateswaran, V. Cannabinoid WIN 55,212-2 induces cell cycle arrest and apoptosis, and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer in a cannabinoid-receptor 2 dependent manner. Prostate 2019, 79, 151–159. [Google Scholar] [CrossRef]
- Borsani, E.; Majorana, A.; Cocchi, M.A.; Conti, G.; Bonadeo, S.; Padovani, A.; Lauria, G.; Bardellini, E.; Rezzani, R.; Rodella, L.F. Epithelial expression of vanilloid and cannabinoid receptors: A potential role in burning mouth syndrome pathogenesis. Histol. Histopathol. 2014, 29, 523–533. [Google Scholar] [CrossRef]
- Hipkaeo, W.; Watanabe, M.; Kondo, H. Localization of Cannabinoid Receptor 1 (CB1) in Submandibular and Sublingual Salivary Glands of Mice throughout Postnatal Development. Int. J. Morphol. 2015, 33, 695–700. [Google Scholar] [CrossRef][Green Version]
- Prestifilippo, J.P.; Fernández-Solari, J.; Cal, C.D.L.; Iribarne, M.; Suburo, A.M.; Rettori, V.; McCann, S.M.; Elverdin, J.C. Inhibition of Salivary Secretion by Activation of Cannabinoid Receptors. Exp. Biol. Med. 2006, 231, 1421–1429. [Google Scholar] [CrossRef]
- Kopach, O.; Vats, J.; Netsyk, O.; Voitenko, N.; Irving, A.; Fedirko, N. Cannabinoid receptors in submandibular acinar cells: Functional coupling between saliva fluid and electrolytes secretion and Ca2+ signalling. J. Cell Sci. 2012, 125 Pt 8, 1884–1895. [Google Scholar] [CrossRef]
- Kakoei, S.; Parirokh, M.; Nakhaee, N.; Jamshidshirazi, F.; Rad, M.; Kakooei, S. Prevalence of toothache and associated factors: A population-based study in Southeast Iran. Iran. Endod. J. 2013, 8, 123–128. [Google Scholar]
- Cohen, L.A.; Bonito, A.J.; Akin, D.R.; Manski, R.J.; Macek, M.D.; Edwards, R.R.; Cornelius, L.J. Toothache pain: A comparison of visits to physicians, emergency departments and dentists. J. Am. Dent. Assoc. 2008, 139, 1205–1216. [Google Scholar] [CrossRef]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Royal Queen Seeds. Can CBD Help Relieve Toothache? 25 November 2019. Available online: https://www.royalqueenseeds.com/blog-can-cbd-help-relieve-toothache-n1062 (accessed on 6 April 2021).
- Mayo Foundation for Medical Education and Research. Burning mouth syndrome. Mayo Clinic, 14 February 2019. Available online: https://www.mayoclinic.org/diseases-conditions/burning-mouth-syndrome/symptoms-causes/syc-20350911 (accessed on 6 April 2021).
- Sun, A.; Wu, K.M.; Wang, Y.P.; Lin, H.P.; Chen, H.M.; Chiang, C.P. Burning mouth syndrome: A review and update. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2013, 42, 649–655. [Google Scholar] [CrossRef]
- Pereira, S.R.; Tello Velasquez, J.; Duggan, S.; Ivanisevic, B.; McKenna, J.P.; McCreary, C.; Downer, E.J. Recent advances in the understanding of the aetiology and therapeutic strategies in burning mouth syndrome: Focus on the actions of cannabinoids. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Study: Cannabis Extracts Mitigate Symptoms of Burning Mouth Syndrome. NORML, 10 May 2021. Available online: https://norml.org/news/2020/11/05/study-cannabis-extracts-mitigate-symptoms-of-burning-mouth-syndrome/ (accessed on 6 April 2021).
- Dowden, A. Cannabis Oil May Help Burning Mouth Syndrome. Labroots.com, 13 November 2020. Available online: https://www.labroots.com/trending/health-and-medicine/19161/cannabis-oil-help-burning-mouth-syndrome (accessed on 6 April 2021).
- Gambino, A.; Cabras, M.; Panagiotakos, E.; Calvo, F.; Macciotta, A.; Cafaro, A.; Suria, M.; Haddad, G.E.; Broccoletti, R.; Arduino, P.G. Evaluating the Suitability and Potential Efficiency of Cannabis sativa Oil for Patients with Primary Burning Mouth Syndrome: A Prospective, Open-Label, Single-Arm Pilot Study. Pain Med. (Malden Mass.) 2021, 22, 142–151. [Google Scholar] [CrossRef]
- Sunariani, J.; Wijaksana, I.K.E.; Setiawatie, E.M. The Role of Vanilloid and Cannabinoid Receptors in Taste and Pain Perception in Burning Mouth Syndrome. In Proceedings of the 7th International Meeting and the 4th Joint Scientific Meeting in Dentistry, Surabaya, Indonesia, 5–7 October 2017. [Google Scholar] [CrossRef]
- Bare, L.C.; Dundes, L. Strategies for combating dental anxiety. J. Dent. Educ. 2004, 68, 1172–1177. [Google Scholar] [CrossRef]
- Berggren, U.; Meynert, G. Dental fear and avoidance: Causes, symptoms, and consequences. J. Am. Dent. Assoc. 1984, 109, 247–251. [Google Scholar] [CrossRef]
- Townend, E.; Dimigen, G.; Fung, D. A clinical study of child dental anxiety. Behav. Res. Ther. 2000, 38, 31–46. [Google Scholar] [CrossRef]
- Minja, I.K.; Kahabuka, F.K. Dental Anxiety and Its Consequences to Oral Health Care Attendance and Delivery. Anxiety Disord. From Childhood Adulthood 2019. [Google Scholar] [CrossRef]
- Cohen, S.M.; Fiske, J.; Newton, J.T. The impact of dental anxiety on daily living. Br. Dent. J. 2000, 189, 385–390. [Google Scholar] [CrossRef]
- Hällström, T.; Halling, A. Prevalence of dentistry phobia and its relation to missing teeth, alveolar bone loss and dental care habits in an urban community sample. Acta Psychiatr. Scand. 1984, 70, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Locker, D.; Liddell, A. Clinical correlates of dental anxiety among older adults. Community Dent. Oral Epidemiol. 1992, 20, 372–375. [Google Scholar] [CrossRef]
- Hakeberg, M.; Berggren, U.; Gröndahl, H.G. A radiographic study of dental health in adult patients with dental anxiety. Community Dent. Oral Epidemiol. 1993, 21, 27–30. [Google Scholar] [CrossRef]
- Appukuttan, D.P. Strategies to manage patients with dental anxiety and dental phobia: Literature review. Clin. Cosmet. Investig. Dent. 2016, 8, 35–50. [Google Scholar] [CrossRef]
- Sharif, M.O. Dental anxiety: Detection and management. J. Appl. Oral Sci. Rev. FOB 2010, 18. [Google Scholar] [CrossRef][Green Version]
- Wide Boman, U.; Carlsson, V.; Westin, M.; Hakeberg, M. Psychological treatment of dental anxiety among adults: A systematic review. Eur. J. Oral Sci. 2013, 121 Pt 2, 225–234. [Google Scholar] [CrossRef]
- Facco, E.; Zanette, G.; Casiglia, E. The role of hypnotherapy in dentistry. SAAD Dig. 2014, 30, 3–6. [Google Scholar]
- Roberts, K. Hypnosis in dentistry. Dent. Update 2006, 33, 312–314. [Google Scholar] [CrossRef]
- Patel, B.; Potter, C.; Mellor, A.C. The use of hypnosis in dentistry: A review. Dent. Update 2000, 27, 198–202. [Google Scholar] [CrossRef]
- Armfield, J.M.; Heaton, L.J. Management of fear and anxiety in the dental clinic: A review. Aust. Dent. J. 2013, 58, 390–531. [Google Scholar] [CrossRef]
- Chebbi, Y. Cbd: A Natural Remedy for Dental Anxiety. 24 July 2020. Available online: https://www.myflossery.com/cbd-a-natural-remedy-for-dental-anxiety/ (accessed on 1 April 2021).
- Cohen, P. Treating Dental Anxiety with Natural Remedies Like CBD. 15 February 2019. Available online: https://www.healthworkscollective.com/treating-dental-anxiety-with-natural-remedies-like-cbd/ (accessed on 6 April 2021).
- Özdemir, B.; Shi, B.; Bantleon, H.P.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Endocannabinoids and inflammatory response in periodontal ligament cells. PLoS ONE 2014, 9, e107407. [Google Scholar] [CrossRef] [PubMed]
- Jäger, A.; Setiawan, M.; Beins, E.; Schmidt-Wolf, I.; Konermann, A. Analogous modulation of inflammatory responses by the endocannabinoid system in periodontal ligament cells and microglia. Head Face Med 2020, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Kozono, S.; Matsuyama, T.; Biwasa, K.K.; Kawahara, K.-I.; Nakajima, Y.; Yoshimoto, T.; Yonamine, Y.; Kadomatsu, H.; Tancharoen, S.; Hashiguchi, T.; et al. Involvement of the endocannabinoid system in periodontal healing. Biochem. Biophys. Res. Commun. 2010, 394, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Van Klingeren, B.; Ten Ham, M. Antibacterial activity of delta9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Dariš, B.; Tancer Verboten, M.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina (Kaunas, Lithuania) 2019, 55, 747. [Google Scholar] [CrossRef]
- Sukumar, K.; Tadepalli, A. Nexus between COVID-19 and periodontal disease. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef]
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case–control study. J. Clin. Periodontol. 2021, 48, 483–491. [Google Scholar] [CrossRef]
- Mucositis. Available online: https://oralcancerfoundation.org/complications/mucositis/ (accessed on 1 April 2021).
- Cuba, L.F.; Salum, F.G.; Cherubini, K.; Figueiredo, M.A.Z. Cannabidiol: An alternative therapeutic agent for oral mucositis? J. Clin. Pharm. Ther. 2017, 42, 245–250. [Google Scholar] [CrossRef]
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar] [CrossRef]
- Hanlon, K.E.; Lozano-Ondoua, A.N.; Umaretiya, P.J.; Symons-Liguori, A.M.; Chandramouli, A.; Moy, J.K.; Kwass, W.K.; Mantyh, P.W.; Nelson, M.A.; Vanderah, T.W. Modulation of breast cancer cell viability by a cannabinoid receptor 2 agonist, JWH-015, is calcium dependent. Breast Cancer 2016, 8, 59–71. [Google Scholar] [CrossRef]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [CrossRef]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef]
- Sharma, M.; Hudson, J.B.; Adomat, H.; Guns, E.; Cox, M.E. In Vitro Anticancer Activity of Plant-Derived Cannabidiol on Prostate Cancer Cell Lines. Pharmacol. Pharm. 2014, 05, 806–820. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Cridge, B.J.; Rosengren, R.J. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag. Res. 2013, 5, 301–313. [Google Scholar] [CrossRef][Green Version]
- McAllister, S.D.; Soroceanu, L.; Desprez, P.Y. The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. J. Neuroimmune Pharmacol. 2015, 10, 255–267. [Google Scholar] [CrossRef]
- Gruetzmacher, K. The Pros and Cons of Using Medical Marijuana to Treat Oral Cancer. 13 September 2017. Available online: https://thefreshtoast.com/cannabis/the-pros-and-cons-of-medical-marijuana-and-oral-cancer/ (accessed on 6 April 2021).
- CBD Oil for Oral Hygiene. 5 April 2018. Available online: https://cbdinstead.com/blogs/cbd-for-general-health/cbd-oil-for-oral-hygiene (accessed on 6 April 2021).
- CBD in Dentistry—Can Cannabis Cure Cavities? 13 October 2017. Available online: https://www.principesactifs.org/cbd-in-dentistry-can-cannabis-cure-cavities/ (accessed on 6 April 2021).
- Rettori, E.; De Laurentiis, A.; Zorrilla Zubilete, M.; Rettori, V.; Elverdin, J.C. Anti-inflammatory effect of the endocannabinoid anandamide in experimental periodontitis and stress in the rat. Neuroimmunomodulation 2012, 19, 293–303. [Google Scholar] [CrossRef]
- Nakajima, Y.; Furuichi, Y.; Biswas, K.K.; Hashiguchi, T.; Kawahara, K.; Yamaji, K.; Uchimura, T.; Izumi, Y.; Maruyama, I. Endocannabinoid, anandamide in gingival tissue regulates the periodontal inflammation through NF-kappaB pathway inhibition. FEBS Lett. 2006, 580, 613–619. [Google Scholar] [CrossRef]
- Arney, K. Cannabis, Cannabinoids and Cancer—The Evidence So Far. Cancer News, 25 July 2012. Available online: https://news.cancerresearchuk.org/2012/07/25/cannabis-cannabinoids-and-cancer-the-evidence-so-far/ (accessed on 6 April 2021).
- Vatican, J. Why Use CBD Oil Before Visiting the Dentist. Medical Daily, 22 May 2019. Available online: https://www.medicaldaily.com/why-use-cbd-oil-visiting-dentist-435364 (accessed on 6 April 2021).
- Burhenne, M. CBD Oil Benefits for Better Dental & Overall Health. 30 August 2019. Available online: https://askthedentist.com/cbd-oil-facts/#what-is-cbd-oil (accessed on 1 April 2021).
- Sybertz, A. CBD for Dental Pain, Anxiety, and Problems: Does It Work? The Healthy, 7 May 2021. Available online: https://www.thehealthy.com/dental/cbd-for-dental-pain-and-anxiety/ (accessed on 6 April 2021).
- What Are the Benefits of Hemp Seed Oil in Toothpaste? Humming Hemp, 3 March 2021. Available online: https://thehumminggroup.com/benefits-hemp-oil-toothpaste/ (accessed on 6 April 2021).
- Administrator. CBD and Bone Fracture Healing: The Best Data Published for Medical Marijuana in Fifty Years. BioTrack: The Leading Seed-to-Sale Cannabis Software. Available online: https://www.biotrack.com/cbd-and-bone-fracture-healing-the-best-data-published-for-medical-marijuana-in-fifty-years/ (accessed on 6 April 2021).
- McNamee, D. Marijuana ‘Helps Bones to Heal’. 20 July 2015. Available online: https://www.medicalnewstoday.com/articles/297012.php#4 (accessed on 6 April 2021).
- Bab, I.; Zimmer, A.; Melamed, E. Cannabinoids and the skeleton: From marijuana to reversal of bone loss. Ann. Med. 2009, 41, 560–567. [Google Scholar] [CrossRef]
- Stahl, V. Cannabis and Derivatives Thereof for the Treatment of Pain and Inflammation Related with Dental Pulp and Bone Regeneration Related to Dental Jaw Bone Defects. U.S. Patent No. WO2019030762A3, 19 March 2019. [Google Scholar]
- Napimoga, M.H.; Benatti, B.B.; Lima, F.O.; Alves, P.M.; Campos, A.C.; Pena-Dos-Santos, D.R.; Severino, F.P.; Cunha, F.Q.; Guimarães, F.S. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int. Immunopharmacol. 2009, 9, 216–222. [Google Scholar] [CrossRef]
- Ways CBD Takes Care of Your Salivary Gland Infection. 31 August 2019. Available online: https://thecbdoil.online/cbd-for-salivary-gland-infection/ (accessed on 6 April 2021).
- Benefits of CBG for Salivary Gland Infection: Salivary Gland Inflammation. 2019. Available online: https://thoughtcloud.net/cbg-for-salivary-gland-infection/ (accessed on 12 May 2019).
- CBD for Temporomandibular Joint Disorder (TMJ). 24 January 2020. Available online: https://hemppedia.org/cbd-for-tmj/ (accessed on 6 April 2021).
- Qian, H.; Jin, Z.; Li, S.; Huo, N.; Han, C.; Sang, H. Activation of CB2 cannabinoid receptors: A novel therapeutic strategy to accelerate osseointegration of dental implants. Med. Hypotheses 2009, 72, 311–313. [Google Scholar] [CrossRef]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Richter, N. What Are Flavonoids? Comprehensive Guide. 10 March 2020. Available online: https://wayofleaf.com/education/what-are-flavonoids (accessed on 28 July 2020).
| Dental and Oral Diseases | Prevalence/Incidence | Reference | |
|---|---|---|---|
| 1. | Dental Caries/Cavities (Tooth Decay) | ||
| 2.3 billion 530 million | [6] | |
| 2. | Gum (Periodontal) Disease including gingivitis | 10% of global population | [7] |
| 3. | Periodontitis (severe periodontal disease) | 20–50% of global population | [8,9] |
| 4. | Oral Cancer (that is, cancers of the lip, oral cavity, and oropharynx) | 657,000 new cases annually | [6] |
| 5. | Oro-dental trauma | Approximately 1 billion people have had traumatic dental injuries (TDIs) at some point in their lives. | [10] |
| Potential Applications of Secondary Metabolites of C. sativa L. in Dentistry | Appropriate Property of Secondary Metabolite | Reference | |
|---|---|---|---|
| Cannabinoids | |||
| 1. | General oral hygiene (Cannabidiol, delta9-tetrahydrocannabinol ajulemic acid, Cannabigerol) | Antifungal Antibacterial | [41,84,93,105,117,118,210] |
| 2. | Toothache (Cannabidiol, HU-320) | Analgesic | [41,151,163] |
| 3. | Dental caries/cavities (Cannabidiol, Cannabigerol and Delta9-tetrahydrocannabinol) | Anti-bacterial Analgesic | [41,47,48,84,93,117,118,191,211] |
| 4. | Abscesses (Cannabidiol and delta9-tetrahydrocannabinol) | Anti-bacterial Anti-pruritic | [191] |
| 5. | Prevention of biofilm attachment on teeth (Cannabidiol and delta9-tetrahydrocannabinol) | Anti-bacterial | [84,191] |
| 6. | Burning Mouth Syndrome (Cannabidiol) | Analgesic | [191] |
| 7. | Oral and Salivary Gland Cancers (Cannabidiol) | Anti-cancer Anti-metastatic | [191] |
| 8. | Periodontitis (most severe form of gum disease) (Cannabidiol, HU-320, delta9-tetrahydrocannabinol, AEA) | Anti-bacterial Anti-inflammatory Analgesic | [84,151,188,191,212,213] |
| 9. | Periodontal (Gum) disease (Cannabidiol, delta9-tetrahydrocannabinol, Cannabigerol and HU-320) | Anti-bacterial Anti-inflammatory Analgesic | [84,93,117,118,151,211] |
| 10. | Gingivitis (Cannabidiol, delta9-tetrahydrocannabinol, Cannabigerol, and HU-320) | Anti-bacterial Anti-inflammatory Analgesic | [84,93,117,118,151,211] |
| 11. | Oral Mucositis and other forms of oral cancer (Cannabidiol, delta9-tetrahydrocannabinol, JWH-133m, WIN-55,212-2, Cannabinol, Cannabicyclol) | Anti-bacterial Anti-cancer Anti-metastatic Anti-inflammatory Analgesic Antioxidant | [84,154,191,192,198,214] |
| 12. | Dental Anxiety (Cannabidiol) | Anxiolytic | [191,215] |
| 13. | Sleep issues resulting from dental anxiety (Cannabidiol and delta-9-tetrahydrocannabinol (THC)) | Relaxant | [216] |
| 14. | Indirect enamel protectant (Cannabidiol and delta-9-tetrahydrocannabinol (THC)) | The anti-bacterial properties of CBD and THC could indirectly protect the enamel by prevent plaque build-up that could ultimately lead to erosion of the enamel. | [84,191] |
| 15. | Remineralization of enamel (Hemp oil) | [211] | |
| 16. | Improvement of tooth sensitivity (Hemp seed oil, Cannabigerol, and CBD oil) | [211,216,217,218] | |
| 17. | Stimulation of jaw bone osteogenesis/regeneration (Cannabidiol and delta-9-tetrahydrocannabinol (THC)) | Stimulates osteogenesis in bone fracture healing | [219,220,221,222] |
| 18. | Decrease in bone resorption in experimental periodontitis in rats (Cannabidiol) | Anti-inflammatory Decreases alveolar bone loss (in rat model) | [223] |
| 19. | Salivary gland bacterial infection (Cannabidiol and delta9-tetrahydrocannabinol, cannabigerol) | Anti-bacterial Anti-inflammatory Analgesic | [93,117,118,191,224,225] |
| 20. | Digestive issues associated with anesthesia and numbing agents (Cannabidiol) | Anti-emetic Anti-nauseant | [216] |
| 21. | Temporomandibular Joint (TMJ) Disorder (Cannabidiol) | Analgesic | [226] |
| 22. | Osseointegration of dental implants (HU-308—a CB2-specific agonist) | Stimulation of osteoblastic bone formation and inhibition of osteoclastic bone resorption via activation of CB2 receptors in osteoblasts and osteoclast, and subsequent maintenance of bone mass. | [227] |
| Flavonoids | |||
| 1. | Toothaches, (Cannflavins A and B) | 30× more analgesic than aspirin Anti-inflammatory | [33,143,144,228] |
| 2. | Oral cancers that are characterized by increased production of reactive oxygen species. (Flavonols (e.g., quercetin and kaempferol)) | Antioxidant; | [229] |
| 3. | Inflammation-based oral diseases such. Oral cancers that are characterized by increased production of reactive oxygen species. (Flavanones) | Antioxidant; anticancer; anti-inflammatory | [229] |
| 4. | Inflammation-based oral diseases. Oral cancers that are characterized by increased production of reactive oxygen species. (Anthocyanins) | Antioxidant and anti-inflammatory | [229] |
| Terpenes | |||
| 1. | Toothache and other oral disorders that cause pain (β- caryophyllene, α-terpineol, Myrcene) | Analgesic | [35,85,86,92,141,142] |
| 2. | Dental Anxiety (E.g. Linalool) | Anxiolytic | [86] |
| 3. | Inflammation-based oral diseases such as gingivitis, periodontal disease and periodontitis. α-terpineol(Linalool, Myrcene, α-Pinene, Ocimene, β- caryophyllene, Limonene) | Anti-inflammatory | [85,92] |
| 4. | Oral cancers that are characterized by increased production of reactive oxygen species. (Myrcene, Limonene, Linalool, α-terpineol, α-Humulene, Ocimene) | Antioxidant Anticancer | [39,86,87,88,89,90,91] |
| 5. | Oral diseases such as gingivitis, periodontal disease, periodontitis and salivary gland infections that are characterized by bacterial-plaque build-up and bacterial infections. (Ocimene, α-terpineol, Linalool, α-Pinene, Limonene) | Anti-microbial (anti-bacterial) | [35,86,87,88,89,90,91,92,141,142] (Cavaleiro et al., 2015) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W.; Nedamat, K. The Current and Potential Application of Medicinal Cannabis Products in Dentistry. Dent. J. 2021, 9, 106. https://doi.org/10.3390/dj9090106
Lowe H, Toyang N, Steele B, Bryant J, Ngwa W, Nedamat K. The Current and Potential Application of Medicinal Cannabis Products in Dentistry. Dentistry Journal. 2021; 9(9):106. https://doi.org/10.3390/dj9090106
Chicago/Turabian StyleLowe, Henry, Ngeh Toyang, Blair Steele, Joseph Bryant, Wilfred Ngwa, and Kaveh Nedamat. 2021. "The Current and Potential Application of Medicinal Cannabis Products in Dentistry" Dentistry Journal 9, no. 9: 106. https://doi.org/10.3390/dj9090106
APA StyleLowe, H., Toyang, N., Steele, B., Bryant, J., Ngwa, W., & Nedamat, K. (2021). The Current and Potential Application of Medicinal Cannabis Products in Dentistry. Dentistry Journal, 9(9), 106. https://doi.org/10.3390/dj9090106






