Oral Pemphigoid Recalcitrant Lesion Treated with PRGF Infiltration. Case Report
Abstract
:1. Introduction
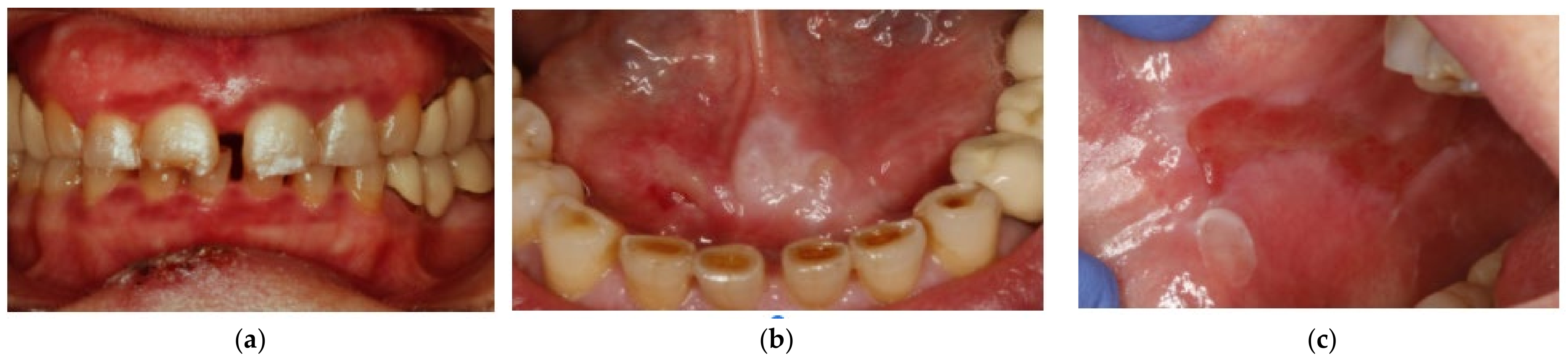
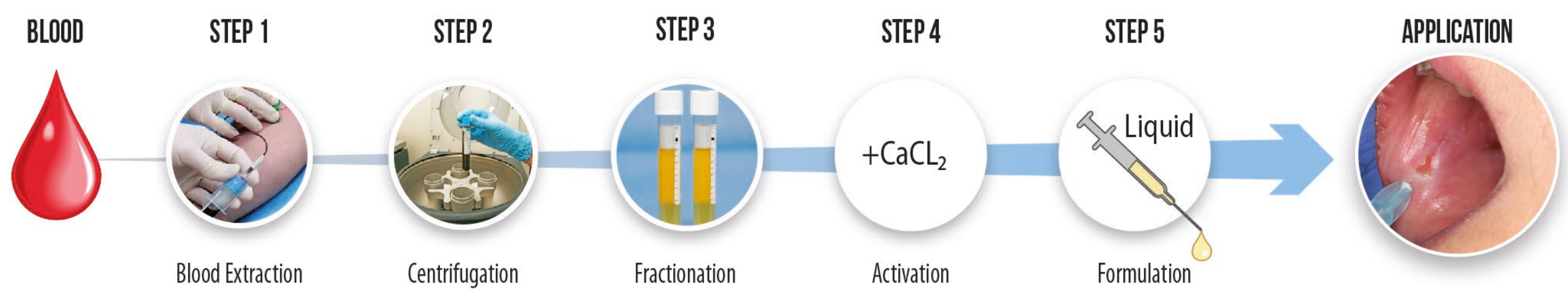
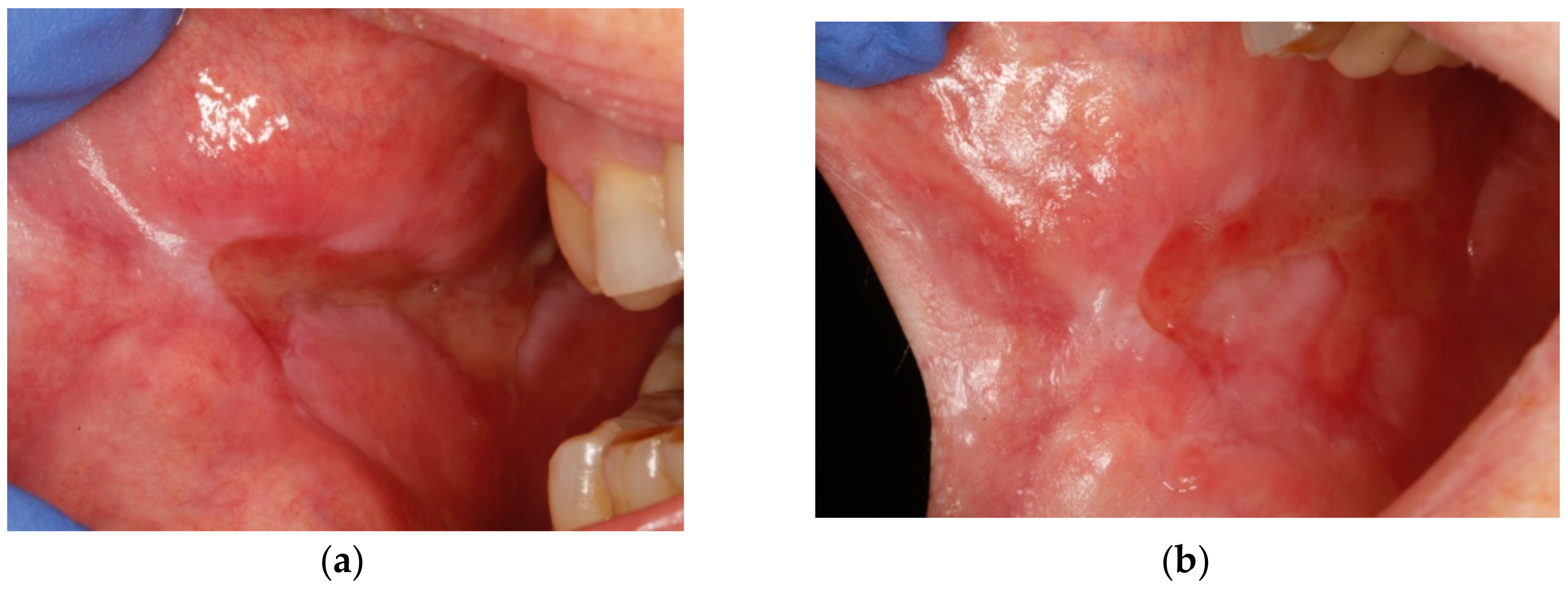
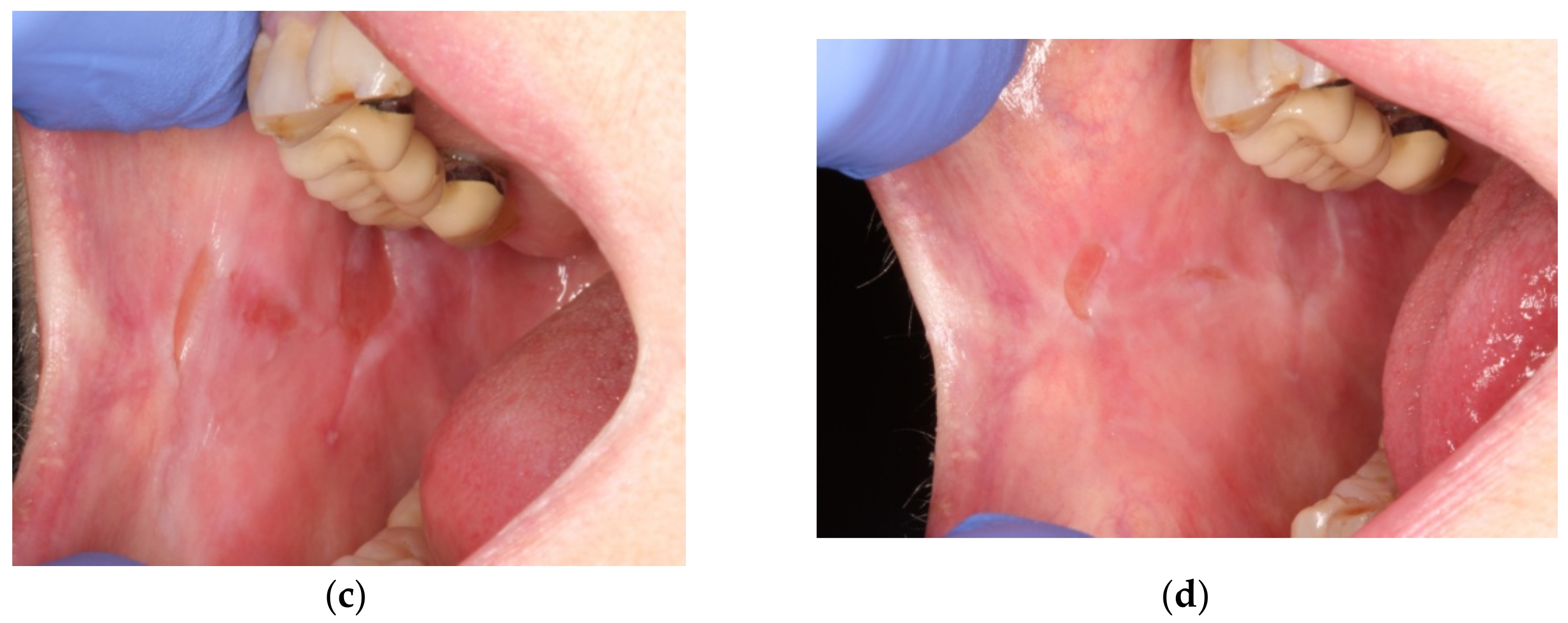
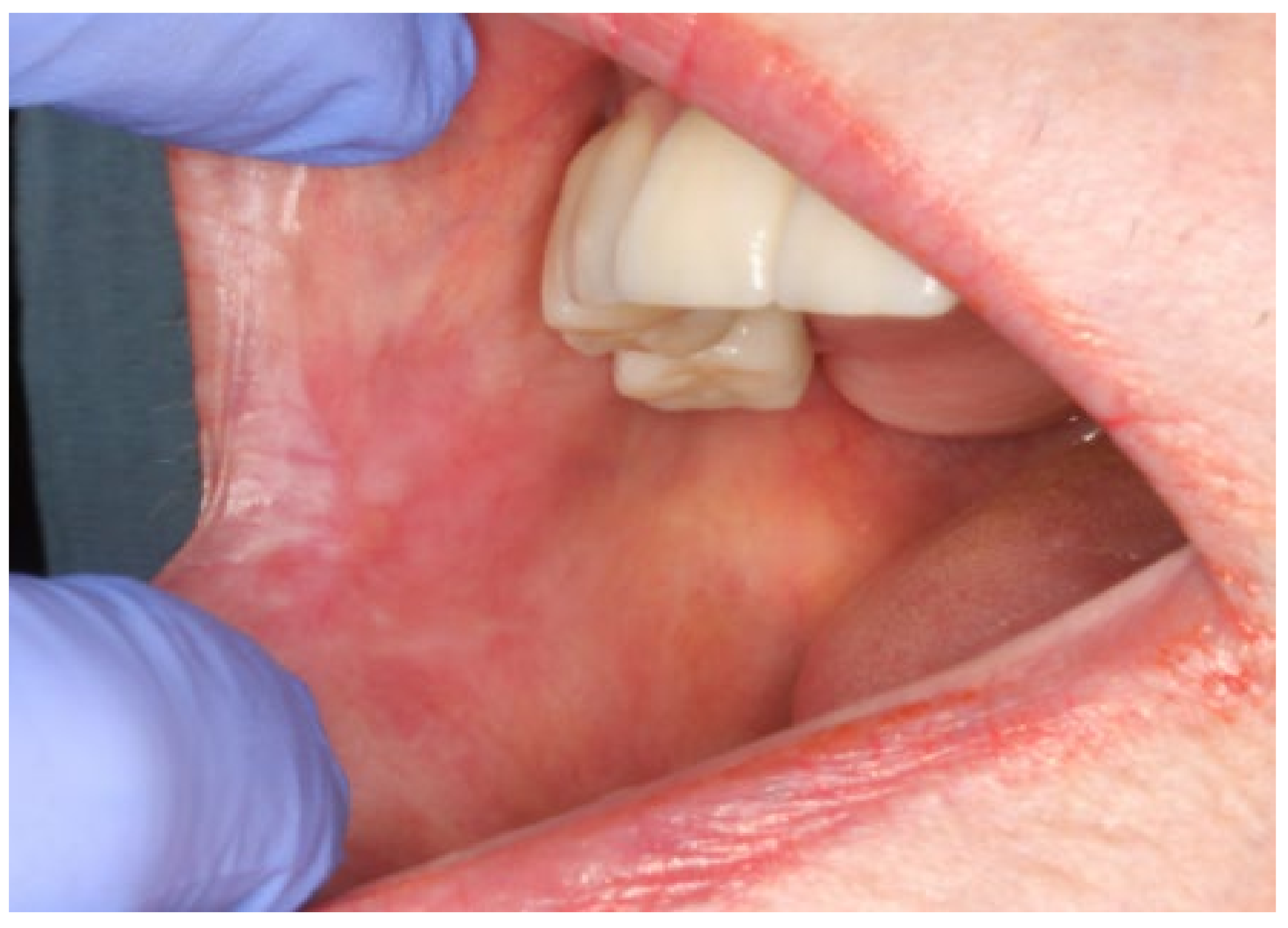
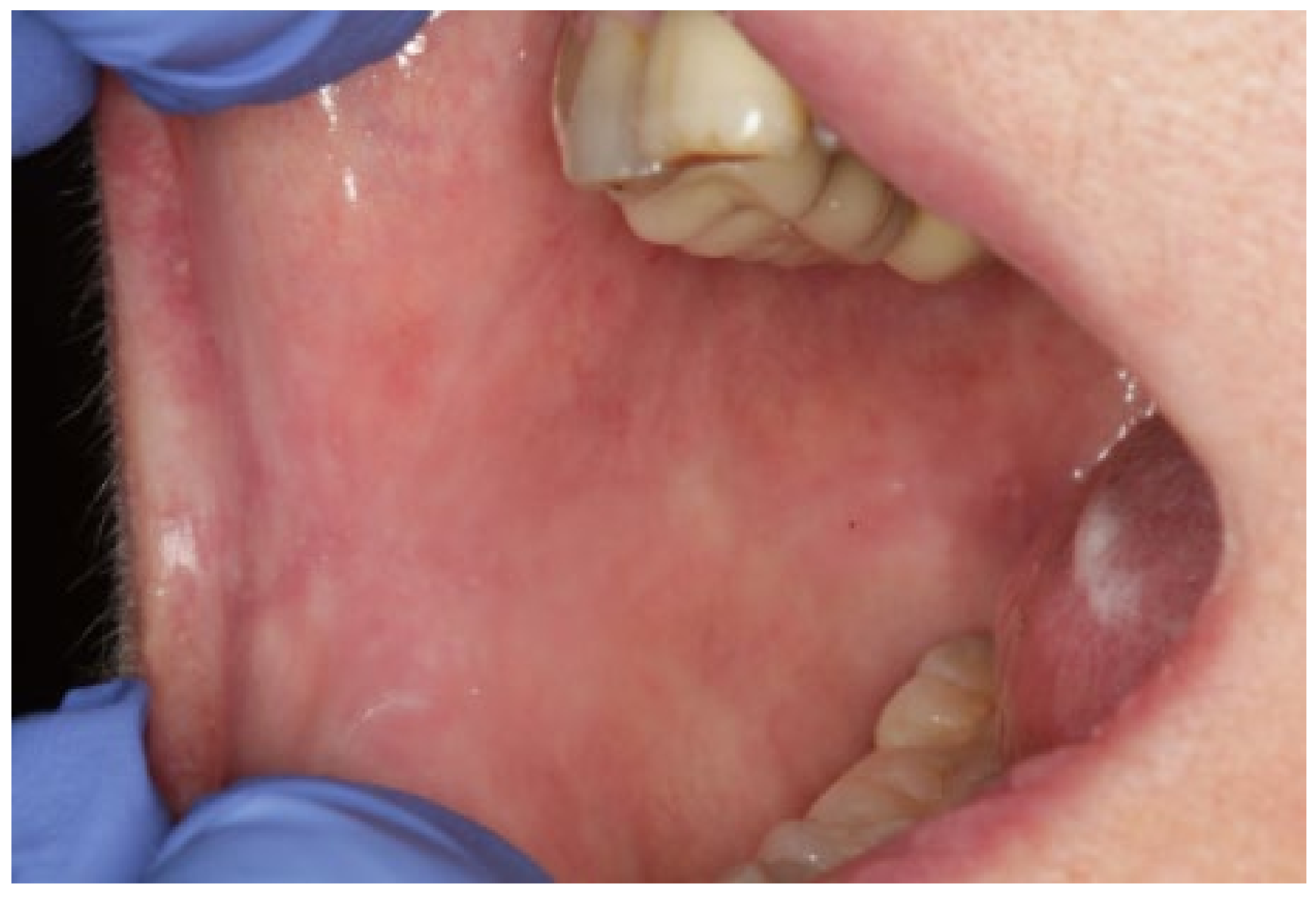
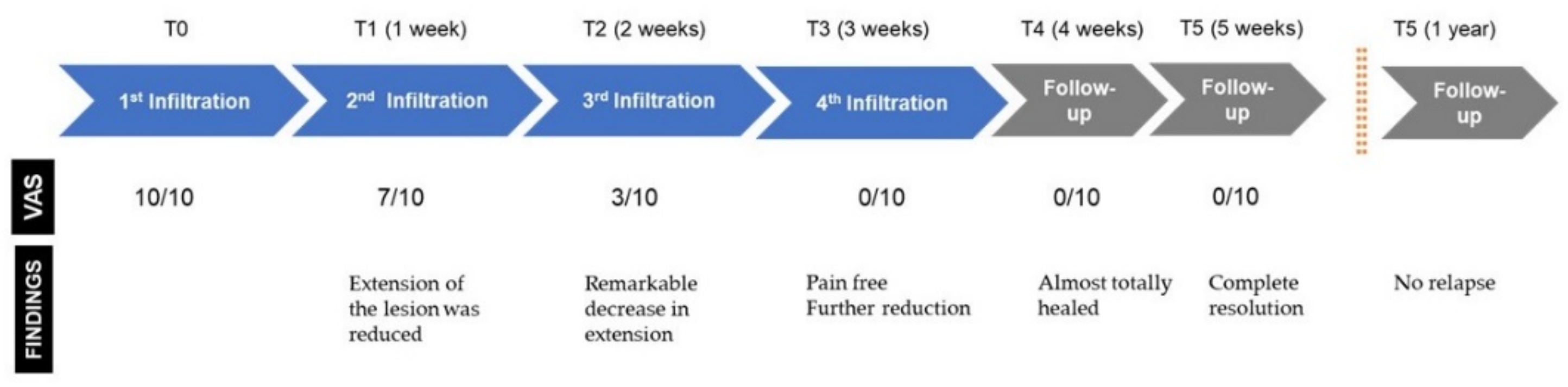
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leuci, S.; Ruoppo, E.; Adamo, D.; Calabria, E.; Mignogna, M.D. Oral autoimmune vesicobullous diseases: Classification, clinical presentations, molecular mechanisms, diagnostic algorithms, and management. Periodontology 2000 2019, 80, 77–88. [Google Scholar] [CrossRef]
- Rashid, H.; Lamberts, A.; Diercks, G.F.H.; Pas, H.H.; Meijer, J.M.; Bolling, M.C.; Horváth, B. Oral Lesions in Autoimmune Bullous Diseases: An Overview of Clinical Characteristics and Diagnostic Algorithm. Am. J. Clin. Derm. 2019, 20, 847–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, B.; Setterfield, J. Mucous membrane pemphigoid and oral blistering diseases. Clin. Exp. Derm. 2019, 44, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Bagan, J.; Jiménez, Y.; Murillo, J.; Bagan, L. Oral mucous membrane pemphigoid: A clinical study of 100 low-risk cases. Oral Dis. 2018, 24, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Montagnon, C.M.; Tolkachjov, S.N.; Murrell, D.F.; Camilleri, M.J.; Lehman, J.S. Subepithelial autoimmune blistering dermatoses: Clinical features and diagnosis. J. Am. Acad. Derm. 2021, 85, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kamaguchi, M.; Iwata, H. The Diagnosis and Blistering Mechanisms of Mucous Membrane Pemphigoid. Front. Immunol. 2019, 10, 34. [Google Scholar] [CrossRef]
- Madgar, O.; Baniel, A.; Yarom, N.; Glikson, E.; Zeeli, T.; Sprecher, E.; Alon, E.E. Mucous membrane pemphigoid-otorhinolaryngological manifestations: A retrospective cohort study. Eur. Arch. Otorhinolaryngol. 2020, 277, 939–945. [Google Scholar] [CrossRef]
- Kuten-Shorrer, M.; Menon, R.S.; Lerman, M.A. Mucocutaneous Diseases. Dent. Clin. N. Am. 2020, 64, 139–162. [Google Scholar] [CrossRef]
- Patel, S.; Kumar, S.; Laudenbach, J.M.; Teruel, A. Mucocutaneous Diseases: Oral Lichen Planus, Mucous Membrane Pemphigoid and Pemphigus Vulgaris. J. Calif. Dent. Assoc. 2016, 44, 561–570. [Google Scholar]
- Feller, L.; Ballyram, R.; Khammissa, R.A.; Altini, M.; Lemmer, J. Immunopathogenic Oral Diseases: An Overview Focusing on Pemphigus Vulgaris and Mucous Membrane Pemphigoid. Oral Health Prev. Dent. 2017, 15, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonavoglia, A.; Leone, P.; Dammacco, R.; Di Lernia, G.; Petruzzi, M.; Bonamonte, D.; Vacca, A.; Racanelli, V.; Dammacco, F. Pemphigus and mucous membrane pemphigoid: An update from diagnosis to therapy. Autoimmun. Rev. 2019, 18, 349–358. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Devillez, R.L.; Henning, J.S. Recalcitrant cicatricial pemphigoid treated with the anti-TNF-alpha agent etanercept. J. Drugs Derm. 2010, 9, 68–70. [Google Scholar]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control. Release 2012, 157, 29–38. [Google Scholar] [CrossRef]
- Barrientos, S.; Brem, H.; Stojadinovic, O.; Tomic-Canic, M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014, 22, 569–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitua, E.; Zalduendo, M.; Troya, M.; Padilla, S.; Orive, G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS ONE 2015, 10, e0121713. [Google Scholar] [CrossRef]
- Sagi, Z.; Hieronymus, T. The Impact of the Epithelial-Mesenchymal Transition Regulator Hepatocyte Growth Factor Receptor/Met on Skin Immunity by Modulating Langerhans Cell Migration. Front. Immunol. 2018, 9, 517. [Google Scholar] [CrossRef] [Green Version]
- Anitua, E.; Muruzabal, F.; de la Fuente, M.; Riestra, A.; Merayo-Lloves, J.; Orive, G. PRGF exerts more potent proliferative and anti-inflammatory effects than autologous serum on a cell culture inflammatory model. Exp. Eye Res. 2016, 151, 115–121. [Google Scholar] [CrossRef]
- Tabatabaei-Panah, P.-S.; Moravvej, H.; Sadaf, Z.; Babaei, H.; Geranmayeh, M.; Hajmanouchehri, S.; Karimi, A.; Sajjadi, F.; Arghand, F.; Ludwig, R.J.; et al. Proinflammatory Cytokine Gene Polymorphisms in Bullous Pemphigoid. Front. Immunol. 2019, 10, 636. [Google Scholar] [CrossRef]
- EL-Komy, M.H.; Hassan, A.S.; Abdel Raheem, H.M.; Doss, S.S.; EL-Kaliouby, M.; Saleh, N.A.; Saleh, M.A. Platelet-rich plasma for resistant oral erosions of pemphigus vulgaris: A pilot study. Wound Repair Regen. 2015, 23, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Piñas, L.; Alkhraisat, M.H.; Fernández, R.S.; Anitua, E. Biological Therapy of Refractory Ulcerative Oral Lichen Planus with Plasma Rich in Growth Factors. Am. J. Clin. Derm. 2017, 18, 429–433. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Alkhraisat, M.H.; Eguia, A.; Piñas, L. Oral Pemphigoid Recalcitrant Lesion Treated with PRGF Infiltration. Case Report. Dent. J. 2021, 9, 137. https://doi.org/10.3390/dj9110137
Anitua E, Alkhraisat MH, Eguia A, Piñas L. Oral Pemphigoid Recalcitrant Lesion Treated with PRGF Infiltration. Case Report. Dentistry Journal. 2021; 9(11):137. https://doi.org/10.3390/dj9110137
Chicago/Turabian StyleAnitua, Eduardo, Mohammad H. Alkhraisat, Asier Eguia, and Laura Piñas. 2021. "Oral Pemphigoid Recalcitrant Lesion Treated with PRGF Infiltration. Case Report" Dentistry Journal 9, no. 11: 137. https://doi.org/10.3390/dj9110137
APA StyleAnitua, E., Alkhraisat, M. H., Eguia, A., & Piñas, L. (2021). Oral Pemphigoid Recalcitrant Lesion Treated with PRGF Infiltration. Case Report. Dentistry Journal, 9(11), 137. https://doi.org/10.3390/dj9110137






