Benefits of Using Fluorescence Induced Theragnosis in Fixed Orthodontic Therapy: Status, Technology and Future Trends
Abstract
:1. Introduction
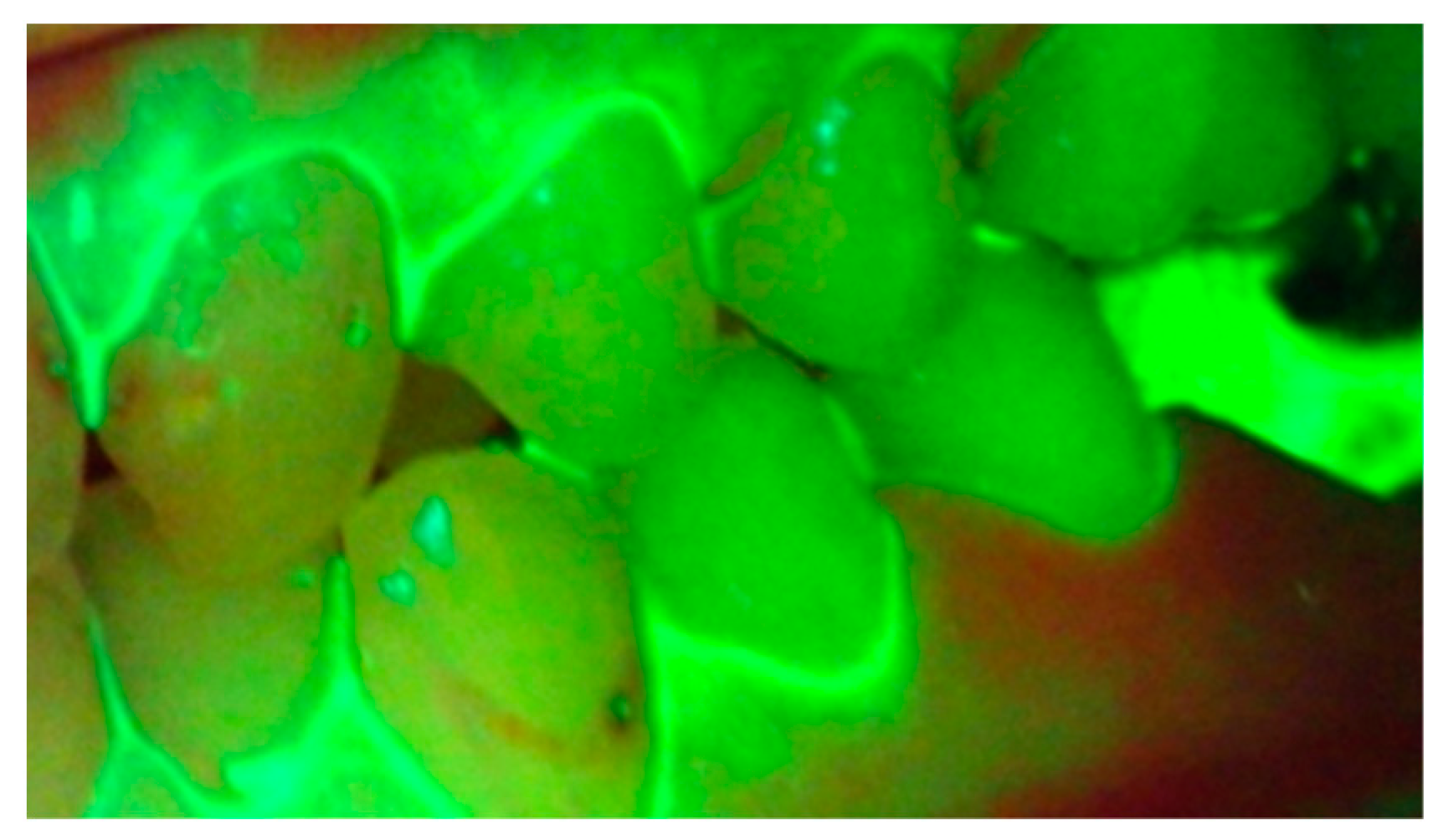
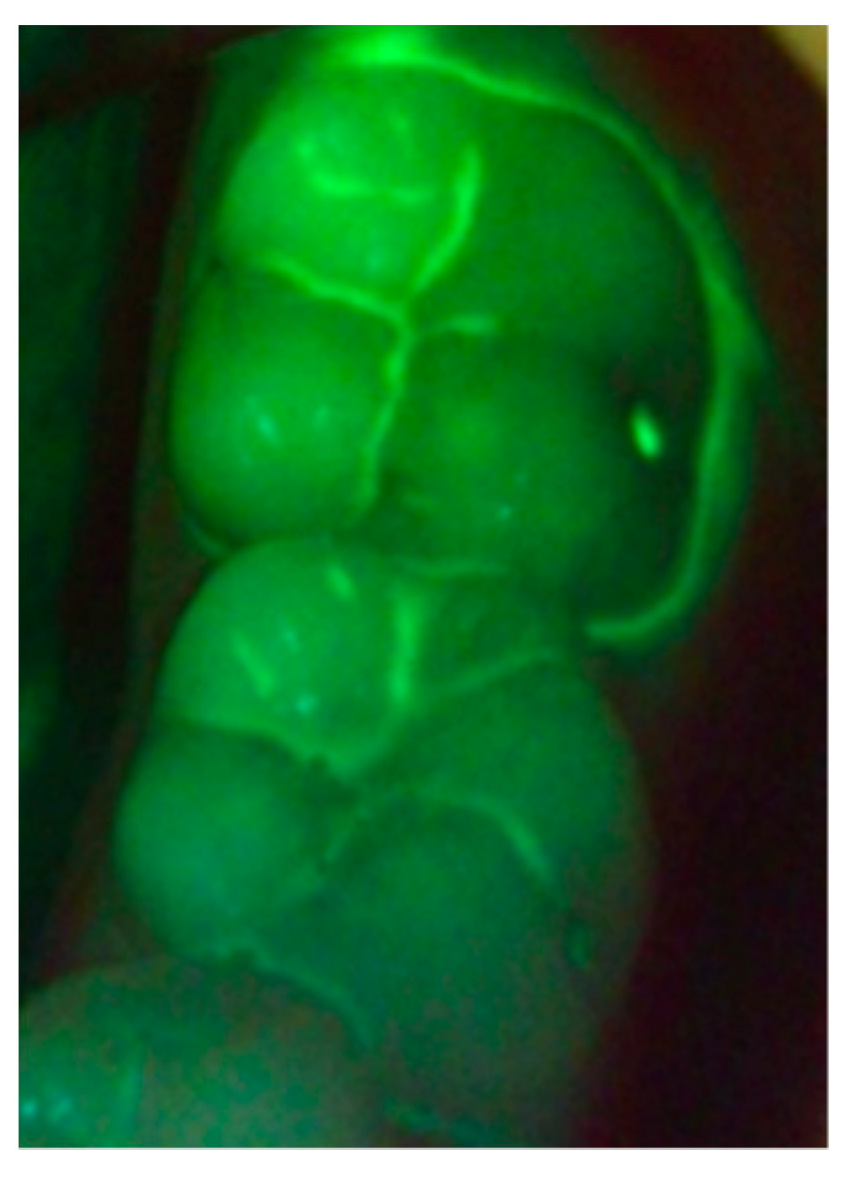
2. Hygiene Motivation and Monitoring during Treatment
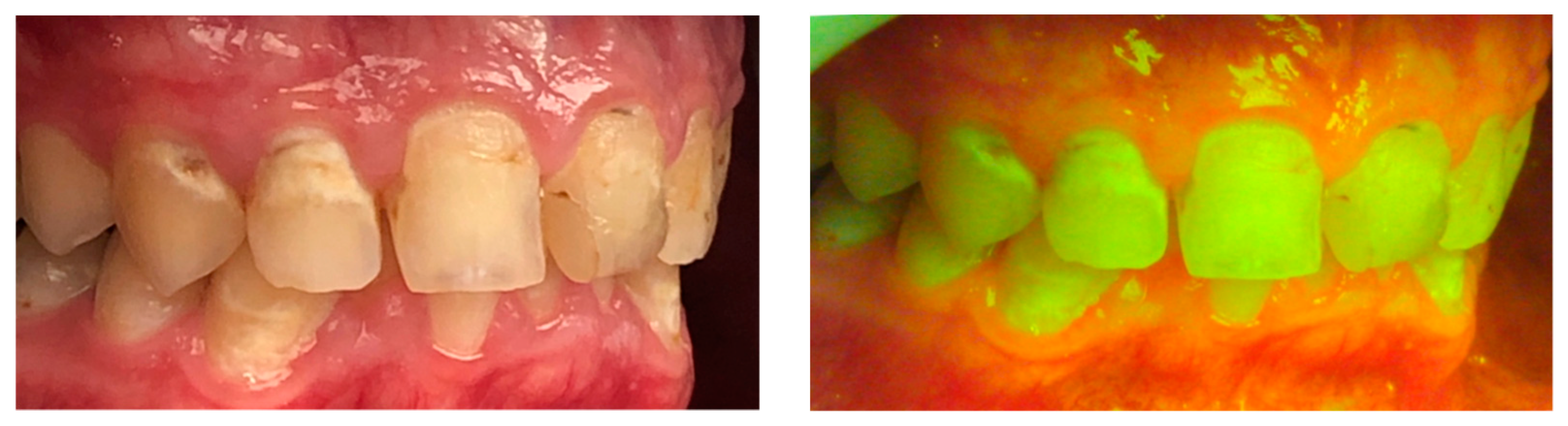
3. Early Demineralization Detection
4. Resin Cement Assessment and White Spot Lesions after Debonding
5. Comparison of Various Orthodontic Materials in Terms of Plaque Accumulation
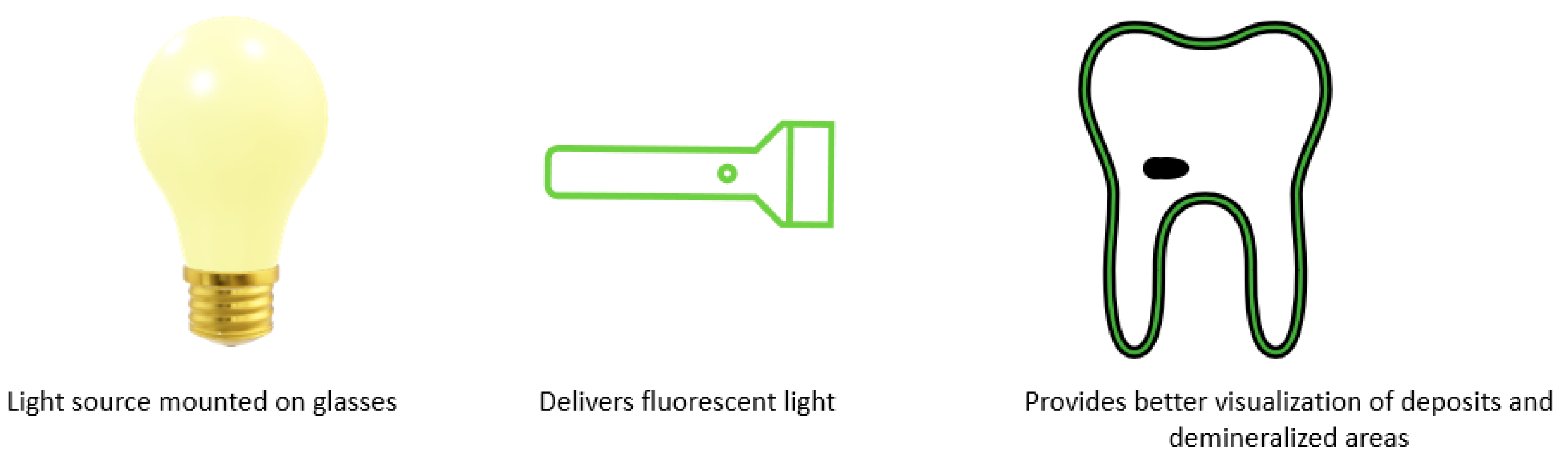
6. Fluorescence Enhanced Theragnosis—The Next Stage of a Targeted Diagnostic Approach?
7. Areas of Improvement
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial Diversity in Human Subgingival Plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolenbrander, P.E. Oral Microbial Communities: Biofilms, Interactions, and Genetic Systems. Annu. Rev. Microbiol. 2000, 54, 413–437. [Google Scholar] [CrossRef]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontology 2000 1997, 14, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000 1994, 5, 78–111. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C.; Robertson, P.B. The Biology, Prevention, Diagnosis and Treatment of Periodontal Diseases: Scientific Advances in the United States. J. Am. Dent. Assoc. 2009, 140, 36–43. [Google Scholar] [CrossRef]
- Offenbacher, S. Periodontal Diseases: Pathogenesis. Ann. Periodontol. 1996, 1, 821–878. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.C.; Qaqish, J.; Klukowska, M.; Grender, J.; Rooney, J. The plaque removal efficacy of a novel power brush head. J. Clin. Dent. 2011, 22, 19–22. [Google Scholar] [PubMed]
- Raggio, D.P.; Braga, M.M.; Rodrigues, J.A.; Freitas, P.M.; Imparato, J.C.P.; Mendes, F. Reliability and discriminatory power of methods for dental plaque quantification. J. Appl. Oral Sci. 2010, 18, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Yavan, M.A.; Kocahan, S.; Ozdemir, S.; Sokucu, O.; Orthodontist, G.P. The Effects of Using Plaque-Disclosing Tablets on the Removal of Plaque and Gingival Status of Orthodontic Patients. Turk. J. Orthod. 2019, 32, 207–214. [Google Scholar] [CrossRef]
- Øgaard, B.; Ten Bosch, J.J. Regression of white spot enamel lesions. A new optical method for quantitative longitudinal evaluation in vivo. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 238–242. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal Disease in Pregnancy II. Correlation Between Oral Hygiene and Periodontal Condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Pretty, I.A.; Pender, N.; Edgar, W.M.; Higham, S.M. The in vitro detection of early enamel de-and remineralization adjacent to bonded orthodontic cleats using quantitative light-induced Fluorescence. Eur. J. Orthod. 2003, 25, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, D.; McIntyre, G. Does oral health promotion influence the oral hygiene and gingival health of patients undergoing fixed appliance orthodontic treatment? A systematic literature review. J. Orthod. 2008, 35, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Marini, I.; Bortolotti, F.; Parenti, S.I.; Gatto, M.R.; Bonetti, G.A. Combined effects of repeated oral hygiene motivation and type of toothbrush on orthodontic patients: A blind randomized clinical trial. Angle Orthod. 2014, 84, 896–901. [Google Scholar] [CrossRef]
- Boyd, R.L. Longitudinal evaluation of a system for self-monitoring plaque control effectiveness in orthodontic patients. J. Clin. Periodontol. 1983, 10, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.D.; Nanda, R.S.; Sinha, P.K.; Smith, D.W.; Currier, G.F. Effect of behavior modification on patient compliance in orthodontics. Angle Orthod. 1998, 68. [Google Scholar] [CrossRef]
- Lalic, M.; Aleksic, E.; Gajic, M.; Milic, J.; Malesevic, D. Does oral health counseling effectively improve oral hygiene of orthodontic patients? Eur. J. Paediatr. Dent. 2012, 13, 181–186. [Google Scholar] [PubMed]
- Eppright, M.; Shroff, B.; Best, A.; Barcoma, E.; Lindauer, S.J. Influence of active reminders on oral hygiene compliance in orthodontic patients. Angle Orthod. 2013, 84, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [Green Version]
- Anil, K.; Anand, A. Fundamentals and Applications of Biophotonics in Dentistry; London Imperial College Press: London, UK, 2007; Volume 105–107, pp. 16–17. [Google Scholar]
- Walsh, L.J.; Shakibaie, F. Ultraviolet-Induced Fluorescence: Shedding New Light on Dental Biofilms and Dental Caries. Australas. Dent. Pract. 2007, 18, 1–7. [Google Scholar]
- Heinrich-Weltzien, R.; Kühnisch, J.; Van Der Veen, M.; Jong, E.D.J.D.; Stösser, L. Quantitative light-induced fluorescence (QLF)—A potential method for the dental practitioner. Quintessence Int. 2003, 34, 181–188. [Google Scholar]
- Volgenant, C.M.; Hoogenkamp, M.A.; Buijs, M.J.; Zaura, E.; ten Cate, J.B.M.; van der Veen, M.H. Red fluorescent biofilm: The thick, the old, and the cariogenic. J. Oral Microbiol. 2016, 8, 30346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biamonte, F.; Buffone, C.; Santamaria, G.; Battaglia, A.M.; Mignogna, C.; Fortunato, L.; Costanzo, F.S.; Giudice, A. Gene expression analysis of autofluorescence margins in leukoplakia and oral carcinoma: A pilot study. Oral Dis. 2020, 27, 193–203. [Google Scholar] [CrossRef]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.; Fortunato, L. Can Autofluorescence Guide Surgeons in the Treatment of Medication-Related Osteonecrosis of the Jaw? A Prospective Feasibility Study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef]
- Karkhanechi, M.; Chow, D.; Sipkin, J.; Sherman, D.; Boylan, R.J.; Norman, R.G.; Craig, R.G.; Cisneros, G.J. Periodontal status of adult patients treated with fixed buccal appliances and removable aligners over one year of active orthodontic therapy. Angle Orthod. 2012, 83, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.A. Effects of orthodontic attachments on the gingival health of permanent second molars. Am. J. Orthod. Dentofac. Orthop. 1991, 100, 337–340. [Google Scholar] [CrossRef]
- Ristic, M.; Svabic, M.V.; Sasic, M.; Zelic, O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod. Craniofacial Res. 2007, 10, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef]
- Preda, C.; Butera, A.; Pelle, S.; Pautasso, E.; Chiesa, A.; Esposito, F.; Oldoini, G.; Scribante, A.; Genovesi, A.; Cosola, S. The Efficacy of Powered Oscillating Heads vs. Powered Sonic Action Heads Toothbrushes to Maintain Periodontal and Peri-Implant Health: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 1468. [Google Scholar] [CrossRef]
- Bollen, A.M.; Cunha-Cruz, J.; Bakko, D.W.; Huang, G.J.; Hujoel, P.P. The effects of orthodontic therapy on periodontal health: A systematic review of controlled evidence. J. Am. Dent. Assoc. 2008, 139, 413–422. [Google Scholar] [CrossRef]
- Miller, C.C.; Burnside, G.; Higham, S.M.; Flannigan, N.L. Quantitative Light-induced Fluorescence-Digital as an oral hygiene evaluation tool to assess plaque accumulation and enamel demineralization in orthodontics. Angle Orthod. 2016, 86, 991–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şen, S.; Erber, R.; Deurer, N.; Orhan, G.; Lux, C.J.; Zingler, S. Demineralization detection in orthodontics using an ophthalmic optical coherence tomography device equipped with a multicolor fluorescence module. Clin. Oral Investig. 2019, 24, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Farahani, M.R.D.; Marino, G.; Matera, C.; Baena, R.R.Y.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, M.M.; Featherstone, J.D. Demineralization, and remineralization around orthodontic appliances: An in vivo study. Am. J. Orthod. Dentofac. Orthop. 1987, 92, 33–40. [Google Scholar] [CrossRef]
- van der Veen, M.H.; de Josselin de Jong, E. Application of quantitative light-induced Fluorescence for assessing early caries lesions. Monogr. Oral Sci. 2000, 17, 144–162. [Google Scholar]
- Al-Khateeb, S.; Forsberg, C.M.; de Josselin de Jong, E.; AngmarMansson, B. A longitudinal laser fluorescence study of white spot lesions in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 1998, 113, 595–602. [Google Scholar] [CrossRef]
- Ren, Y.; Jongsma, M.A.; Mei, L.; Van Der Mei, H.C.; Busscher, H.J. Orthodontic treatment with fixed appliances and biofilm formation—A potential public health threat? Clin. Oral Investig. 2014, 18, 1711–1718. [Google Scholar] [CrossRef]
- Fontana, M.; Young, D.A.; Wolff, M.S. Evidence-based caries, risk assessment, and treatment. Dent. Clin. N. Am. 2009, 53, 149–161. [Google Scholar] [CrossRef]
- Lussi, A.; Hibst, R.; Paulus, R. DIAGNOdent: An Optical Method for Caries Detection. J. Dent. Res. 2004, 83, 80–83. [Google Scholar] [CrossRef]
- Pignatta, L.M.B.; Júnior, S.D.; Santos, E.C.A. Evaluation of enamel surface after bracket debonding and polishing. Dent. Press J. Orthod. 2012, 17, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.G.; Millett, D.T.; Cronin, M. Bonded molar tubes: A survey of their use by specialist orthodontists. J. Orthod. 2012, 39, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-E. Quantitative light-induced Fluorescence: A potential tool for dental hygiene process. J. Dent. Hyg. Sci. 2013, 13, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steier, L. Reveal: Fluorescence Enhanced Theragnosis by Designs for Vision. Eur. J. Dent. 2020, 14, 186–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marya, A.; Steier, L.; Karobari, M.I.; Venugopal, A. Benefits of Using Fluorescence Induced Theragnosis in Fixed Orthodontic Therapy: Status, Technology and Future Trends. Dent. J. 2021, 9, 90. https://doi.org/10.3390/dj9080090
Marya A, Steier L, Karobari MI, Venugopal A. Benefits of Using Fluorescence Induced Theragnosis in Fixed Orthodontic Therapy: Status, Technology and Future Trends. Dentistry Journal. 2021; 9(8):90. https://doi.org/10.3390/dj9080090
Chicago/Turabian StyleMarya, Anand, Liviu Steier, Mohmed Isaqali Karobari, and Adith Venugopal. 2021. "Benefits of Using Fluorescence Induced Theragnosis in Fixed Orthodontic Therapy: Status, Technology and Future Trends" Dentistry Journal 9, no. 8: 90. https://doi.org/10.3390/dj9080090
APA StyleMarya, A., Steier, L., Karobari, M. I., & Venugopal, A. (2021). Benefits of Using Fluorescence Induced Theragnosis in Fixed Orthodontic Therapy: Status, Technology and Future Trends. Dentistry Journal, 9(8), 90. https://doi.org/10.3390/dj9080090








