Influence of the Use of a Collagen Membrane Placed on the Bone Window after Sinus Floor Augmentation—An Experimental Study in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design and Experimental Animals
2.3. Randomization and Allocation Concealment
2.4. Sample Size
2.5. Biomaterials
2.6. Clinical Procedures
2.7. Maintenance Care
2.8. Euthanasia
2.9. Preparation of Paraffin Sections
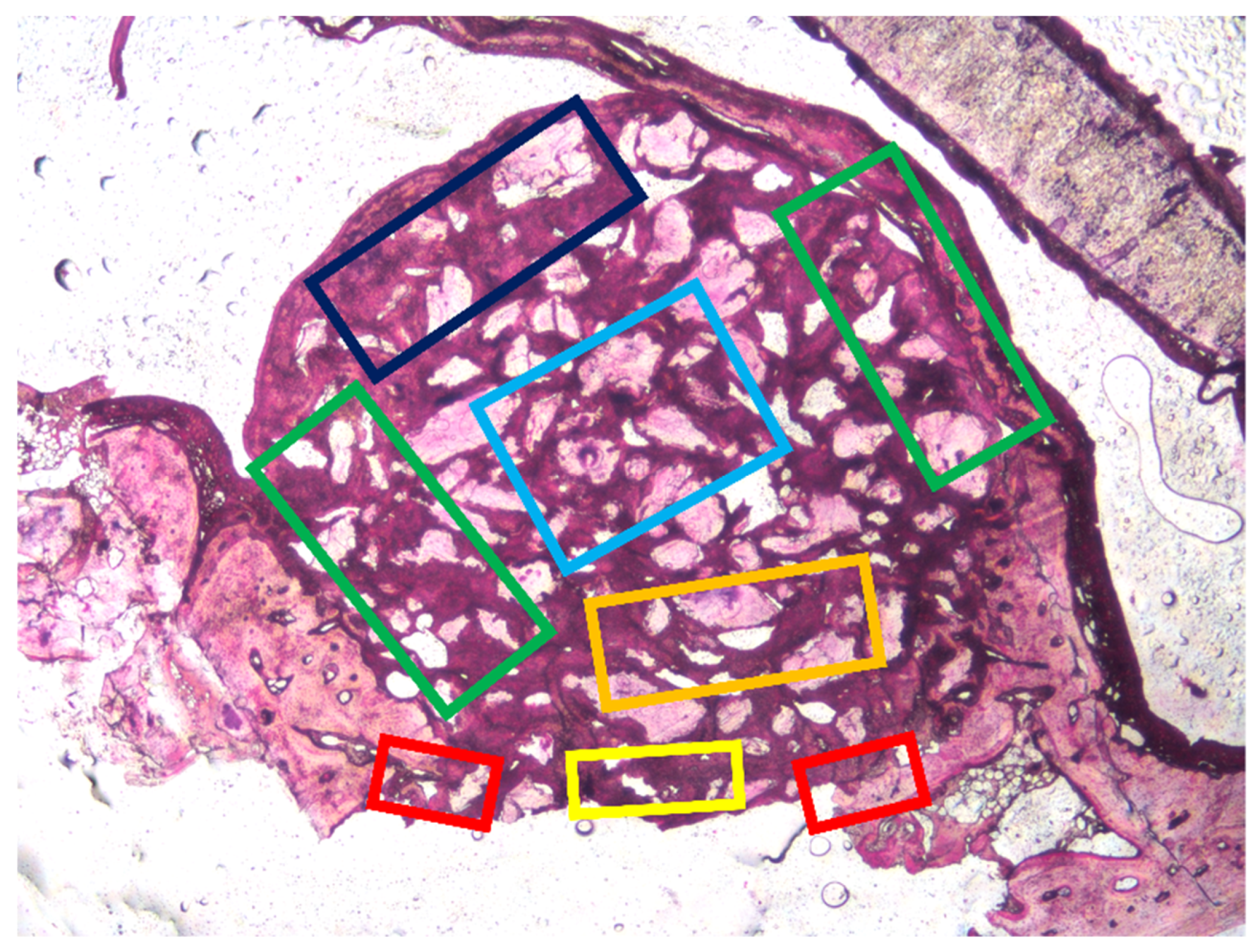
2.10. Histo-Morphometric Analysis
2.11. Data Analysis
3. Results
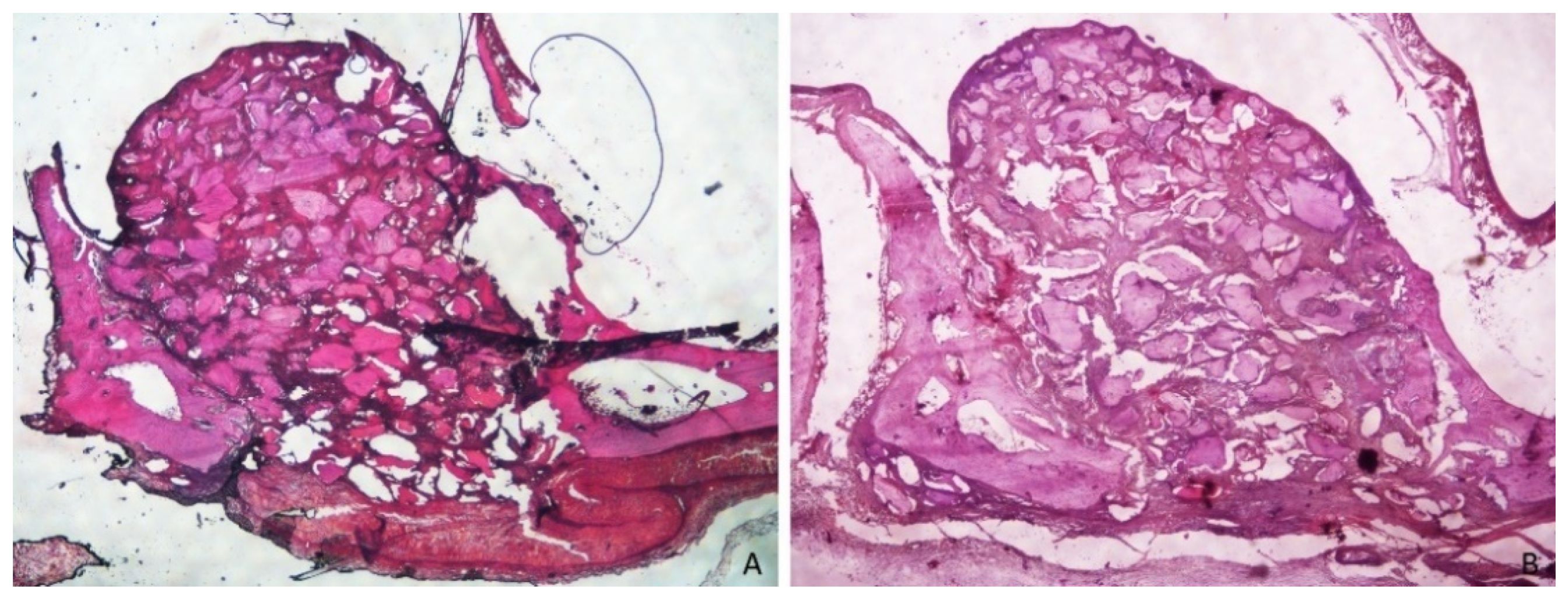
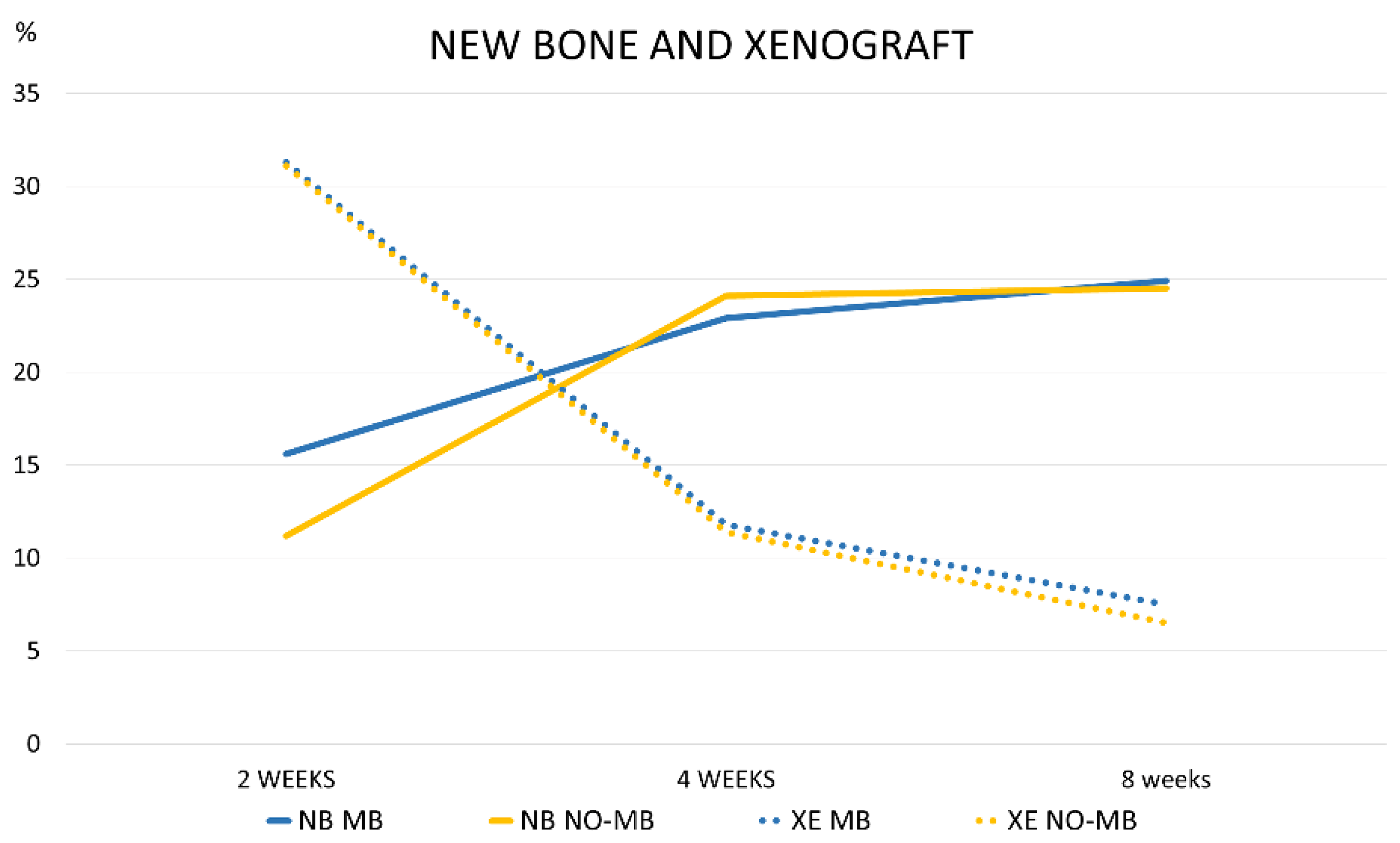
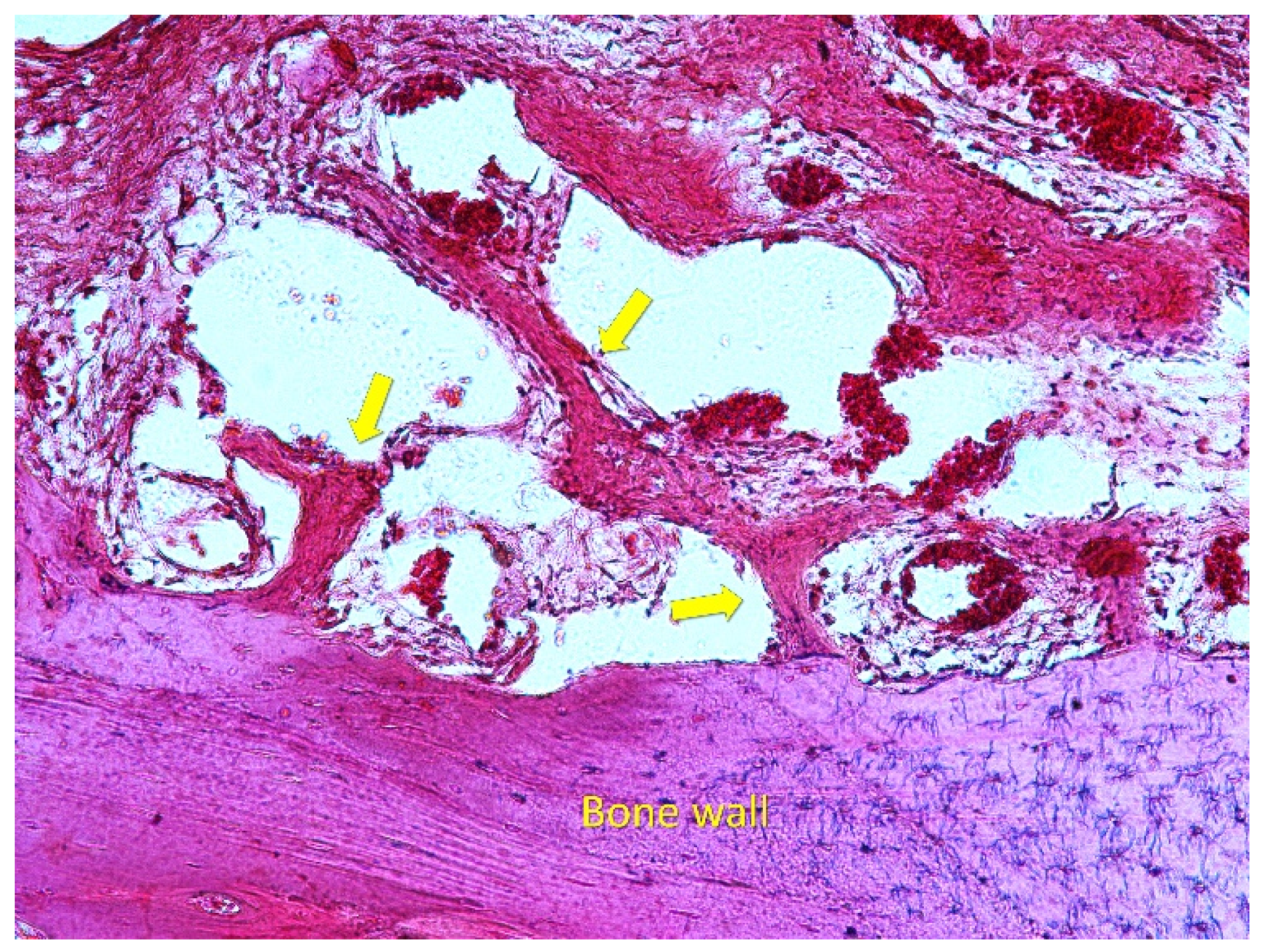
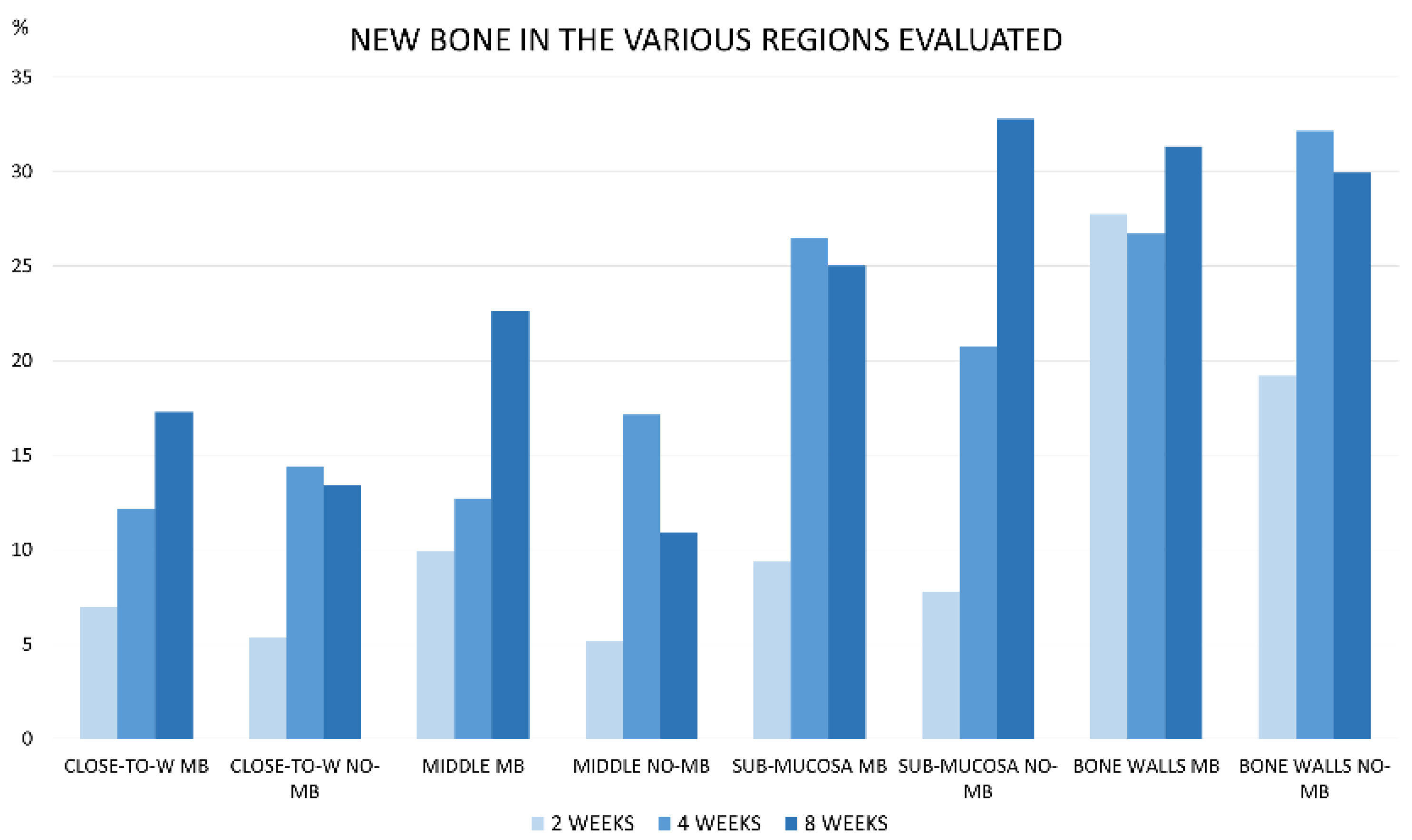
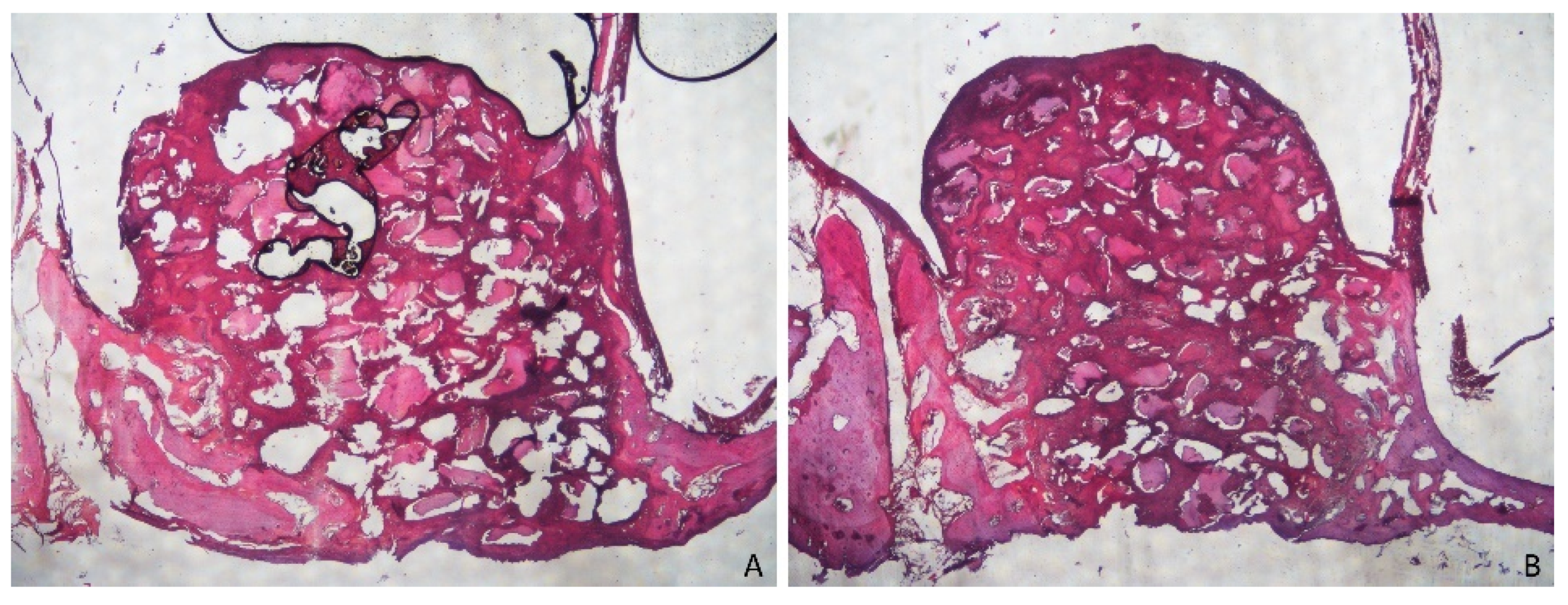
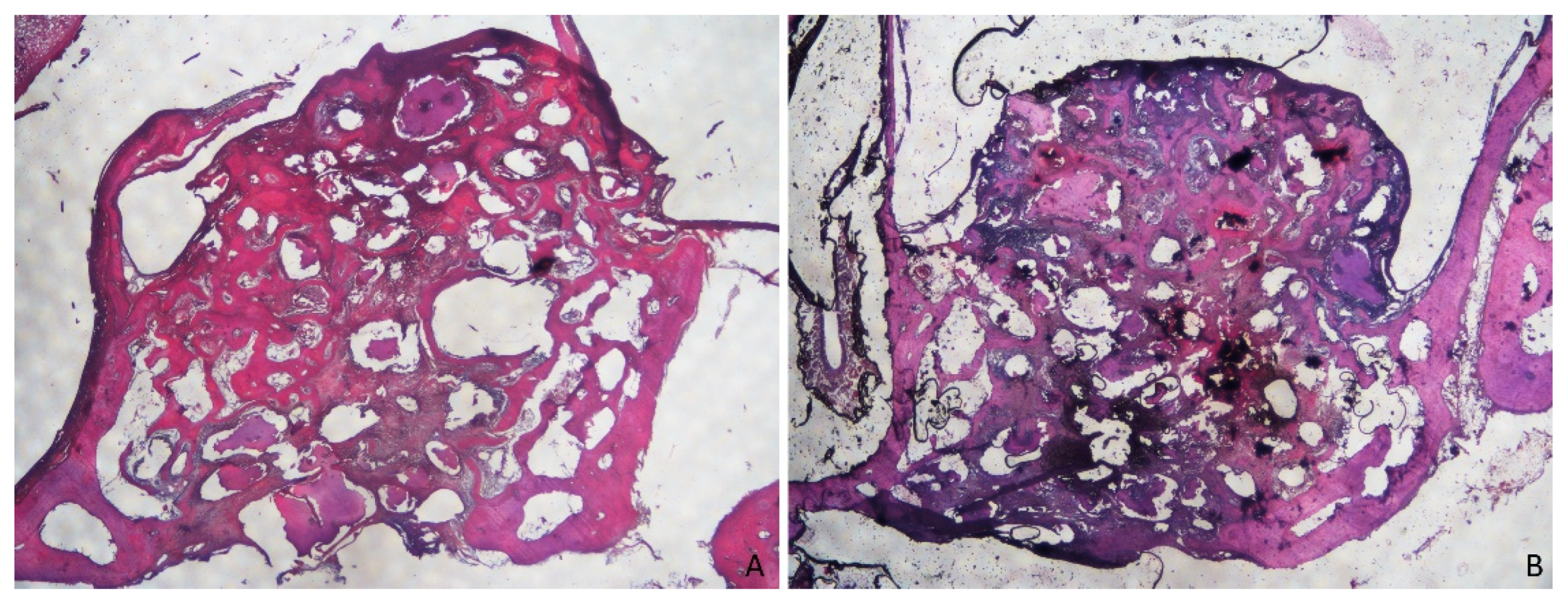
3.1. Grafted Region
3.2. Osteotomy Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tatum, H., Jr. Maxillary sinus grafting for endosseous implants. In Lecture Presented at the Annual Meeting of the Alabama Implant Study Group; Birmingham, AL, USA, 1977. [Google Scholar]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 216–240. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Wallace, S.S.; Testori, T. Long-term implant survival in the grafted maxillary sinus: A systematic review. Int. J. Periodontics Restor. Dent. 2013, 33, 773–783. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Del Fabbro, M. Long-term outcomes for the treatment of atrophic posterior maxilla: A systematic review of literature. Clin. Implant Dent. Relat. Res. 2015, 17, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Aludden, H.; Hallman, M.; Dahlin, C.; Christensen, A.E.; Mordenfeld, A. A systematic review and meta-analysis of long-term studies (five or more years) assessing maxillary sinus floor augmentation. Int. J. Oral Maxillofac. Surg. 2018, 47, 103–116. [Google Scholar] [CrossRef]
- Juzikis, E.; Gaubys, A.; Rusilas, H. Uses of maxillary sinus lateral wall bony window in an open window sinus lift procedure: Literature review. Stomatologija 2018, 20, 14–21. [Google Scholar] [PubMed]
- Perini, A.; Ferrante, G.; Sivolella, S.; Urbizo Velez, J.; Bengazi, F.; Botticelli, D. Bone plate repositioned over the antrostomy after sinus floor elevation: An experimental study in sheep. Int. J. Implant Dent. 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- García-Denche, J.T.; Wu, X.; Martinez, P.P.; Eimar, H.; Ikbal, D.J.; Hernández, G.; López-Cabarcos, E.; Fernandez-Tresguerres, I.; Tamimi, F. Membranes over the lateral window in sinus augmentation procedures: A two-arm and split-mouth randomized clinical trials. J. Clin. Periodontol. 2013, 40, 1043–1051. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Wallace, S.S.; Froum, S.J.; Rohrer, M.D.; Cho, S.C. Histologic and clinical comparison of bilateral sinus floor elevations with and without barrier membrane placement in 12 patients: Part 3 of an ongoing prospective study. Int. J. Periodontics Restor. Dent. 2000, 20, 117–125. [Google Scholar]
- Wallace, S.S.; Froum, S.J.; Cho, S.C.; Elian, N.; Monteiro, D.; Kim, B.S.; Tarnow, D.P. Sinus augmentation utilizing anorganic bovine bone (Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: Histomorphometric and clinical analyses. Int. J. Periodontics Restor. Dent. 2005, 25, 551–559. [Google Scholar]
- Suárez-López Del Amo, F.; Ortega-Oller, I.; Catena, A.; Monje, A.; Khoshkam, V.; Torrecillas-Martínez, L.; Wang, H.L.; Galindo-Moreno, P. Effect of barrier membranes on the outcomes of maxillary sinus floor augmentation: A meta-analysis of histomorphometric outcomes. Int. J. Oral Maxillofac. Implant 2015, 30, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.S.; Kan, J.Y.; Boyne, P.J.; Goodacre, C.J.; Lozada, J.L.; Rungcharassaeng, K. The effects of resorbable membrane on human maxillary sinus graft: A pilot study. Int. J. Oral Maxillofac. Implant 2009, 24, 73–80. [Google Scholar]
- Barone, A.; Ricci, M.; Covani, U.; Nannmark, U.; Azarmehr, I.; Calvo-Guirado, J.L. Maxillary sinus augmentation using prehydrated corticocancellous porcine bone: Hystomorphometric evaluation after 6 months. Clin. Implant Dent. Relat. Res. 2013, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchi, G.; Lollobrigida, M.; Laurito, D.; Di Nardo, D.; Berlutti, F.; Passariello, C.; Serafini, G.; Testarelli, L.; De Biase, A. Microbiological and FE-SEM Assessment of d-PTFE Membrane Exposed to Oral Environment after Alveolar Socket Preservation Managed with Granular nc-HA. J. Contemp. Dent. Pract. 2020, 21, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lin, G.-H.; Monje, A.; Chan, H.-L.; Wang, H.-L. Wound healing complications following guided bone regeneration for ridge augmentation: A Systematic Review and meta-Analysis. Int. J. Oral Maxillofac. Implant 2018, 33, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.-L.; Kapila, Y.; Lin, G.-H. Effects of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implant Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, S.; Botticelli, D.; Nakajima, Y.; Sakuma, S.; Baba, S. Anatomical analyses for maxillary sinus floor augmentation with a lateral approach: A cone beam computed tomography study. Ann. Anat. 2019, 226, 29–34. [Google Scholar] [CrossRef]
- Sakuma, S.; Ferri, M.; Imai, H.; Fortich Mesa, N.; Blanco Victorio, D.J.; Apaza Alccayhuaman, K.A.; Botticelli, D. Involvement of the maxillary sinus ostium (MSO) in the edematous processes after sinus floor augmentation: A cone-beam computed tomographic study. Int. J. Implant Dent. 2020, 6, 35. [Google Scholar] [CrossRef]
- Testori, T.; Weinstein, T.; Taschieri, S.; Wallace, S. Risk factors in lateral window sinus elevation surgery. Periodontology 2000 2019, 81, 91–123. [Google Scholar] [CrossRef]
- Alhammadi, M.S.; Al-Mashraqi, A.A.; Alnami, R.H.; Ashqar, N.M.; Alamir, O.H.; Halboub, E.; Reda, R.; Testarelli, L.; Patil, S. Accuracy and Reproducibility of Facial Measurements of Digital Photographs and Wrapped Cone Beam Computed Tomography (CBCT) Photographs. Diagnostics 2021, 11, 757. [Google Scholar] [CrossRef]
- De Stavola, L.; Fincato, A.; Albiero, A.M. A computer guided bone block harvesting procedure: A proof-of-principle case report and technical notes. Int. J. Oral Maxillofac. Implant 2015, 30, 1409–1413. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Shimizu, Y.; Asai, S.; Ooya, K. Experimental sinus grafting with the use of deproteinized bone particles of different sizes. Clin. Oral Implant Res. 2003, 14, 548–555. [Google Scholar] [CrossRef]
- Iida, T.; Silva, E.R.; Lang, N.P.; Apaza Alccayhuaman, K.A.; Botticelli, D.; Xavier, S.P. Histological and micro-computed tomography evaluations of newly formed bone after maxillary sinus augmentation using a xenograft with similar density and mineral content of bone: An experimental study in rabbits. Clin. Exp. Dent. Res. 2018, 4, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Omori, Y.; Ricardo Silva, E.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Lang, N.P.; Xavier, S.P. Reposition of the bone plate over the antrostomy in maxillary sinus augmentation: A histomorphometric study in rabbits. Clin. Oral Implant Res. 2018, 29, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, D.A.; Choi, I.G.G.; Arita, E.S.; Cortes, A.R.G. Estimating bone mineral density using MRI in medicine and dentistry: A literature review. Oral Radiol. 2021, 37, 366–375. [Google Scholar] [CrossRef]
- Masuda, K.; Silva, E.R.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Xavier, S.P. Antrostomy preparation for maxillary sinus floor augmentation using drills or a sonic instrument: A microcomputed tomography and histomorphometric study in rabbits. Int. J. Oral Maxillofac. Implant 2019, 34, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Busenlechner, D.; Huber, C.D.; Vasak, C.; Dobsak, A.; Gruber, R.; Watzek, G. Sinus augmentation analysis revised: The gradient of graft consolidation. Clin. Oral Implant Res. 2009, 20, 1078–1083. [Google Scholar] [CrossRef]
- Scala, A.; Botticelli, D.; Garcia Rangel, I., Jr.; de Oliveira, J.A.; Okamoto, R.; Lang, N.P. Early healing after elevation of the maxillary sinus floor applying a lateral access: A histological study in monkeys. Clin. Oral Implant Res. 2010, 21, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Botticelli, D.; Faeda, R.S.; Garcia Rangel, I., Jr.; Américo de Oliveira, J.; Lang, N.P. Lack of influence of the Schneiderian membrane in forming new bone apical to implants simultaneously installed with sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant Res. 2013, 23, 175–181. [Google Scholar] [CrossRef]
- Scala, A.; Lang, N.P.; de Carvalho Cardoso, L.; Pantani, F.; Schweikert, M.; Botticelli, D. Sequential healing of the elevated sinus floor after applying autologous bone grafting: An experimental study in minipigs. Clin. Oral Implant Res. 2015, 26, 419–425. [Google Scholar] [CrossRef]
- Favero, V.; Lang, N.P.; Canullo, L.; Urbizo Velez, J.; Bengazi, F.; Botticelli, D. Sinus floor elevation outcomes following perforation of the Schneiderian membrane. Exp. Study Sheep. Clin. Oral Implant Res. 2015, 27, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Lang, N.P.; Garcia Rangel, I.J.; Ferreira, S.; Caneva, M.; De Santis, E.; Botticelli, D. Sinus mucosa elevation using Bio-Oss® or Gingistat® collagen sponge: An experimental study in rabbits. Clin. Oral Implant Res. 2017, 28, e21–e30. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Lang, N.P. Dynamics of osseointegration in various human and animal models—A comparative analysis. Clin. Oral Implant Res. 2017, 28, 742–748. [Google Scholar] [CrossRef] [PubMed]

| New Mineralized Bone | Xenograft | |
|---|---|---|
| 2 WEEKS MB | 15.6 ± 7.3 18.5 (11.3; 21.2) | 31.3 ± 8.1 29.7 (25.6; 36.5) |
| 2 WEEKS NO-MB | 11.2 ± 4.5 10.9 (8.8; 12.9) | 31.1 ± 3.5 30.9 (28.5; 32.1) |
| 4 WEEKS MB | 22.9 ± 6.1 23.0 (17.7; 28.0) | 11.8 ± 5.4 10.7 (9.7; 14.0) |
| 4 WEEKS NO-MB | 24.1 ± 5.7 24.6 (21.3; 28.8) | 11.4 ± 3.1 11.3 (9.8; 12.0) |
| 8 WEEKS MB | 24.9 ± 12.0 28.0 (14.9; 33.0) | 7.5 ± 3.5 7.7 (6.7; 8.5) |
| 8 WEEKS NO-MB | 24.5 ± 4.9 24.7 (23.3; 27.6) | 6.5 ± 7.0 3.2 (1.7; 9.6) |
| 2 WEEKS | 4 WEEKS | 8 WEEKS | ||||
|---|---|---|---|---|---|---|
| New Mineralized Bone % | Xenograft | New Mineralize Bone % | Xenograft | New Mineralize Bone % | Xenograft | |
| BONE WALLS MB | 27.8 ± 12.0 32.0 (22.1; 35.0) | 18.6 ± 8.2 15.9 (14.7; 19.1) | 27.9 ± 7.6 * 25.7 (24.9; 29.4) | 7.4 ± 5.1 7.2 (3.7; 11.3) | 31.3 ± 8.9 35.9 (23.9; 38.7) | 3.5 ± 2.2 3.5 (2.3; 5.1) |
| BONE WALLS NO-MB | 19.2 ± 5.2 19.3 (15.7; 22.1) | 20.6 ± 11.3 17.1 (13.9; 25.1) | 32.9 ± 9.5 * 30.6 (28.2; 38.4) | 5.4 ± 3.0 7.0 (4.7; 7.2) | 29.9 ± 9.1 32.9 (23.3; 37.4) | 2.1 ± 3.1 0.6 (0.0; 3.1) |
| SUB-MUCOSA MB | 9.4 ± 5.7 8.0 (5.7; 12.5) | 42.5 ± 6.8 39.8 (37.8; 47.0) | 26.4 ± 9.0 27.7 (19.3; 34.9) | 13.1 ± 8.4 11.7 (8.5; 15.6) | 25.0 ± 10.1 23.3 (19.1; 32.8) | 9.5 ± 3.1 10.6 (8.0; 11.7) |
| SUB-MUCOSA NO-MB | 7.8 ± 5.2 5.5 (4.3; 10.9) | 36.1 ± 8.9 37.1 (29.3; 42.4) | 21.4 ± 9.6 23.9 (12.4; 27.5) | 15.1 ± 4.9 14.3 (10.9; 18.4) | 32.8 ± 9.1 34.0 (30.8; 38.2) | 9.2 ± 8.3 7.9 (2.4; 15.9) |
| MIDDLE MB | 9.9 ± 5.9 12.7 (6.7; 13.1) | 32.6 ± 15.8 38.8 (25.4; 42.0) | 15.3 ± 8.8 11.2 (8.7; 19.9) | 17.5 ± 8.4 16.4 (13.3; 25.3) | 22.6 ± 21.6 15.5 (5.0; 36.6) | 10.5 ± 5.0 11.9 (7.6; 13.6) |
| MIDDLE NO-MB | 5.2 ± 4.0 5.1(2.5; 8.1) | 38.7 ± 10.0 36.1 (32.9; 44.0) | 20.0 ± 11.9 18.2 (10.4; 24.1) | 17.8 ± 10.6 14.8 (10.4; 20.5) | 10.9 ± 11.5 9.2 (2.3; 15.8) | 8.2 ± 10.3 6.8 (0.5; 10.3) |
| CLOSE-TO-WINDOW MB | 7.0 ± 5.1 6.3 (3.7; 10.5) | 33.7 ± 16.9 36.8 (23.6; 46.0) | 13.9 ± 6.3 11.5 (9.6; 17.2) | 14.8 ± 8.4 11.6 (9.9; 16.8) | 17.3 ± 14.0 21.3 (4.5; 29.2) | 9.9 ± 11.0 5.9 (2.0; 14.3) |
| CLOSE-TO-WINDOW NO-MB | 5.3 ± 4.5 6.3 (1.3; 8.6) | 42.8 ± 13.7 40.0 (33.1; 50.4) | 13.6 ± 8.0 12.2 (7.8; 14.8) | 13.1 ± 6.8 13.9 (8.2; 18.0) | 13.4 ± 9.8 16.1 (6.6; 18.7) | 9.4 ± 13.8 4.9 (0.9; 10.3) |
| 2 WEEKS | 4 WEEKS | 8 WEEKS | |||
|---|---|---|---|---|---|
| MEMBRANE | NO MEMBRANE | MEMBRANE | NO MEMBRANE | MEMBRANE | NO MEMBRANE |
| 26.4 ± 15.4 27.1 (18.4; 32.2) | 18.2 ± 5.9 18.3 (14.9; 21.2) | 27.4 ± 10.1 22.6 (20.4; 31.9) | 32.2 ± 10.6 31.3 (24.2; 35.7) | 21.8 ± 11.6 28.2 (13.8; 30.1) | 19.1 ± 6.4 20.1 (16.7; 22.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perini, A.; Viña-Almunia, J.; Carda, C.; Martín de Llano, J.J.; Botticelli, D.; Peñarrocha-Diago, M. Influence of the Use of a Collagen Membrane Placed on the Bone Window after Sinus Floor Augmentation—An Experimental Study in Rabbits. Dent. J. 2021, 9, 131. https://doi.org/10.3390/dj9110131
Perini A, Viña-Almunia J, Carda C, Martín de Llano JJ, Botticelli D, Peñarrocha-Diago M. Influence of the Use of a Collagen Membrane Placed on the Bone Window after Sinus Floor Augmentation—An Experimental Study in Rabbits. Dentistry Journal. 2021; 9(11):131. https://doi.org/10.3390/dj9110131
Chicago/Turabian StylePerini, Alessandro, Jose Viña-Almunia, Carmen Carda, José Javier Martín de Llano, Daniele Botticelli, and Miguel Peñarrocha-Diago. 2021. "Influence of the Use of a Collagen Membrane Placed on the Bone Window after Sinus Floor Augmentation—An Experimental Study in Rabbits" Dentistry Journal 9, no. 11: 131. https://doi.org/10.3390/dj9110131
APA StylePerini, A., Viña-Almunia, J., Carda, C., Martín de Llano, J. J., Botticelli, D., & Peñarrocha-Diago, M. (2021). Influence of the Use of a Collagen Membrane Placed on the Bone Window after Sinus Floor Augmentation—An Experimental Study in Rabbits. Dentistry Journal, 9(11), 131. https://doi.org/10.3390/dj9110131







