Therapeutic Effect of Polidocanol Sclerotherapy on Oral Vascular Malformations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
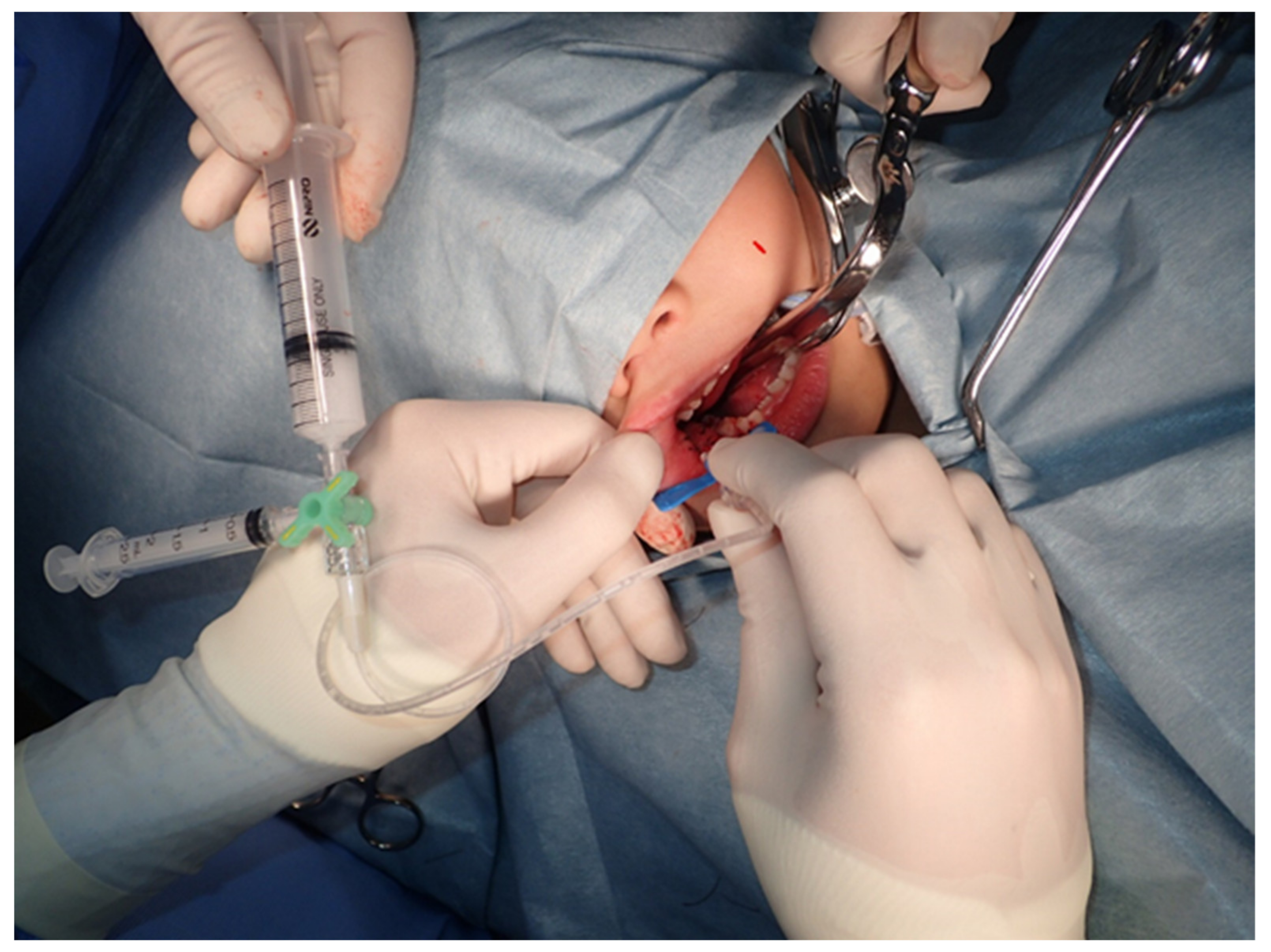
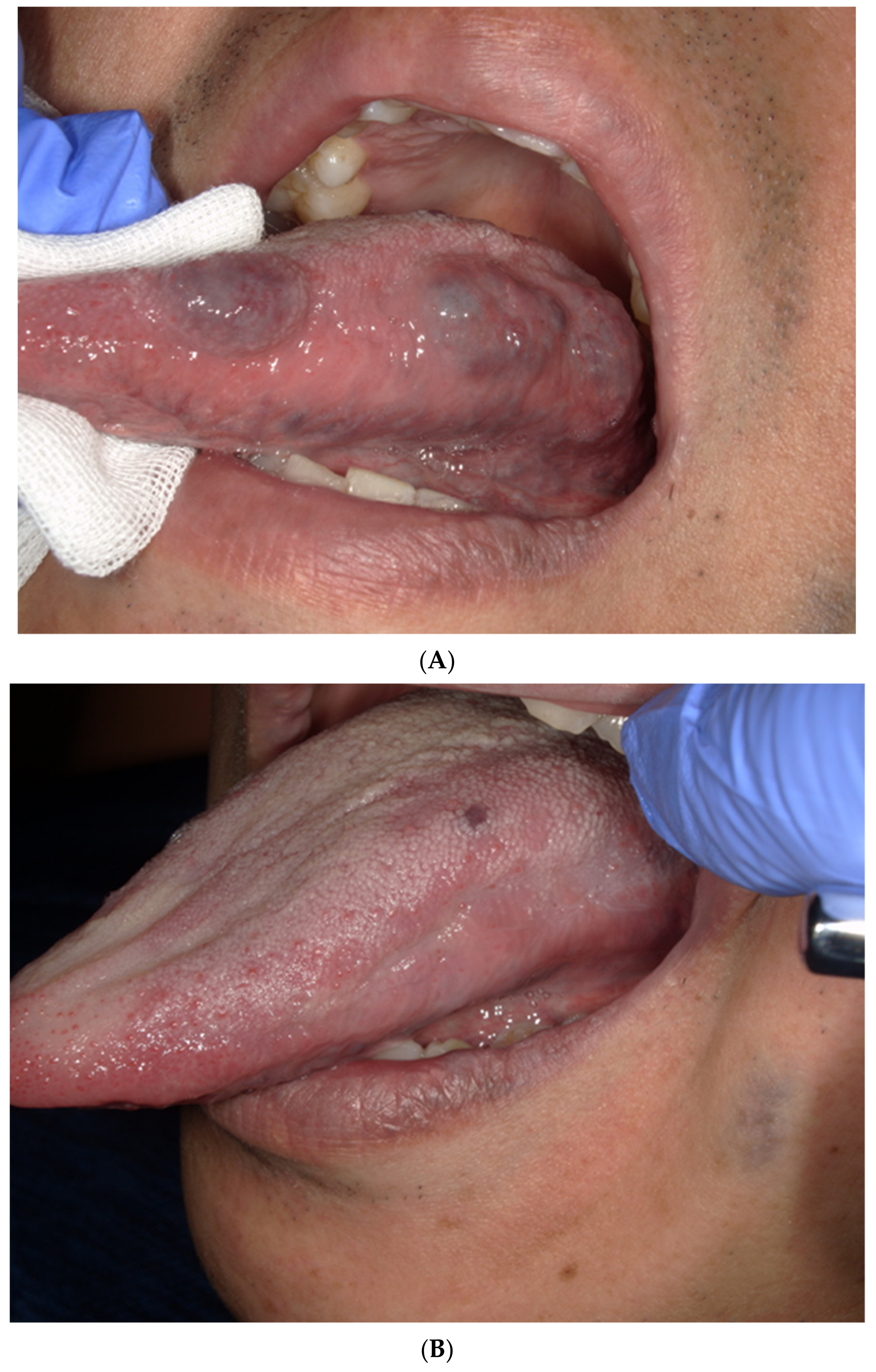
3.2. The Treatment and Side Effects
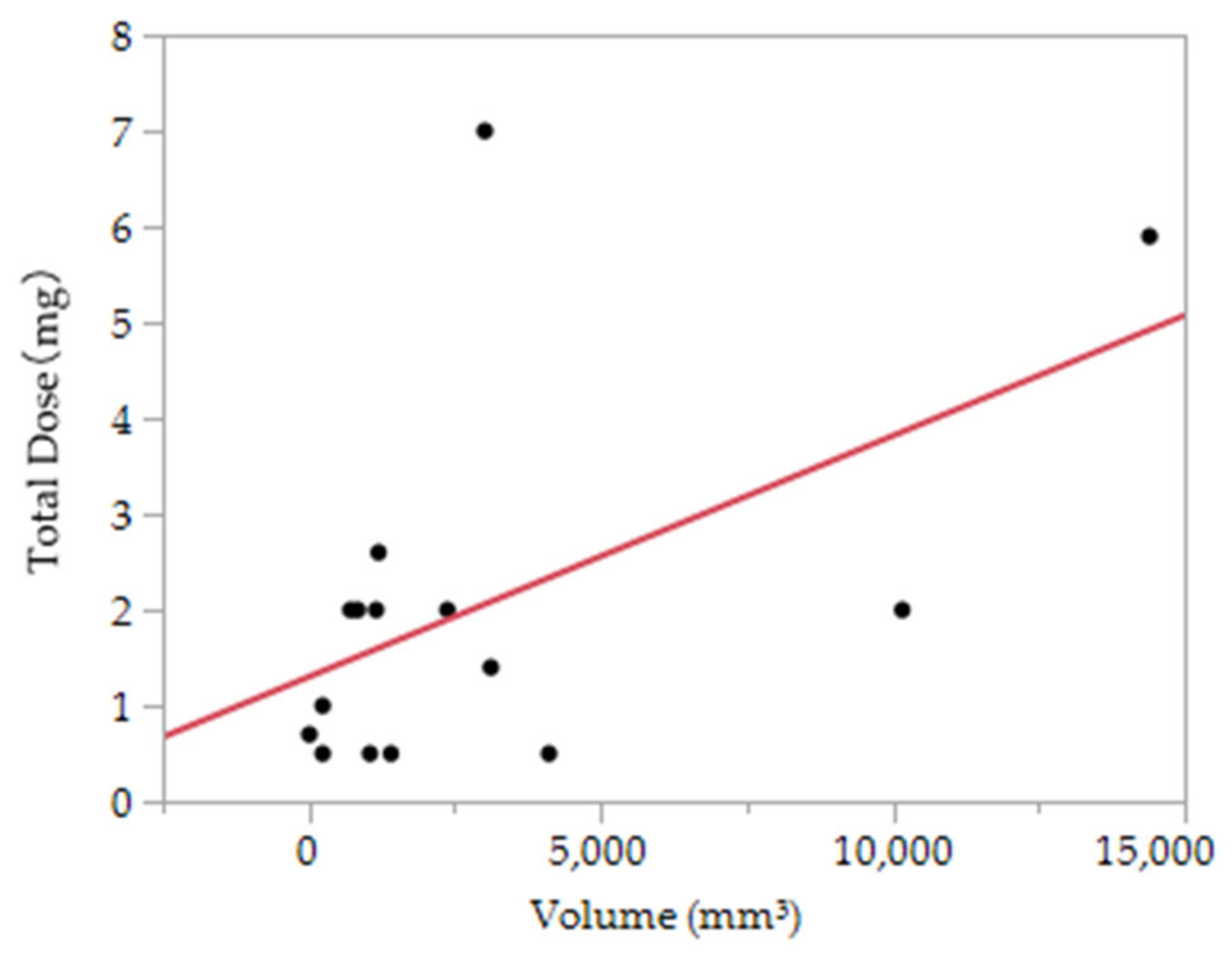
3.3. The Association between the Dose of Polidocanol and the Therapeutic Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulliken, J.B.; Glowacki, J. Hemangiomas and Vascular Malformations in Infants and Children: A Classification Based on Endothelial Characteristics. Plast. Reconstr. Surg. 1982, 69, 412–422. [Google Scholar] [CrossRef]
- Mulliken, J.B. Classification of Vascular Birthmarks. In Vascular Birthmarks-Hemangiomas Malformations; Mulliken, J.B., Young, A.E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 1988; pp. 24–37. [Google Scholar]
- Jackson, I.T.; Carreno, R.; Potparic, Z.; Hussain, K. Hemangioma, Vascular Malformations, and Lymphovenous Malformations: Classification and Methods of Treatment. Plast. Recon. Str. Surg. 1993, 91, 1216–1230. [Google Scholar] [CrossRef]
- Dubois, J.; Garel, L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr. Radiol. 1999, 29, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Soulez, G.; Oliva, V.L.; Berthiaume, M.J.; Lapierre, C.; Therasse, E. Soft-Tissue Venous Malformations in Adult Patients: Imaging and Therapeutic Issues. RadioGraphics 2001, 21, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Legiehn, G.M.; Heran, M.K. Venous Malformations: Classification, Development, Diagnosis, and Interventional Radiologic Management. Radiol. Clin. N. Am. 2008, 46, 545–597. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, B.; Burrows, P.E.; Zurakowski, D.; Mulliken, J.B. Sclerotherapy of Craniofacial Venous Malformations: Complications and Results. Plast. Reconstr. Surg. 1999, 104, 1–11. [Google Scholar] [CrossRef]
- Mulliken, J.B.; Fishman, S.J.; Burrows, P.E. Vascular Anomalies. Curr. Probl. Surg. 2000, 37, 517–584. [Google Scholar] [CrossRef]
- Tessari, L. Nouvelle technique d’obtention de la scléromousee. Phlébologie 2000, 53, 129. [Google Scholar]
- Hammer, F.D.; Boon, L.M.; Mathurin, P.; Vanwijck, R.R. Ethanol Sclerotherapy of Venous Malformations; Evaluation of Systemic Alcohol Contamination. J. Vasc. Interv. Radiol. 2001, 12, 595–600. [Google Scholar] [CrossRef]
- Doganci, S.; Yildirim, V.; Erol, G.; Yesildal, F.; Karabacak, K.; Kadan, M.; Ozkan, G.; Ince, M.E.; Ozgurtas, T. Polido-canol (Lauromacrogol 400) has anti-angiogenic effects in vitro and in vivo. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1384–1389. [Google Scholar]
- Rabe, E.; Pannier-Fischer, F.; Gerlach, H.; Breu, F.X.; Guggenbichler, S.; Zabel, M. Guidelines for sclerotherapy of varicose veins (ICD 10: I83.0, I83.1, I83.2, and I83.9). Dermatol. Surg. 2004, 30, 687–693. [Google Scholar]
- Kobayashi, S.; Crooks, S.; Eckmann, D.M. Dose- and time-dependent liquid sclerosant effects on endothelial cell death. Dermatol. Surg. 2006, 32, 1444–1452. [Google Scholar] [PubMed]
- Gemmete, J.J.; Pandey, A.S.; Kasten, S.J.; Chaudhary, N. Endovascular Methods for the Treatment of Vascular Anomalies. Neuroimaging Clin. N. Am. 2013, 23, 703–728. [Google Scholar] [CrossRef]
- Yakes, W.F. Extremity Venous Malformations: Diagnosis and Management. Semin. Interv. Radiol. 1994, 11, 332–339. [Google Scholar] [CrossRef]
- Li, N.; Li, J.; Huang, M.; Zhang, X. Efficacy and safety of polidocanol in the treatment of varicose veins of lower extremities. Medicine 2021, 100, e24500. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Zhang, A.Q.; Jiang, L.C.; Li, K.Y.; Liu, F.Z.; Yuan, D.Y.; Xu, K.; Fan, Q.C.; Liu, X.B.; Zou, B.; et al. The efficary of polidocanol sclerotherapy in mucocele of the minor salivary gland. J. Oral. Pathol. Med. 2018, 47, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.S.; Shin, K.H.; Na, J.B.; Won, J.Y.; Hahn, S.B. Venous Malformations: Sclerotherapy with a Mixture of Ethanol and Lipiodol. Cardiovasc. Interv. Radiol. 1997, 20, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Shireman, P.K.; McCarthy, W.J.; Yao, J.S.; Vogelzang, R.L. Treatment of Venous Malformations by Direct Injection with Ethanol. J. Vasc. Surg. 1997, 26, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.B.; Kim, D.I.; Huh, S.; Kim, H.H.; Choo, I.W.; Byun, H.S.; Do, Y.S. New Experiences with Absolute Ethanol Sclerotherapy in the Management of a Complex Form of Congenital Venous Malformation. J. Vasc. Surg. 2001, 33, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Goyal, M.; Causer, P.A.; Armstrong, D. Venous Vascular Malformations in Pediatric Patients: Comparison of Results of Alcohol Sclerotherapy with Proposed MR Imaging Classification. Radiology 2002, 223, 639–644. [Google Scholar] [CrossRef]
- Mimura, H.; Kanazawa, S.; Yasui, K.; Fujiwara, H.; Hyodo, T.; Mukai, T.; Dendo, S.; Iguchi, T.; Hiraki, T.; Koshima, I.; et al. Percutaneous sclerotherapy for venous malformations using polidocanol under fluoroscopy. Acta Med. Okayama 2003, 57, 227–234. [Google Scholar]
- Mimura, H.; Fujiwara, H.; Hiraki, T.; Gobara, H.; Mukai, T.; Hyodo, T.; Iguchi, T.; Yasui, K.; Kimata, Y.; Kanazawa, S. Polidocanol Sclerotherapy for Painful Venous Malformations: Evaluation of Safety and Effi-cacy in Pain Relief. Eur. Radiol. 2009, 19, 2474–2480. [Google Scholar] [CrossRef]
- Hou, F.; Chen, J.; Xia, M.; Ding, K.; Zeng, Q.; Liu, W. Percutaneous sclerotherapy with polidocanol under the guidance of ultrasound for venous malformations in children–A retrospective cohort study from a single tertiary medical center. Medicine 2020, 99, e18839. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, H.; Lin, X.; Hu, X.; Jin, Y.; Ma, G. Outcomes and Complications of Sclerotherapy for Venous Malformations. Vasc. Endovasc. Surg. 2013, 47, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Marrocco-Trischitta, M.M.; Guerrini, P.; Abeni, D.; Stillo, F. Reversible Cardiac Arrest after Polidocanol Sclerotherapy of Peripheral Venous Malformation. Dermatol. Surg. 2002, 28, 153–155. [Google Scholar] [PubMed]
- Shimo, T.; Hidaka, K.; Yanagawa, S.; Kadota, W.; Kawakami, S.; Tsuchida, H. Two Episodes of Cardiac Arrest in a Boy Receiving Sclerotherapy with Polydocanol—A Case Report. Masui 2005, 54, 57–59. [Google Scholar] [PubMed]
- Cabrera, J.; Cabrera, J., Jr.; Garcia-Olmedo, M.A.; Redondo, P. Treatment of Venous Malformations with Sclerosant in Microfoam Form. Arch. Dermatol. 2003, 139, 1409–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaki, T.; Nozaki, M.; Sakurai, H.; Takeuchi, M.; Soejima, K.; Kono, T. Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J. Vasc. Surg. 2008, 47, 578–584. [Google Scholar] [CrossRef] [Green Version]
| Case | Age (Years) | Sex | Height (cm) | Body Weight (Kg) | Primary | Volume (mm3) | Total Dose (mL) | Administration Method | Number of Administrations | NRS Score the Next Day | NRS Score after 1 Week | Effect Mesurement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | F | 113 | 20 | Tongue | 3016 | 7 | Foam | 3 | 4 | 0 | PR |
| 2 | 42 | M | 164 | 62 | Right tongue | 1408 | 0.5 | Foam | 1 | 6 | 0 | CR |
| 3 | 42 | M | 164 | 62 | Left tongue | 1050 | 0.5 | Foam | 1 | 6 | 0 | CR |
| 4 | 42 | M | 164 | 62 | Buccal mucosa | 4116 | 0.5 | Foam | 1 | 6 | 0 | CR |
| 5 | 44 | F | 161 | 61 | Oral floor | 2912 | 4.5 | Foam | 2 | 5 | 0 | SD |
| 6 | 44 | F | 161 | 61 | Tongue | 1800 | 1 | Local injection | 1 | 3 | 1 | SD |
| 7 | 44 | F | 161 | 61 | Lip | 240 | 0.5 | Local injection | 1 | 0 | 0 | CR |
| 8 | 23 | F | 157 | 47 | Lip | 240 | 1 | Local injection | 1 | 5 | 0 | CR |
| 9 | 23 | F | 157 | 47 | Tongue | 10,166 | 2 | Local injection | 2 | - | - | PR |
| 10 | 81 | F | 162 | 61 | Tongue | 14,400 | 5.9 | Local injection | 2 | 2 | 0 | PR |
| 11 | 81 | F | 162 | 61 | Lip | 1197 | 2.6 | Local injection | 2 | 0 | 0 | CR |
| 12 | 81 | M | 153 | 49 | Tongue | 840 | 2 | Local injection | 1 | 0 | 0 | CR |
| 13 | 41 | M | 165 | 80 | Tongue | 1155 | 2 | Local injection | 1 | 0 | 0 | CR |
| 14 | 41 | M | 165 | 80 | Tongue | 2376 | 2 | Local injection | 1 | 0 | 0 | CR |
| 15 | 41 | M | 165 | 80 | Buccal mucosa | 3120 | 1.4 | Local injection | 1 | 0 | 0 | CR |
| 16 | 83 | F | 152 | 44 | Lip | 715 | 2 | Foam | 1 | 0 | 0 | CR |
| 17 | 75 | F | 149 | 49 | ip | 12 | 0.7 | Local injection | 1 | 1 | 0 | CR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuzawa, S.; Yamagata, K.; Okubo-Sato, M.; Terada, K.; Uchida, F.; Ishibashi-Kanno, N.; Bukawa, H. Therapeutic Effect of Polidocanol Sclerotherapy on Oral Vascular Malformations. Dent. J. 2021, 9, 119. https://doi.org/10.3390/dj9100119
Fukuzawa S, Yamagata K, Okubo-Sato M, Terada K, Uchida F, Ishibashi-Kanno N, Bukawa H. Therapeutic Effect of Polidocanol Sclerotherapy on Oral Vascular Malformations. Dentistry Journal. 2021; 9(10):119. https://doi.org/10.3390/dj9100119
Chicago/Turabian StyleFukuzawa, Satoshi, Kenji Yamagata, Makiko Okubo-Sato, Kazuhiro Terada, Fumihiko Uchida, Naomi Ishibashi-Kanno, and Hiroki Bukawa. 2021. "Therapeutic Effect of Polidocanol Sclerotherapy on Oral Vascular Malformations" Dentistry Journal 9, no. 10: 119. https://doi.org/10.3390/dj9100119
APA StyleFukuzawa, S., Yamagata, K., Okubo-Sato, M., Terada, K., Uchida, F., Ishibashi-Kanno, N., & Bukawa, H. (2021). Therapeutic Effect of Polidocanol Sclerotherapy on Oral Vascular Malformations. Dentistry Journal, 9(10), 119. https://doi.org/10.3390/dj9100119







