Integration of Dental Implants in Conjunction with EDTA-Conditioned Dentin Grafts: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Anesthesia
2.2. Preparation of Dentin Grafts for Surgery
2.3. Preparation of Dentin Grafts for Histology, X-ray, and Elemental Analysis
2.4. Baseline Surgery—Graft Preparation and Placement
2.5. Second Surgery—Implant Placement
2.6. Histological and Histomorphometric Analysis
2.7. Statistical Evaluation
3. Results
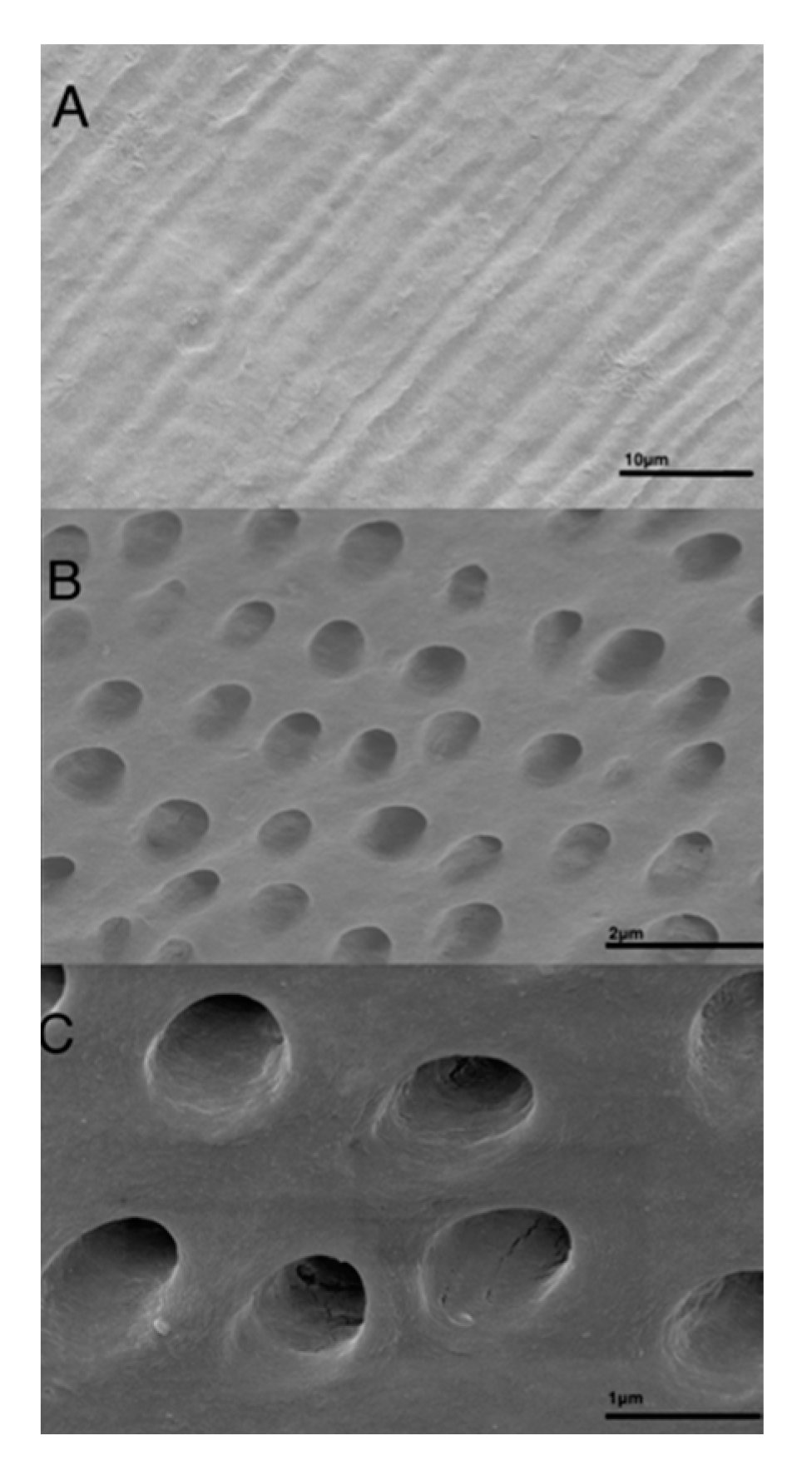
3.1. Descriptive Characterization of Dentin Block
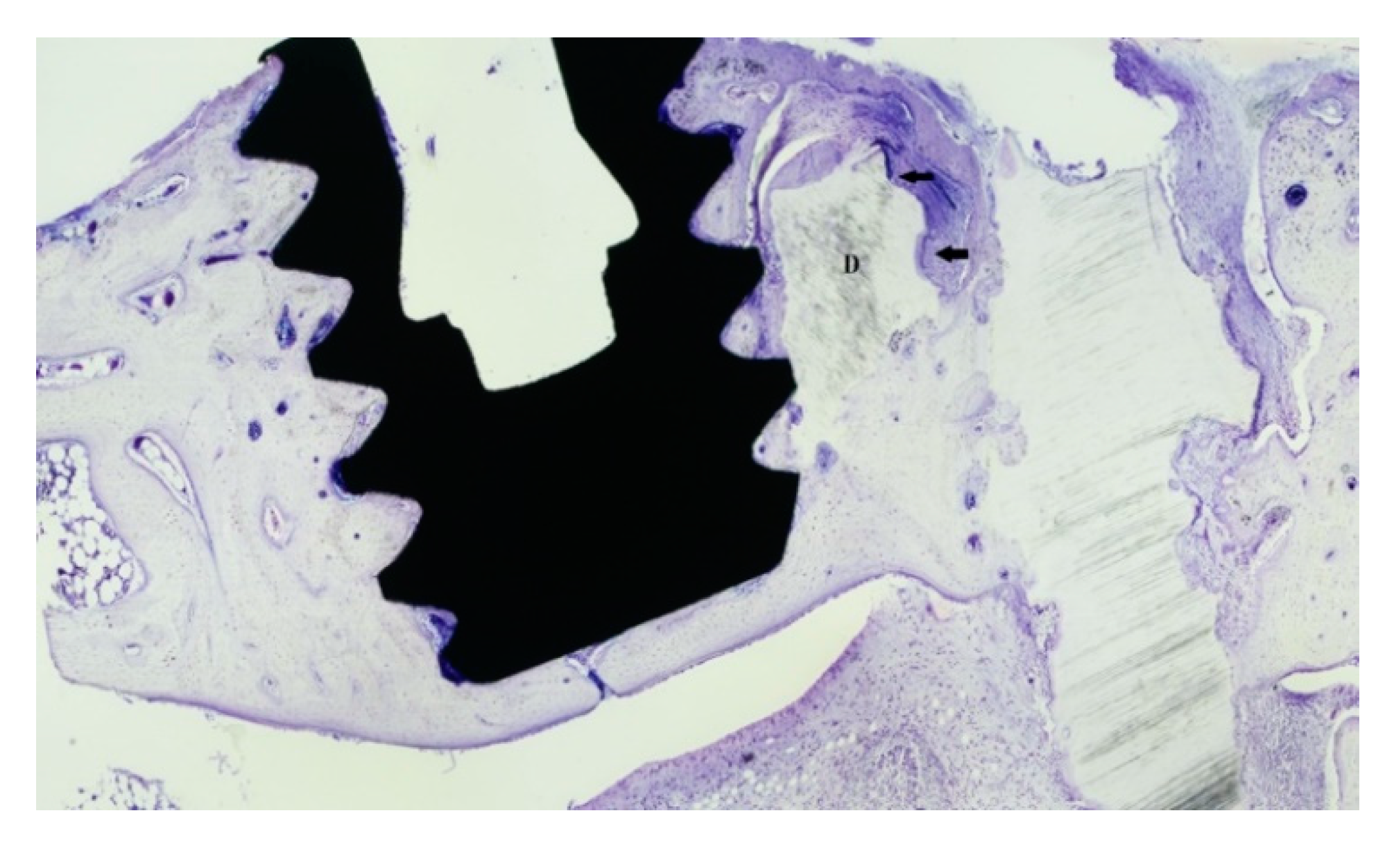
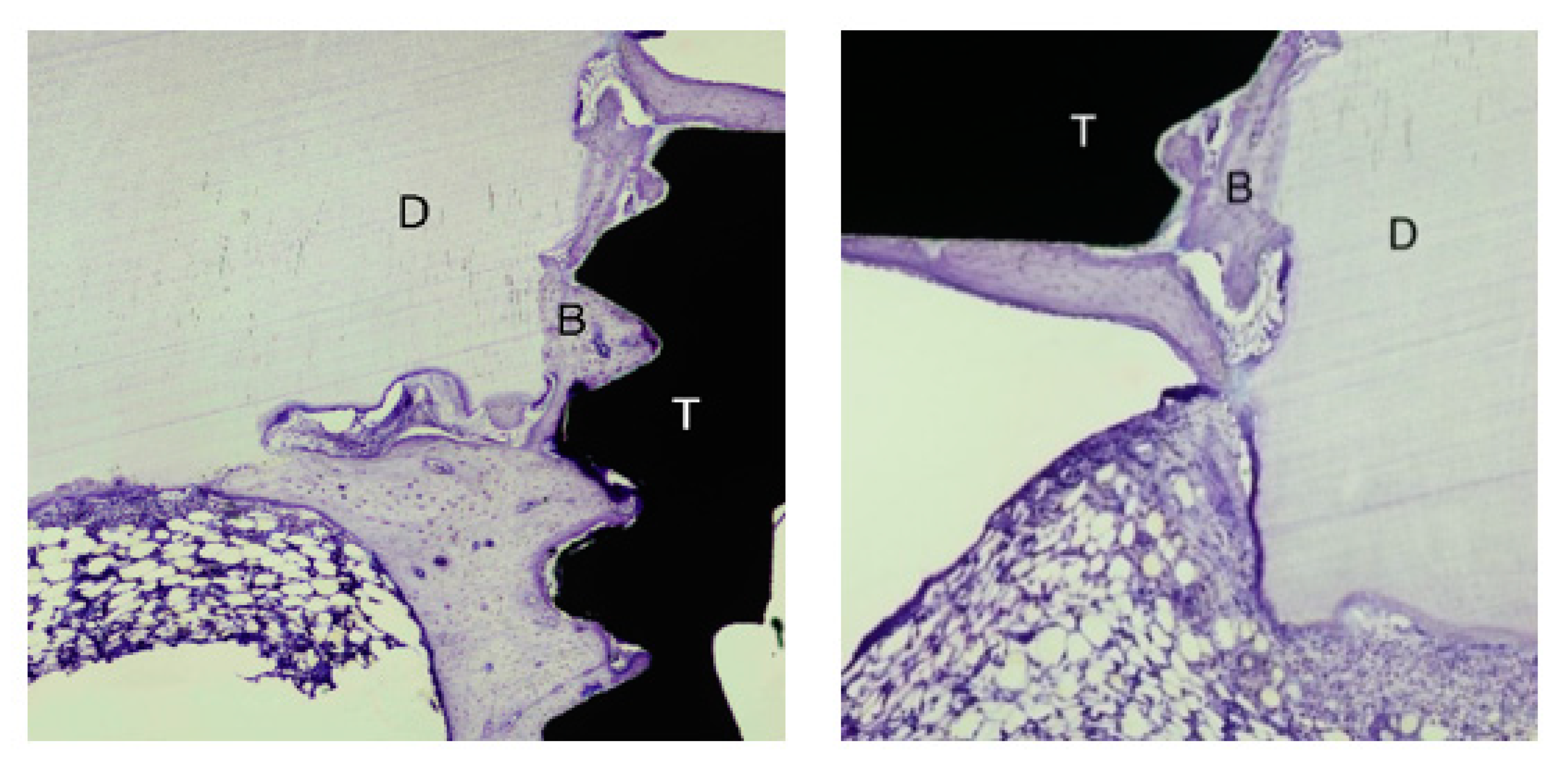
3.2. Bone Healing
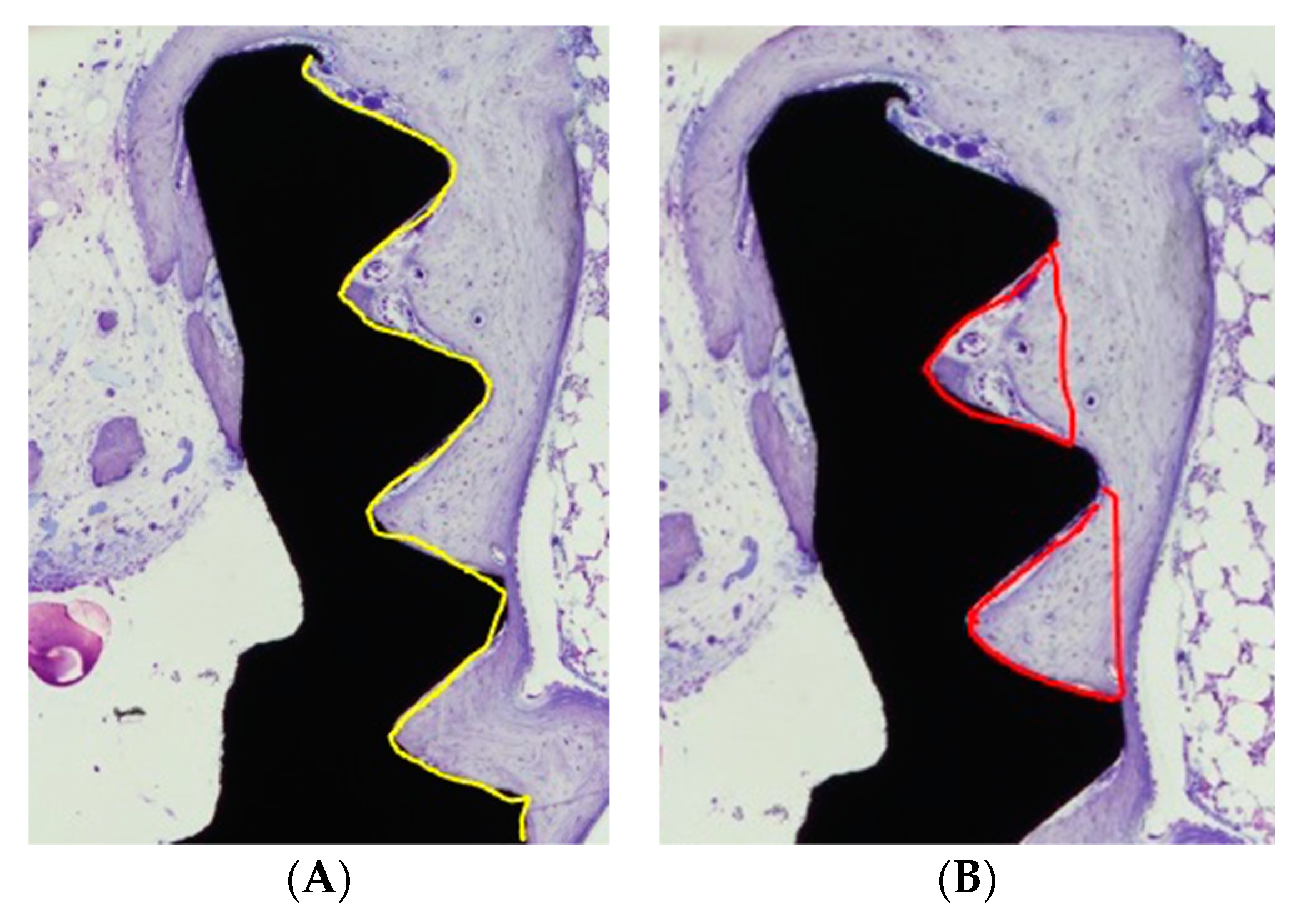
3.3. Histomorphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciu, M. Sandblasted and acid etched titanium dental implant surfaces systematic review and comical microscopy evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Carlsson, G. Responses of jawbone to pressure. Gerodontology 2004, 21, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Su-Gwan, K.; Hak-Kyun, K.; Sung-Schul, L. Combined implantation of particulate dentine, plaster of Paris, and a bone xenograft (Bio-Oss ®) for bone regeneration in rats. J. Cranio-Maxillofac. Surg. 2001, 29, 282–288. [Google Scholar] [CrossRef]
- Carvalho, V.; Tosello Dde, O.; Salgado, M.; Gomes, M. Histomorphometric analysis of homogenous demineralized dentin matrix as osteopromotive material in rabbit mandibles. Int. J. Oral Maxillofac. Implant. 2004, 5, 679–686. [Google Scholar]
- Kim, Y.; Kim, S.; Byeon, J.; Lee, H.; Um, I.; Lim, S.; Kim, S. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 496–503. [Google Scholar] [CrossRef]
- Murata, M.; Akazawa, T.; Takahata, M.; Ito, M.; Tazaki, J.; Nakamura, K.; Iwasaki, N.; Shibata, T.; Arisue, M. Bone induction of human tooth and bone crushed by newly developed automatic mill. J. Ceram. Soc. Jpn. 2010, 118, 434–437. [Google Scholar] [CrossRef][Green Version]
- Atiya, B.K.; Shanmuhasuntharam, P.; Huat, S.; Abdulrazzak, S.; Oon, H. Liquid nitrogen-treated autogenous dentin as bone substitute: An experimental study in a rabbit model. Oral Craniofac. Tissue Eng. 2012, 2, 215–220. [Google Scholar] [CrossRef][Green Version]
- Kim, E. Autogenous fresh demineralized tooth graft prepared at chairside for dental implant. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 1–6. [Google Scholar] [CrossRef]
- Pang, K.; Um, I.; Kim, Y.; Woo, J.; Kim, S.; Lee, J. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implant. Res. 2016, 28, 809–815. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Um, I.; Cho, W. Guided bone regeneration using demineralized dentin matrix: Long term follow-up. J. Oral Maxillofac. Surg. 2016, 74, 515.e1–515.e9. [Google Scholar] [CrossRef]
- Kim, Y.; Pang, K.; Yun, P.; Leem, D.; Um, I. Long-term follow-up of autogenous tooth bone graft blocks with dental implants. Clin. Case Rep. 2017, 5, 108–118. [Google Scholar] [CrossRef]
- Bang, G.; Johannessen, J. The effect of physical treatment on the induction of heterotopic bone formation by demineralized dentin in guinea pigs. J. Oral Pathol. 1972, 1, 231–243. [Google Scholar] [CrossRef]
- Bang, G.; Urist, M. Bone induction in excavation chambers in matrix of decalcified dentin. Arch. Surg. 1967, 94, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Wedenberg, C.; Langius-Östlin, G.; Roosaar, A. Osteoinductive properties of different dentin preparations. Eur. J. Musculoskelet. Res. 1994, 3, 123–128. [Google Scholar]
- Machado, M.; Souza, A.; Araújo, V. Histological evaluation of the osteoinduction capability of human dentine. Int. Endod. J. 2006, 11, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Al-Asfour, A.; Farzad, P.; Andersson, L.; Joseph, B.; Dahlin, C. Host tissue reactions of non-demineralized autogenic and xenogenic dentin blocks implanted in a non-osteogenic environment. An experimental study in rabbits. Dent. Traumatol. 2014, 30, 198–203. [Google Scholar] [CrossRef]
- Al-Asfour, A.; Farzad, P.; Al-Musawi, A.; Dahlin, C.; Andersson, L. Demineralized Xenogenic Dentin and Autogenous Bone as Onlay Grafts to Rabbit Tibia. Implant. Dent. 2017, 2, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Urist, M. Bone histogenesis and morphogenesis in implants of demineralised enamel and dentin. J. Oral Surg. 1971, 29, 88–102. [Google Scholar]
- Bang, G. Induction of heterotopic bone formation by demineralized dentin in guinea pigs: Antigenicity of the dentin matrix. J. Oral Pathol. 1972, 4, 172–185. [Google Scholar] [CrossRef]
- Mordenfeld, A.; Hallman, M.; Lindskog, S. Tissue reactions to subperiosteal onlays of demineralized xenogenous dentin blocks in rats. Dent. Traumatol. 2011, 27, 446–451. [Google Scholar] [CrossRef]
- Farzad, P. On Tissue Reactions to Dentin as a Bone Subtitute Material. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2017. [Google Scholar]
- Alberius, P.; Klinge, B.; Isaksson, S. Management of craniotomy in young rabbits. Lab. Anim. 1989, 23, 70–72. [Google Scholar] [CrossRef]
- Salata, L.; Craig, G.; Brook, I. Bone healing following the use of hydroxyapatite or ionomeric bone substitutes alone or combined with a guided bone regeneration technique: An animal study. Int. J. Oral Maxillofac. Implant. 1998, 13, 44–51. [Google Scholar]
- Andersson, L.; Ramzi, A.; Joseph, B. Studies on dentin grafts to bone defects in rabbit tibia and mandible; development of an experimental model. Dent. Traumatol. 2009, 25, 78–83. [Google Scholar] [CrossRef]
- Alberius, P.; Gordh, M.; Lindberg, L.; Johnell, O. Influence of surrounding soft tissues on onlay bone graft incorporation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 22–33. [Google Scholar] [CrossRef]
- Park, S.-M.; Kim, D.-H.; Pang, E.-K. Bone formation of demineralized human dentin block graft with different demineralization time: In vitro and in vivo study. J. Cranio-Maxillofac. Surg. 2017, 45, 903–912. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, J.Y.; Suh, J.S.; Lee, G.; Chung, C.P.; Park, Y.J.; Yoon, J. The mineralization inducing peptide derived from dentin sialophosphoprotein for bone regeneration. J. Biomed. Mater. Res. Part A 2013, 101, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Abreu, P.; Morosolli, A.; Araújo, M.; Goulart, M. Densitometric analysis of the autogenous demineralized dentin matrix on the dental socket wound healing process in humans. Braz. Oral Res. 2006, 20, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.; Kim, S.; Lim, S. Guided bone regeneration using demineralized allogenic bone matrix with calcium sulfate: Case series. J. Adv. Prosthodont. 2013, 5, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.; Consolaro, A.; Sant’Ana, A.; Damante, C.; Greghi, S.; Passanezi, E. Demineralization of the contacting surfaces in autologous onlay bone grafts improves bone formation and bone consolidation. J. Periodontol. 2014, 85, e121–e129. [Google Scholar] [CrossRef] [PubMed]
- Blomlöf, J.; Blomlöf, L.; Lindskog, S. Effect of different concentrations of EDTA on smear removal and collagen exposure in periodontitis-affected root surfaces. J. Clin. Periodontol. 1997, 8, 534–537. [Google Scholar] [CrossRef]
- Kiviranta, I.; Tammi, M.; Lappalainen, R.; Kuusela, T.; Helminen, H.J. The rate of calcium extraction during EDTA decalcification from thin bone slices as assessed with atomic absorption spectrophotometry. Histochemistry 1980, 68, 119–127. [Google Scholar] [CrossRef]
- Pucher, J.; Daniel, J. The effects of chlorhexidine digluconate on human fibroblasts in vitro. J. Periodontol. 1992, 63, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.; Aranha, A.; Nogueira, I.; Giro, E.; Hebling, J.; Costa, C. Toxicity of chlorhexidine on odontoblast-like cells. J. Appl. Oral Sci. 2010, 18, 50–58. [Google Scholar] [CrossRef]
- Solheim, E.; Pinholt, E.; Bang, G.; Sudmann, E. Ethylene oxide gas sterilization does not reduce the osteoinductive potential of demineralized bone in rats. J. Craniofac. Surg. 1995, 6, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L. Dentin xenografts to experimental bone defects in rabbit tibia are ankylosed and undergo osseous replacement. Dent. Traumatol. 2010, 26, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Al-Asfour, A.; Andersson, L.; Kamal, M.; Joseph, B. New bone formation around xenogenic dentin grafts to rabbit tibia marrow. Dent. Traumatol. 2013, 29, 455–460. [Google Scholar] [CrossRef] [PubMed]


| C | N | O | P | Ca | Au | |
|---|---|---|---|---|---|---|
| Spectrum 1 | 57.95 | 23.68 | 14.70 | −0.35 | 0.16 | 3.85 |
| Spectrum 2 | 52.97 | 28.23 | 15.09 | 0.10 | 0.48 | 3.13 |
| Spectrum 3 | 55.11 | 27.28 | 15.30 | 0.04 | 0.18 | 2.08 |
| Spectrum 4 | 54.40 | 27.18 | 14.47 | 0.21 | 0.29 | 3.35 |
| Mean | 55.13 | 26.59 | 14.89 | 0.00 | 0.28 | 3.10 |
| Std.deviation | 2.08 | 2.00 | 0.38 | 0.24 | 0.14 | 0.75 |
| Max | 57.95 | 28.23 | 15.30 | 0.21 | 0.48 | 3.85 |
| Min | 52.97 | 23.68 | 14.47 | −0.35 | 0.16 | 2.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farzad, P.; Lundgren, T.; Al-Asfour, A.; Andersson, L.; Dahlin, C. Integration of Dental Implants in Conjunction with EDTA-Conditioned Dentin Grafts: An Experimental Study. Dent. J. 2021, 9, 63. https://doi.org/10.3390/dj9060063
Farzad P, Lundgren T, Al-Asfour A, Andersson L, Dahlin C. Integration of Dental Implants in Conjunction with EDTA-Conditioned Dentin Grafts: An Experimental Study. Dentistry Journal. 2021; 9(6):63. https://doi.org/10.3390/dj9060063
Chicago/Turabian StyleFarzad, Payam, Ted Lundgren, Adel Al-Asfour, Lars Andersson, and Christer Dahlin. 2021. "Integration of Dental Implants in Conjunction with EDTA-Conditioned Dentin Grafts: An Experimental Study" Dentistry Journal 9, no. 6: 63. https://doi.org/10.3390/dj9060063
APA StyleFarzad, P., Lundgren, T., Al-Asfour, A., Andersson, L., & Dahlin, C. (2021). Integration of Dental Implants in Conjunction with EDTA-Conditioned Dentin Grafts: An Experimental Study. Dentistry Journal, 9(6), 63. https://doi.org/10.3390/dj9060063






