Contact Angle and Cell Adhesion of Micro/Nano-Structured Poly(lactic-co-glycolic acid) Membranes for Dental Regenerative Therapy
Abstract
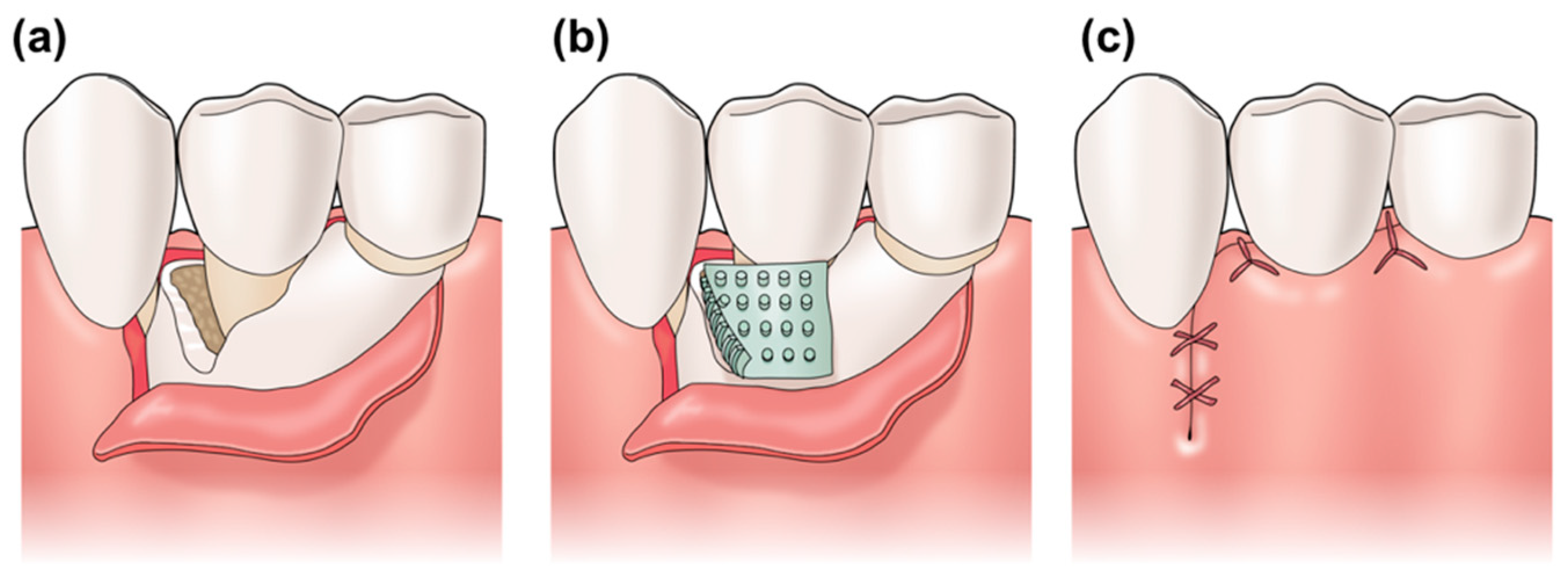
:1. Introduction
2. Materials and Methods
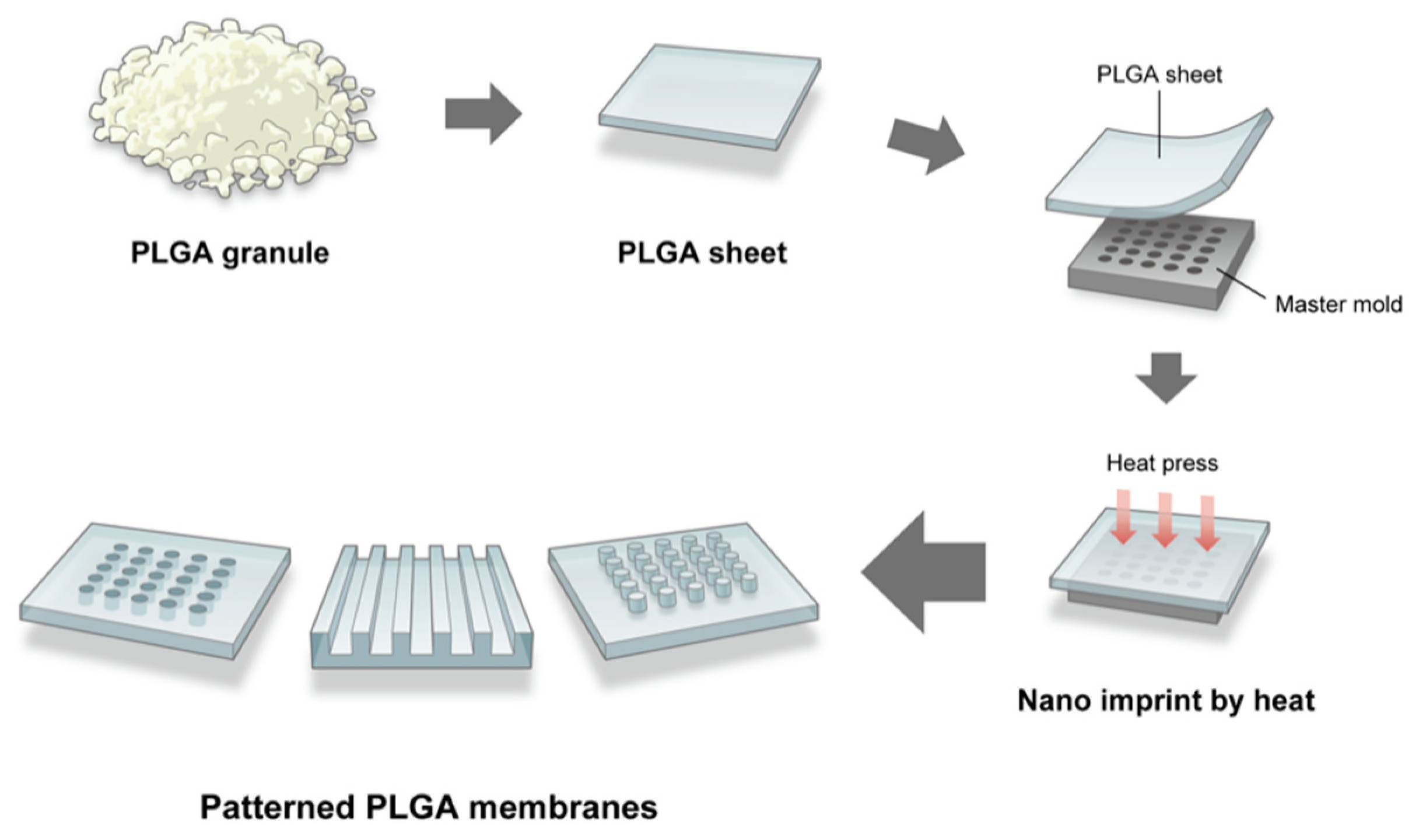
2.1. Fabrication of the Micro/Nano-Structured PLGA Membrane
2.2. Scanning Electron and Laser Microscopy
2.3. Contact Angle of the Micro/Nano-Structured PLGA Membranes
2.4. Cell Culture
2.5. Cell Adhesion Test
2.6. Confocal Laser Scanning Microscopy
2.7. Statistical Analysis
3. Results
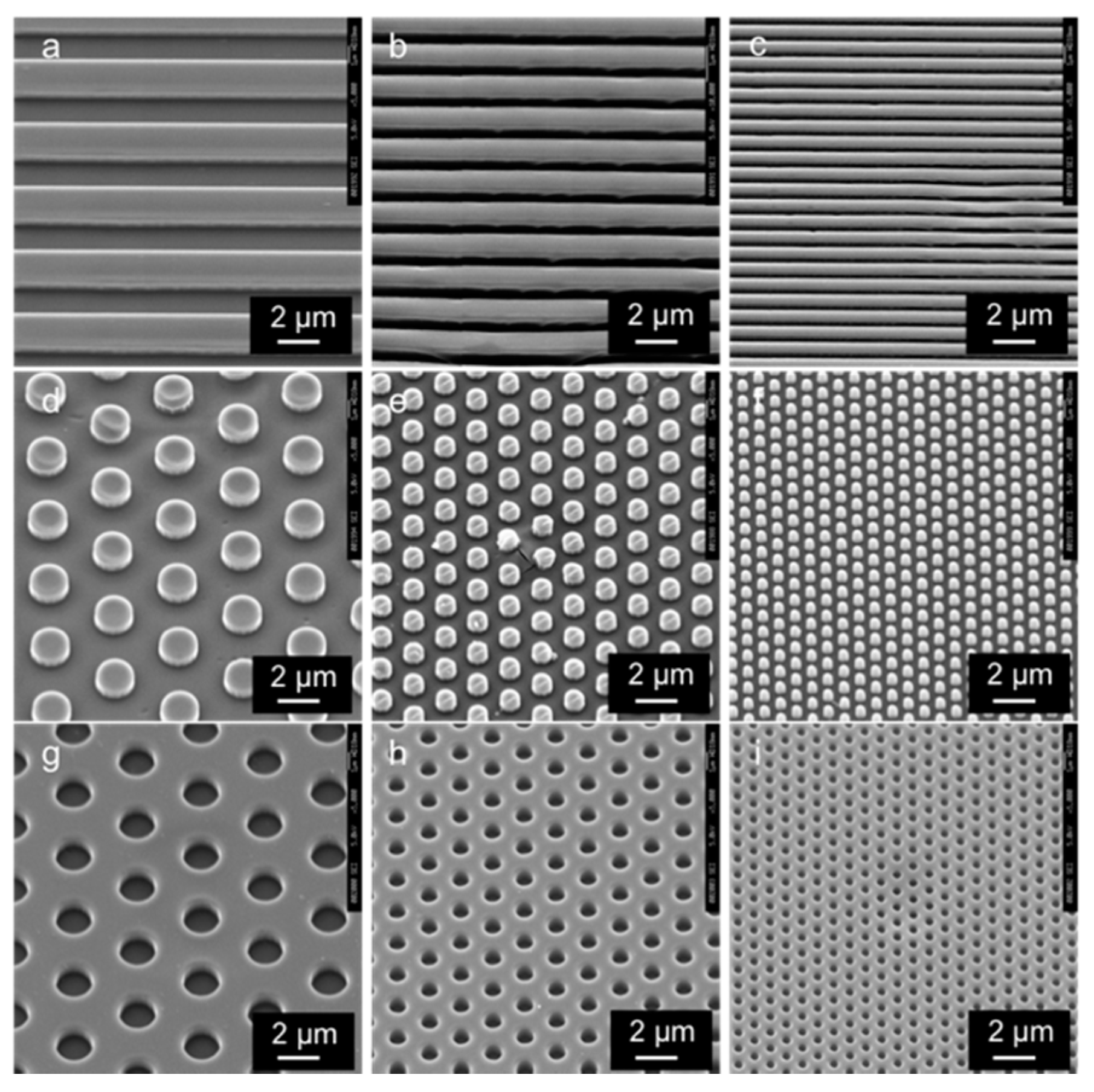
3.1. SEM Images of the Patterned PLGA Membranes
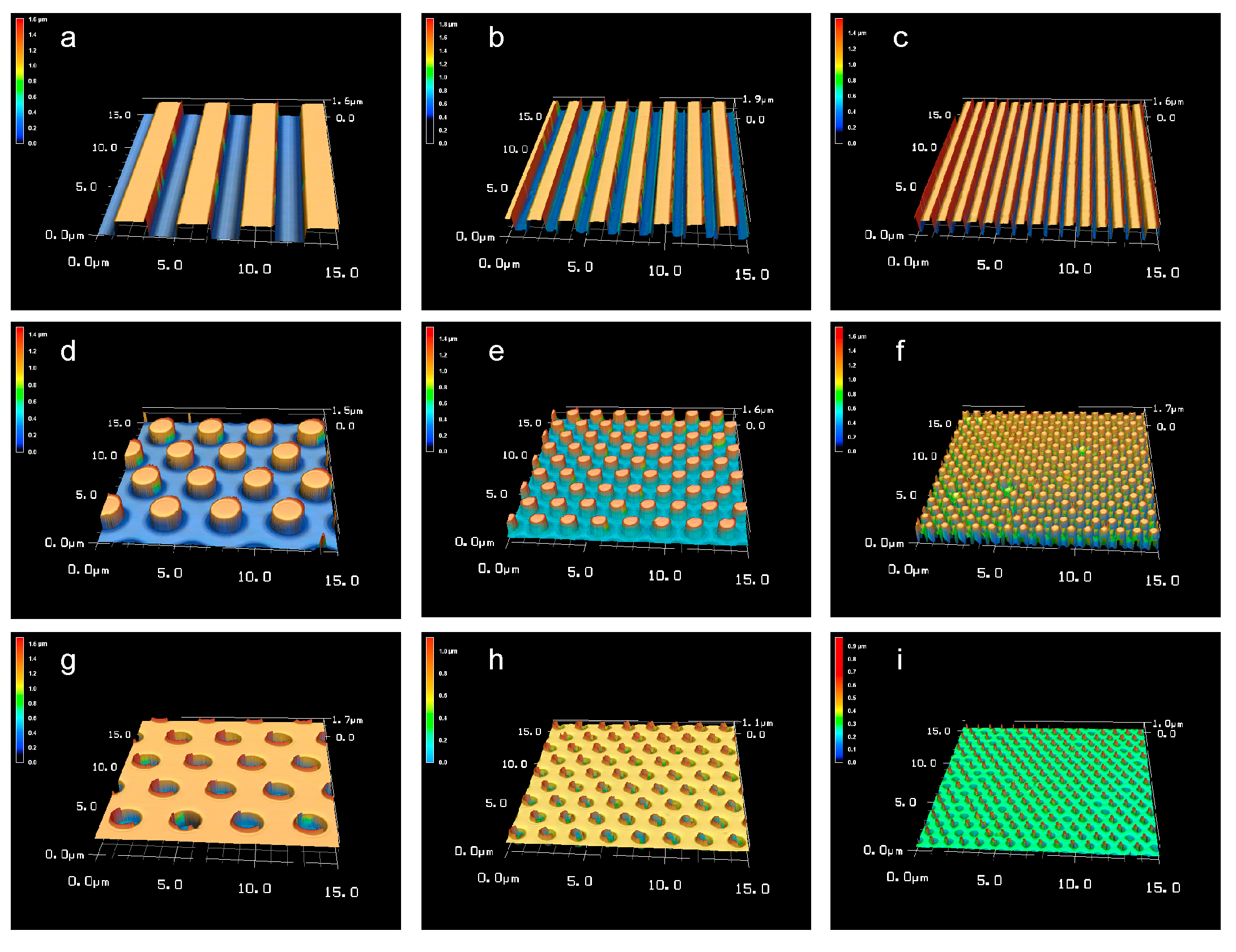
3.2. Laser Microscope Images of the Patterned PLGA Membranes
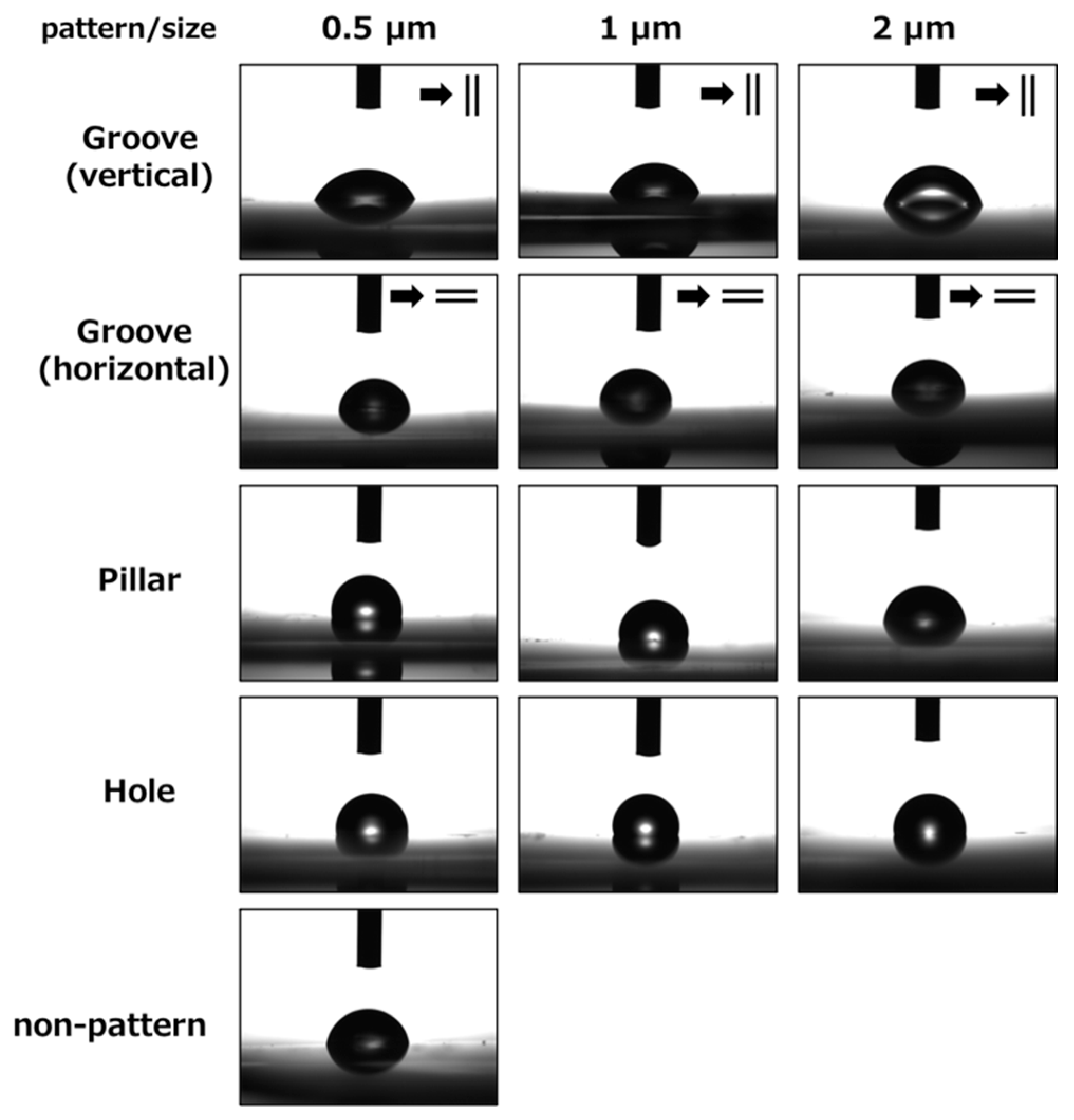
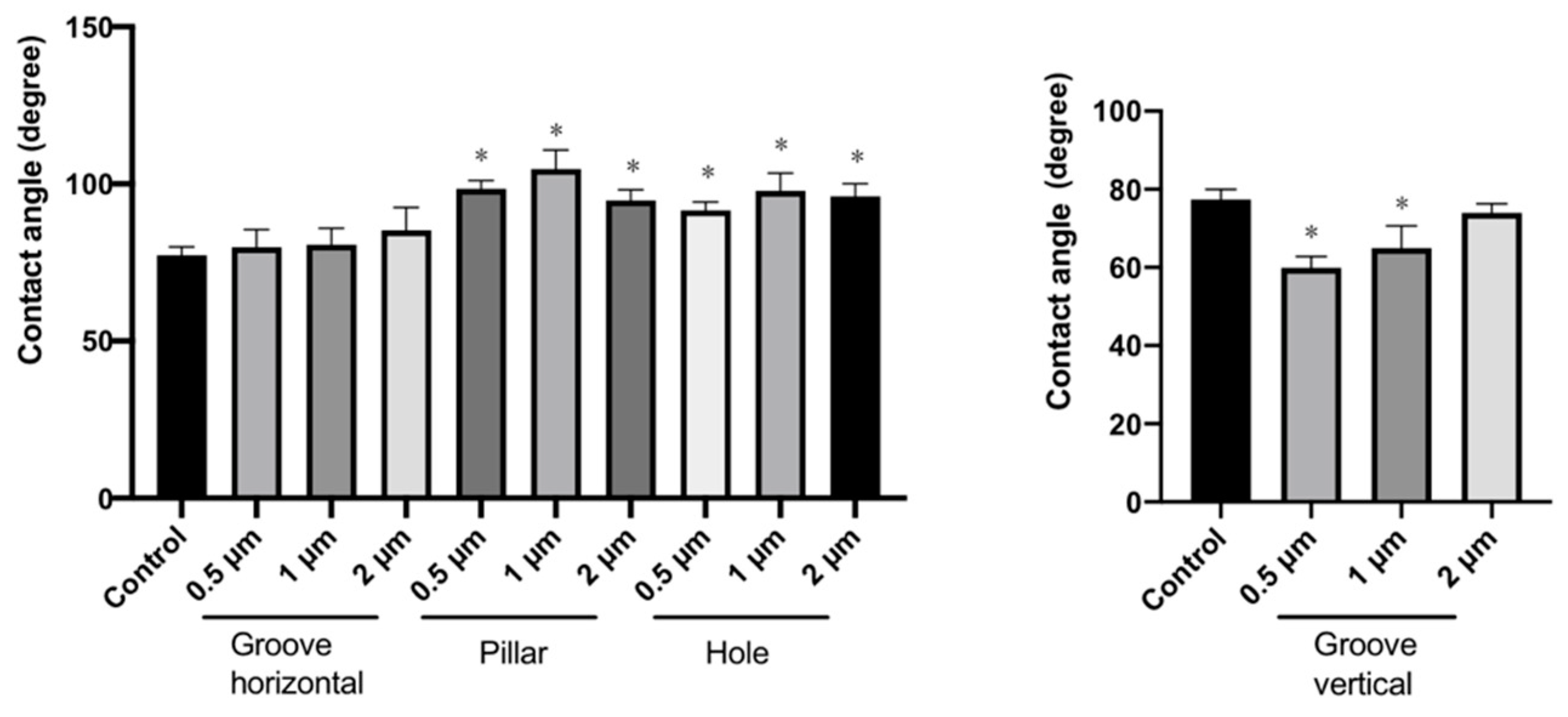
3.3. Measurement of Contact Angles of the Patterned PLGA Membranes
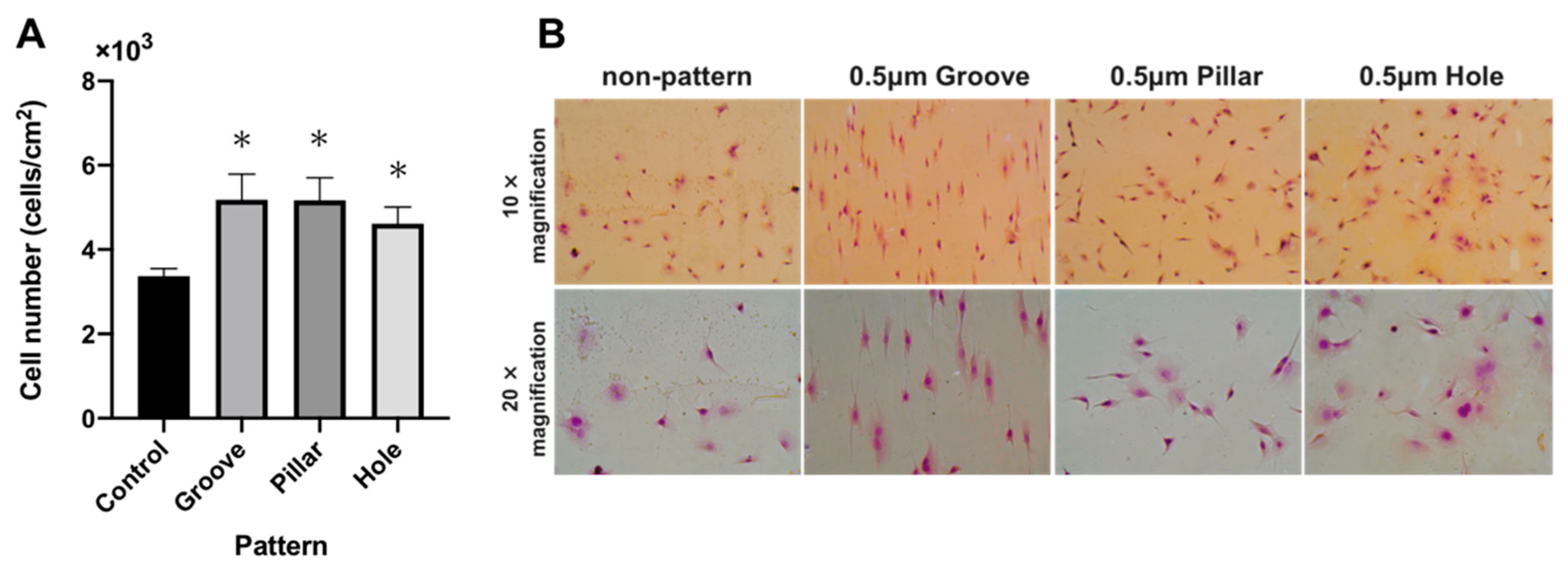
3.4. Cell Adhesion test on the Patterned PLGA Membranes
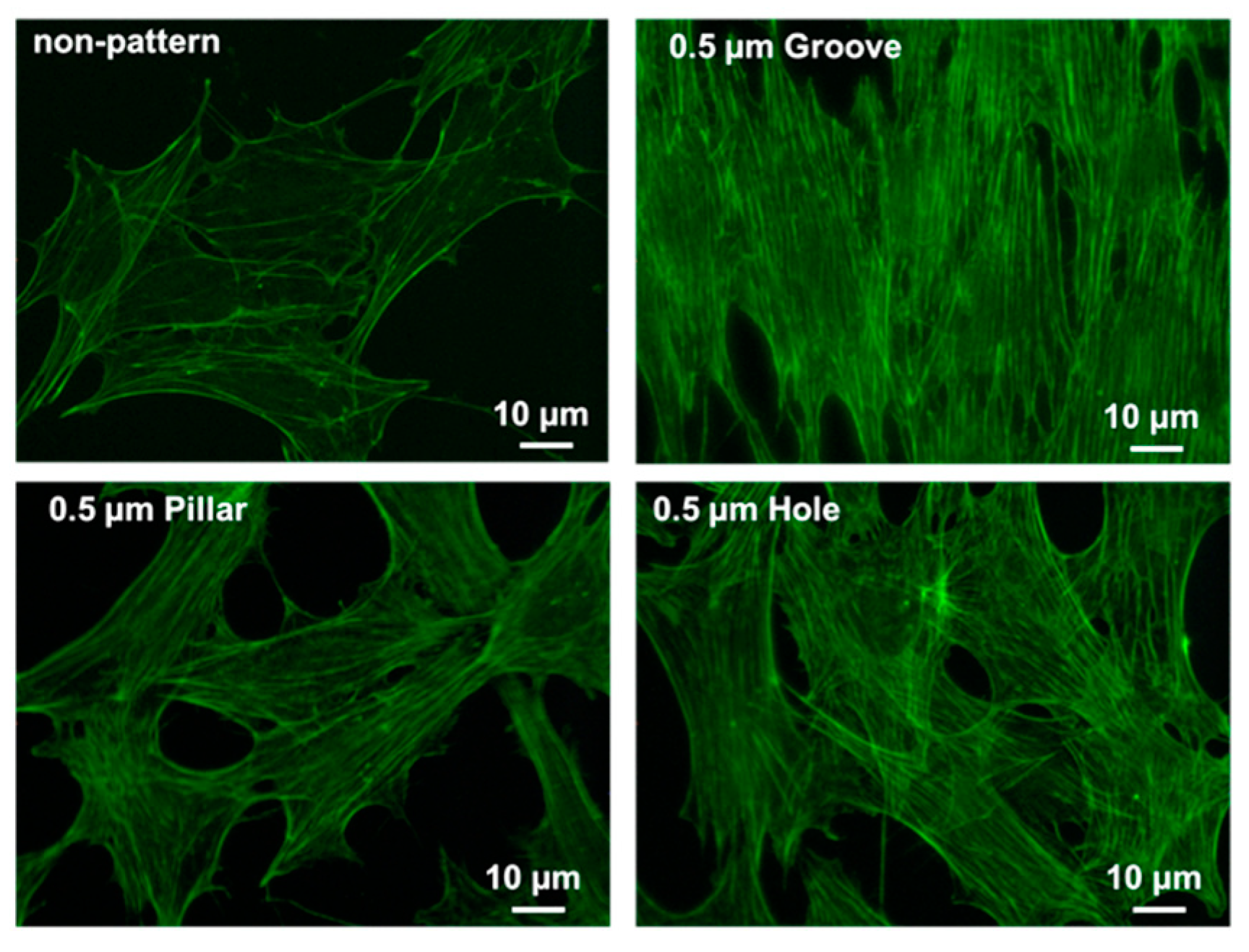
3.5. CLSM Images of Actin Filaments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villar, C.C.; Cochran, D.L. Regeneration of periodontal tissues: Guided tissue regeneration. Dent. Clin. N. Am. 2010, 54, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Gunsolley, J.C. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann. Periodontol. 2003, 8, 266–302. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. BioTechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Bombonato-Prado, K.F.; Wimmers Ferreira, M.R.; Rosa, A.L.; de Oliveira, P.T.; Jahno, V.D.; da Silva, J.B.; Ligabue, R.; Einloft, S. Human alveolar bone-derived cell-culture behaviour on biodegradable poly(L-lactic Acid). J. Biomater. Sci. Polym. Ed. 2009, 20, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Stock, U.A.; Nagashima, M.; Khalil, P.N.; Nollert, G.D.; Herdena, T.; Sperling, J.S.; Mohan, A.; Lien, J.; Martin, D.P.; Schoen, F.J.; et al. Tissue-engineered valved conduits in the pulmonary circulation. J. Thorac. Cardiovasc. Surg. 2000, 119, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Yin, A.; Zhang, K.; McClure, M.J.; Huang, C.; Wu, J.; Fang, J.; Mo, X.; Bowlin, G.L.; Al-Dayeb, S.S.; El-Newehy, M. Electrospinning collagen/chitosan/poly(L-lactic acid-co-ϵ-caprolactone) to form a vascular graft: Mechanical and biological characterization. J. Biomed. Mater. Res. A 2013, 101, 1292–1301. [Google Scholar] [CrossRef]
- Yoshimoto, I.; Sasaki, J.I.; Tsuboi, R.; Yamaguchi, S.; Kitagawa, H.; Imazato, S. Development of layered PLGA membranes for periodontal tissue regeneration. Den. Mater. 2018, 34, 538–550. [Google Scholar] [CrossRef]
- Kasaj, A.; Reichert, C.; Gotz, H.; Rohrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal regeneration–intrabony defects: A systematic review from the AAP regeneration workshop. J. Periodontol. 2015, 86, S77–S104. [Google Scholar] [CrossRef]
- Stachewiza, U.; Qiao, T.; Rawlinson, S.C.F.; Almeida, F.A.; Li, W.-Q.; Cattell, M.; Barber, A.H. 3D imaging of cell interactions with electrospun PLGA nanofiber membranes for bone regeneration. Acta Biomater. 2015, 27, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Sencadas, V.; Areias, A.C.; Gama, F.M.; Lanceros-Méndez, S. Surface roughness dependent osteoblast and fibroblast response on poly(L-lactide) films and electrospun membranes. J. Biomed. Mater. Res. Part. A 2015, 103, 2260–2268. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Shiozawa, M.; Takeuchi, H.; Akiba, Y.; Eguchi, K.; Akiba, N.; Aoyagi, Y.; Nagasawa, M.; Kuwae, H.; Izumi, K.; Uoshima, K.; et al. Biological reaction control using topography regulation of nanostructured titanium. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Akasaka, T.; Miyaji, H.; Imamura, T.; Kaga, N.; Yokoyama, A.; Yoshida, Y. Submicro-patterning of curable dental materials by molding methods: A screening trial. Dig. J. Nanomater. Biostruct. 2017, 12, 281–292. [Google Scholar]
- Kaga, N.; Horiuchi, R.; Yokoyama, A.; Akasaka, T.; Yoshida, Y. Effect of micro/nano-patterned surfaces on cell adhesion of Ca9-22 cells. e-J. Surf. Sci. Nanotechnol. 2017, 15, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.Y.; Wu, T.H.; Tsai, W.B.; Kuo, W.H.; Wang, M.J. Grooved PLGA films incorporated with RGD/YIGSR peptides for potential application on skeletal muscle tissue engineering. Colloids Surf. B 2013, 110, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Mastumoto, S.; Yamamura, S. Control of cell adhesion and detachment on a nanostructured scaffold composed of light-responsive gas-generating film. Sens. Mater. 2019, 31, 89–98. [Google Scholar]
- Mancini, L.; Romandini, M.; Fratini, A.; Americo, L.M.; Panda, S.; Marchetti, E. Biomaterials for Periodontal and Peri-Implant Regeneration. Materials 2021, 14, 3319. [Google Scholar] [CrossRef] [PubMed]
- Naenni, N.; Lim, H.C.; Strauss, F.J.; Jung, R.E.; Hämmerle, C.H.; Thoma, D.S. Local tissue effects of various barrier membranes in a rat subcutaneous model. J. Periodontal Implant. Sci. 2020, 50, 327. [Google Scholar] [CrossRef]
- Owen, G.R.; Jackson, J.; Chehroudi, B.; Burt, H.; Brunette, D.M. A PLGA membrane controlling cell behaviour for promoting tissue regeneration. Biomaterials 2005, 26, 7447–7456. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, Q.; Zheng, Y.; Nan, L.; Liao, N.; Mo, S. Potential Role of Integrin α5β1/Focal Adhesion Kinase (FAK) and Actin Cytoskeleton in the Mechanotransduction and Response of Human Gingival Fibroblasts Cultured on a 3-Dimension Lactide-Co-Glycolide (3D PLGA) Scaffold. Med. Sci. Monit. 2020, 26, e921626-1. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, E.; Mancini, L.; Bernardi, S.; Bianchi, S.; Cristiano, L.; Torge, D.; Marzo, G.; Macchiarelli, G. Evaluation of different autologous platelet concentrate biomaterials: Morphological and biological comparisons and considerations. Materials 2020, 13, 2282. [Google Scholar] [CrossRef]
- Prager-Khoutorsky, M.; Lichtenstein, A.; Krishnan, R.; Rajendran, K.; Mayo, A.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011, 13, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Kwak, S.; Haider, A.; Gupta, K.C.; Kim, S.; Kang, I.K. Micro/nano multilayered scaffolds of PLGA and collagen by alternately electrospinning for bone tissue engineering. Nanoscale Res. Lett. 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watzinger, F.; Luksch, J.; Millesi, W.; Schopper, C.; Neugebauer, J.; Moser, D.; Ewers, R. Guided bone regeneration with titanium membranes: A clinical study. Br. J. Oral Maxillofac. Surg. 2000, 38, 312–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Masui, S.; Ishihata, H. New microperforated pure titanium membrane created by laser processing for guided regeneration of bone. Br. J. Oral Maxillofac. Surg. 2018, 56, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Kaneko, T.; Endo, M.; Kanno, C.; Yamazaki, M.; Yaginuma, S.; Igarashi, H.; Honma, H.; Masui, S.; Suto, M.; et al. Comparing the efficacy of a microperforated titanium membrane for guided bone regeneration with an existing mesh retainer in dog mandibles. Materials 2021, 14, 3358. [Google Scholar] [CrossRef]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restor. Dent. 2011, 31, 265. [Google Scholar]
- Biggs, M.J.P.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.; Oreffo, R.O.C.; Dalby, M.J. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials 2016, 30, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- Kaga, N.; Akasaka, T.; Matsuura, T.; Yokoyama, A.; Yoshida, Y. Proliferation of Saos-2 and Ca9-22 cells on grooved and pillared titanium surfaces. Biomed. Mater. Eng. 2020, 30, 559–567. [Google Scholar] [CrossRef]
- Takata, R.; Akasaka, T.; Tamai, M.; Yoshimura, Y.; Taira, T.; Miyaji, H.; Tagawa, Y.; Yamagata, S.; Iida, J.; Yoshida, Y. Effect of a nano-scale fine hole pattern of the differentiation of RAW264.7 cells into osteoclasts. Dig. J. Nanomater. Biostruct. 2018, 13, 451–458. [Google Scholar]
- Makita, R.; Akasaka, T.; Tamagawa, S.; Yoshida, Y.; Miyata, S.; Miyaji, H.; Sugaya, T. Preparation of micro/nanopatterned gelatins crosslinked with genipin for biocompatible dental implants. Beilstein J. Nanotechnol. 2018, 9, 1735–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldemir, D.B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A novel bilayer polycaprolactone membrane for guided bone regeneration: Combining electrospinning and emulsion templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef] [Green Version]
| Pattern Type | Groove | Pillar | Hole | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | ||
| Pattern size | Width (μm) | 2.0 ± 0.01 | 1.0 ± 0.01 | 0.5 ± 0.02 | 2.0 ± 0.01 | 1.0 ± 0.03 | 0.5 ± 0.04 | 2.0 ± 0.03 | 1.0 ± 0.04 | 0.5 ± 0.05 |
| Height (μm) | 1.0 ± 0.01 | 1.0 ± 0.01 | 1.0 ± 0.03 | 1.0 ± 0.02 | 1.0 ± 0.06 | 0.9 ± 0.05 | 1.0 ± 0.07 | 0.9 ± 0.05 | 0.9 ± 0.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaga, N.; Fujimoto, H.; Morita, S.; Yamaguchi, Y.; Matsuura, T. Contact Angle and Cell Adhesion of Micro/Nano-Structured Poly(lactic-co-glycolic acid) Membranes for Dental Regenerative Therapy. Dent. J. 2021, 9, 124. https://doi.org/10.3390/dj9110124
Kaga N, Fujimoto H, Morita S, Yamaguchi Y, Matsuura T. Contact Angle and Cell Adhesion of Micro/Nano-Structured Poly(lactic-co-glycolic acid) Membranes for Dental Regenerative Therapy. Dentistry Journal. 2021; 9(11):124. https://doi.org/10.3390/dj9110124
Chicago/Turabian StyleKaga, Naoyuki, Hiroki Fujimoto, Sho Morita, Yuichiro Yamaguchi, and Takashi Matsuura. 2021. "Contact Angle and Cell Adhesion of Micro/Nano-Structured Poly(lactic-co-glycolic acid) Membranes for Dental Regenerative Therapy" Dentistry Journal 9, no. 11: 124. https://doi.org/10.3390/dj9110124
APA StyleKaga, N., Fujimoto, H., Morita, S., Yamaguchi, Y., & Matsuura, T. (2021). Contact Angle and Cell Adhesion of Micro/Nano-Structured Poly(lactic-co-glycolic acid) Membranes for Dental Regenerative Therapy. Dentistry Journal, 9(11), 124. https://doi.org/10.3390/dj9110124





